Abstract
Introduction
The activation of the plasmatic coagulation system is a significant contributor to acute myocardial infarction (AMI). This study aimed to investigate the association between the levels of tissue plasminogen activator-inhibitor complex (t-PAIC), thrombin-antithrombin complex (TAT), plasmin-α2 plasmin-inhibitor complex (PIC), and thrombomodulin (TM) with clinical outcomes in patients with AMI.
Methods
Blood samples were collected from 368 patients presenting with acute myocardial infarction in the emergency department to assess levels of t-PAIC, TAT, PIC, and TM. Patients were subsequently followed up for a period of 6 months.
Results
t-PAIC levels were significantly elevated in AMI patients who died compared to those who survived (P < 0.0001). Specifically, of the 368 patients, 48 died and had higher t-PAIC levels above the determined cut-off value of 15.3 ng/mL, while 320 survived and had levels below this threshold (P < 0.001). Furthermore, among the survivors, t-PAIC levels were greater in the major adverse cardiovascular events (MACE) group than in the non-MACE group throughout the 6-month follow-up. Linear regression analysis indicated that high levels of t-PAIC were linked to mortality following acute myocardial infarction and raised the likelihood of MACE in survivors. The ROC curve study revealed that t-PAIC has predictive value for mortality following AMI, with an AUC of 0.871 (95% CI: 0.833–0.904), sensitivity of 81.25%, and specificity of 88.75%. Analysis of the ROC curve and Kaplan–Meier survival curve demonstrated that t-PAIC was able to forecast MACE in individuals who had experienced an AMI, with an AUC of 0.671 (95% CI: 0.620—0.719) for 6-month MACE occurrences.
Conclusion
Our findings suggest that increased t-PAIC levels are correlated with mortality in patients with AMI and the incidence of MACE within a six-month period in survivors.
Keywords: Tissue plasminogen activator-inhibitor complex, Acute myocardial infarction, Major adverse cardiovascular events
Background
Coronary heart disease (CHD) arises from atherosclerotic lesions, leading to stenosis or occlusion of the coronary arteries and consequently causing myocardial ischemia, hypoxia, and tissue damage. The incidence of CHD has been escalating, notably affecting a younger demographic, which significantly impacts patients’ quality of life and safety [1]. AMI, the most severe manifestation of CHD, is characterized by a high incidence of complications and mortality rates. Individuals who survive AMI are at an increased risk of subsequent cardiac events [2]. Thus, predicting the prognosis of MI patients and enhancing secondary prevention measures, particularly for those prone to recurrence, is of paramount importance.
Research indicates that hypercoagulability and thrombosis are pivotal risk factors for CHD [3]. Markers such as the tissue plasminogen activator-inhibitor complex (t-PAIC), thrombin-antithrombin complex (TAT), plasmin-α2 plasmin-inhibitor complex (PIC), and thrombomodulin (TM) provide a comprehensive reflection of the coagulation-fibrinolytic system’s status and the extent of vascular endothelial injury [4]. Current literature suggests that these prothrombotic markers, TAT, PIC, t-PAIC, and TM, possess predictive value for disseminated intravascular coagulation, thrombus formation in malignant tumors, and acute myocardial infarction [5–12].
The prognostic significance of these markers in myocardial infarction remains uncertain. This study aims to elucidate the predictive importance of these four prothrombotic markers in assessing the prognosis of myocardial infarction patients, thereby providing valuable insights for clinical management.
Methods
Study population
A total of 368 patients diagnosed with acute myocardial infarction (AMI) from October 2022 to February 2023 were enrolled in this study. Inclusion criteria: ①The study considers individuals who meet the diagnostic standards as defined by the Fourth Universal Definition of Myocardial Infarction (2018). ②Enrollment is limited to individuals hospitalized within 24 h post-onset of symptoms. ③The age of participation is 18 years of age or older. ④A complete set of clinical medical records is necessary for each participant. ⑤Voluntary informed consent must be obtained from the patient and their family. Exclusion criteria: ①Individuals with cardiomyopathy, valvular heart disease, congenital heart disease, and other heart disorders. ②Patients with malignant tumors, infectious diseases, endocrine disorders, and other significant organ or systemic diseases. ③Those with various thrombotic conditions, a recent history of trauma or surgery. ④Individuals with psychological disorders or impaired cognitive function. ⑤Pregnant or lactating women. This study has been reviewed and granted approval by the Ethics Committee of The Affiliated Hospital of Xuzhou Medical University (Approval No. 2022–056).
Study protocol
All enrolled patients underwent venous blood collection within 10 min of their initial presentation to the emergency department, followed by centrifugation of blood samples at 1000g for 5 min. TAT, PIC, t-PAIC, and TM were quantified using chemiluminescence enzyme immunoassay, strictly following the laboratory’s guidelines. Demographic and clinical characteristics of the patients, including gender, age, body mass index (BMI), family medical history, history of hypertension, and diabetes mellitus, were meticulously recorded. The incidence of MACE, encompassing stable angina pectoris, recurrent myocardial infarction, stent thrombosis, and target vessel revascularization, was assessed throughout a 6-month follow-up period.
Statistical analysis
Statistical analysis was conducted using SPSS version 22.0. Data are presented as the mean ± standard deviation (SD) for normally distributed continuous variables, median ± interquartile range (IQR) for non-normally distributed continuous variables, and frequency (%) for categorical variables. The Shapiro–Wilk test was applied to assess the distribution of values. Continuous variables were compared based on their distribution using the Student’s t-test and Mann–Whitney U-test. Qualitative data were analyzed using either the χ2 test or Fisher’s exact test, depending on the circumstances. The diagnostic performance was assessed using receiver operating characteristic (ROC) curves, and the optimal cutoffs were determined using Youden’s index. Univariate and multivariate logistic regression analyses were performed to evaluate the impact of variables on patient outcomes. Kaplan–Meier curves were generated to compare MACE-free survival between two groups, and the log-rank test was applied to evaluate the differences. A two-tailed p-value of less than 0.05 was deemed statistically significant for all tests.
Results
Clinical characteristics and initial analysis
Table 1 displays the clinical characteristics of our patient cohort. The study encompassed 368 individuals diagnosed with AMI, of which 320 survived. Significant disparities were observed in diabetes mellitus, previous AMI, prior percutaneous coronary intervention (PCI), Killip grade, hs-CRP, NLR, cTnI, TAT, TM, and t-PAIC between the deceased and surviving groups. The surviving cohort was further divided into MACE and non-MACE groups based on the occurrence of MACE events within 6 months post-AMI. Statistical analysis revealed significant differences in diabetes, TM, and t-PAIC between these two groups.
Table 1.
Baseline characteristics
| Variables | Death (48) | Survivor (320) | P value | MACE (70) | Non-MACE (250) | P value |
|---|---|---|---|---|---|---|
| Age | 63.68 ± 11.9 | 63.84 ± 12.0 | 0.931 | 65.48 ± 10.72 | 63.38 ± 12.2 | 0.193 |
| Male, n (%) | 33(68.7) | 223(69.7) | 0.895 | 48(68.5) | 175(70.0) | 0.818 |
| Hypertension, n (%) | 20(41.6) | 147(45.9) | 0.579 | 35(50.0) | 102(40.8) | 0.169 |
| Diabetes, n (%) | 18(37.5) | 61(19.1) | 0.004 | 26(37.1) | 35(14.0) | < 0.001 |
| Active smoker, n (%) | 14(29.1) | 97(30.3) | 0.872 | 20(28.5) | 96(38.4) | 0.131 |
| BMI | 24.84 ± 3.45 | 25.06 ± 3.25 | 0.672 | 24.66 ± 3.62 | 25.17 ± 3.14 | 0.25 |
| Previous AMI, n (%) | 10(20.8) | 24(7.50) | 0.003 | 7(10.0) | 15(6.0) | 0.367 |
| Previous PCI, n (%) | 12(25.0) | 36(11.2) | 0.008 | 11(15.7) | 24(9.60) | 0.147 |
| Killip grade, n | ||||||
| I/II | 18 | 303 | 4 | 6 | ||
| III/IV | 30 | 17 | < 0.001 | 66 | 244 | 0.308 |
| hs-CRP | 25.78 ± 5.02 | 6.06 ± 4.65 | < 0.001 | 7.32 ± 5.17 | 6.47 ± 4.49 | 0.177 |
| NLR | 7.89 ± 1.44 | 4.45 ± 1.15 | < 0.001 | 4.55 ± 1.10 | 4.43 ± 1.17 | 0.444 |
| Ca2+ | 2.26 ± 0.23 | 2.24 ± 0.19 | 0.183 | 2.31 ± 0.27 | 2.29 ± 0.32 | 0.391 |
| Glucose | 7.21 ± 0.91 | 7.09 ± 1.28 | 0.548 | 7.16 ± 1.04 | 7.07 ± 1.16 | 0.278 |
| TC | 3.5 ± 0.65 | 3.4 ± 0.87 | 0.405 | 3.6 ± 0.73 | 3.5 ± 0.92 | 0.221 |
| TG | 1.6 ± 0.58 | 1.5 ± 0.64 | 0.019 | 1.6 ± 0.49 | 1.5 ± 0.83 | 0.053 |
| LDL-C | 2.57 ± 0.77 | 2.49 ± 0.93 | 0.303 | 2.38 ± .84 | 2.41 ± 1.03 | 0.063 |
| HDL-C | 1.01 ± 0.18 | 1.01 ± 0.23 | 0.404 | 0.99 ± 0.29 | 1.00 ± 0.26 | 0.436 |
| cTnT | 6819.00(5178.25,9134.00) | 627.55(199.00,1882.75) | < 0.0001 | 657.85(209.65, 2497.25) | 621.15(184.80, 1717.75) | 0.3536 |
| PIC | 0.69(0.48, 1.23) | 0.65(0.48, 0.85) | 0.3065 | 0.61(0.44, 0.84) | 0.65(0.48, 0.91) | 0.2025 |
| TAT | 4.70(2.78, 13.62) | 2.76(1.58, 5.24) | < 0.0001 | 3.85(1.79, 6.98) | 2.66(1.55, 4.79) | 0.0153 |
| TM | 9.96(7.11, 14.46) | 8.01(6.77, 10.08) | 0.001 | 8.07(6.87, 11.65) | 8.00(6.76, 9.84) | 0.1755 |
| t-PAIC | 23.69(18.06, 32.04) | 9.63(6.60, 12.48) | < 0.0001 | 11.98(8.33, 15.87) | 9.26(6.35, 11.58) | < 0.0001 |
| Drugs, n | ||||||
| DAPT | 46 | 306 | 0.947 | 64 | 242 | 0.052 |
| Aspirin | 45 | 312 | 0.333 | 67 | 245 | 0.279 |
| Clopidogrel | 21 | 105 | 0.136 | 25 | 80 | 0.559 |
| Ticagrelor | 20 | 196 | 0.01 | 42 | 154 | 0.655 |
| Tirofiban | 32 | 197 | 0.496 | 47 | 150 | 0.08 |
| OAC | 10 | 56 | 0.575 | 16 | 40 | 0.106 |
| DTB (min) | 82.4 ± 9.1 | 81.6 ± 8.4 | 0.263 | 81.2 ± 7.9 | 81.7 ± 6.7 | 0.219 |
| PCI times (min) | 28.6 ± 14.4 | 29.9 ± 12.7 | 0.084 | 30.1 ± 13.6 | 29.6 ± 14.1 | 0.164 |
Bolded data are statistically significant
CRP C-reaction protein, DAPT Dual antiplatelet therapy, DTB Door-to-balloon, NLR Neutrophil-to-lymphocyte ratio, TC Total cholesterol, TG Triglyceride, LDL-C Low density lipoprotein cholesterol, HDL-C High density lipoprotein cholesterol, cTnI Cardiac troponin I, OAC Oral anticoagulant, PIC Plasmin-α2 plasmin-inhibitor complex, TAT Thrombin-antithrombin complex, TM Thrombomodulin, t-PAIC Tissue plasminogen activator-inhibitor complex
Logistic regression analysis for mortality
Univariate logistic regression analysis was conducted to explore the relationship between differentially expressed variables and mortality, as detailed in Table 1. The analysis indicated that diabetes, previous AMI, previous PCI, elevated Killip grade, hs-CRP, TAT, TM, and t-PAIC levels were associated with an increased risk of death, as detailed in Table 2. Even after adjusting for diabetes, prior AMI, prior PCI, Killip grade, and hs-CRP using multivariate regression analysis, elevated levels of TAT, TM, and t-PAIC remained significantly associated with an increased risk of death, with odds ratios (OR) of 1.02 (95% CI: 1.00 to 1.05), 1.15 (95% CI: 1.08 to 1.23), and 1.49 (95% CI: 1.28 to 1.97), respectively.
Table 2.
Variables examined in the regression analysis for death
| Variables | Death (Model 1 univariate analysis) | Death (Model 2 multivariate analysis) | ||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Diabetes | 2.54 (1.33–4.86) | 0.005 | ||
| Previous AMI | 3.24 (1.44–7.30) | 0.004 | ||
| Previous PCI | 2.63 (1.25–5.51) | 0.01 | ||
| Killip grade | 10.69 (4.99–22.90) | < 0.001 | ||
| hs-CRP | 6.82 (2.11–22.03) | 0.002 | ||
| TAT | 1.03 (1.01–1.06) | < 0.01 | 1.02 (1.00–1.05) | 0.03 |
| TM | 1.14 (1.07–1.22) | < 0.001 | 1.15 (1.08–1.23) | 0.01 |
| t-PAIC | 1.46 (1.24–2.01) | < 0.001 | 1.49 (1.28–1.97) | < 0.001 |
Bolded data are statistically significant. Model 1: unadjusted. Model 2: adjusted diabetes, Previous AMI, Previous PCI, Killip grade, hs-CRP
OR Odds Ratio, CI Confidence Interval
Risk factors for MACE post-AMI
To delve into the risk factors for MACE following AMI, a 6-month follow-up of survivors was conducted, and logistic regression analysis was applied to the observed variables. The results demonstrated that diabetes and elevated levels of TG, LDL-C, TAT, TM, and t-PAIC heightened the risk of MACE. After adjusting for diabetes, TG, and LDL-C through multivariate logistic analysis, elevated levels of TAT, TM, and t-PAIC continued to increase the risk of MACE, with OR values of 1.08 (95% CI: 1.01 to 1.28), 1.13 (95% CI: 1.07 to 1.46), and 1.41 (95% CI: 1.26 to 1.67) (Table 3).
Table 3.
Variables examined in the regression analysis for MACE
| Variables | MACE (Model 1 univariate analysis) | MACE (Model 2 multivariate analysis) | ||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Diabetes | 3.63 (1.98–6.62) | < 0.001 | ||
| TG | 1.06 (1.01–1.33) | 0.03 | ||
| LDL-C | 1.07 (1.03–1.11) | 0.02 | ||
| TAT | 1.12 (1.05–1.36) | 0.02 | 1.08 (1.01–1.28) | 0.01 |
| TM | 1.08 (1.01–1.26) | < 0.001 | 1.13 (1.07–1.46) | 0.02 |
| t-PAIC | 1.44 (1.21–1.88) | < 0.001 | 1.41 (1.26–1.67) | < 0.001 |
Bolded data are statistically significant. Model 1: unadjusted. Model 2: adjusted diabetes, TG, LDL-C
OR Odds Ratio, CI Confidence Interval
Diagnostic efficacy of biomarkers
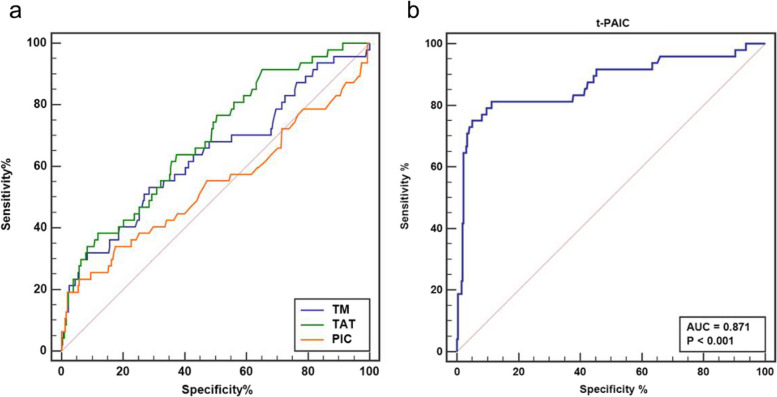
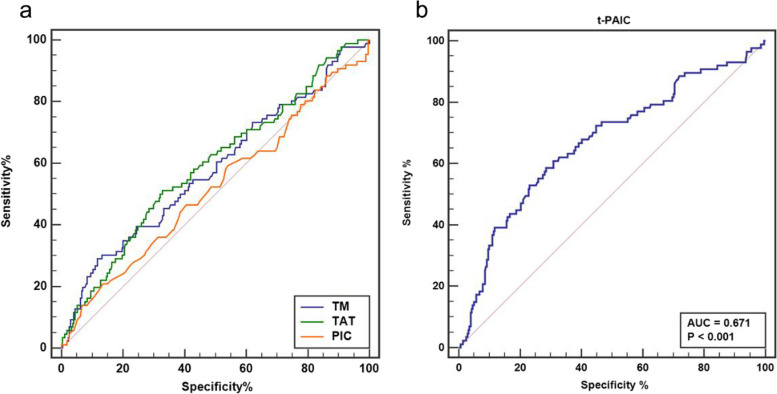
The ROC curve analysis was employed to assess the diagnostic efficacy of TM, TAT, PIC, and t-PAIC as prognostic markers for mortality in AMI patients. t-PAIC exhibited the highest diagnostic efficacy among the four, with an AUC for predicting death subsequent to AMI of 0.871 (95% CI: 0.833 to 0.904), accompanied by a sensitivity of 81.25% and a specificity of 88.75% (Fig. 1). The AUC values for TM, TAT, and PIC were 0.635 (95%CI: 0.583 to 0.685), 0.686 (95%CI: 0.635 to 0.734), and 0.538 (95%CI: 0.485 to 0.591), respectively. The optimal cut-off value for t-PAIC was 15.3 ng/mL, with a positive predictive value of 52.0% and a negative predictive value of 96.9%. For 6-month MACE occurrences in AMI survivors, t-PAIC again showed the highest diagnostic efficacy, with an AUC of 0.671 (95% CI: 0.620 to 0.719), sensitivity 58.62%, specificity 71.53%, as depicted in Fig. 2. The AUC values for TM, TAT, and PIC were 0.581 (95%CI: 0.528 to 0.633), 0.593 (95%CI: 0.540 to 0.644), and 0.519 (95%CI: 0.466 to 0.572), respectively. The cut-off value for t-PAIC was 11.9, with a positive predictive value of 38.9% and a negative predictive value of 84.8%.
Fig. 1.
a: ROC curves for TM, TAT, and PIC in predicting death; b: ROC curves for t-PAIC in predicting death
Fig. 2.
a: ROC curves for TM, TAT, and PIC in predicting MACE; b: ROC curves for t-PAIC in predicting MACE
Prognostic grouping and survival analysis
Based on the 6-month MACE cut-off value for t-PAIC, patients were categorized into high and low t-PAIC groups, with MACE as the primary outcome measure. Kaplan–Meier survival curve analysis revealed significant statistical differences in MACE occurrence between the two groups over a 6-month period (Fig. 3). Furthermore, Kaplan–Meier survival curve analysis was also applied to analyze coronary angiography (CAG) patients, showing a significant statistical disparity in MACE occurrence between the CAG and non-CAG groups.
Fig. 3.
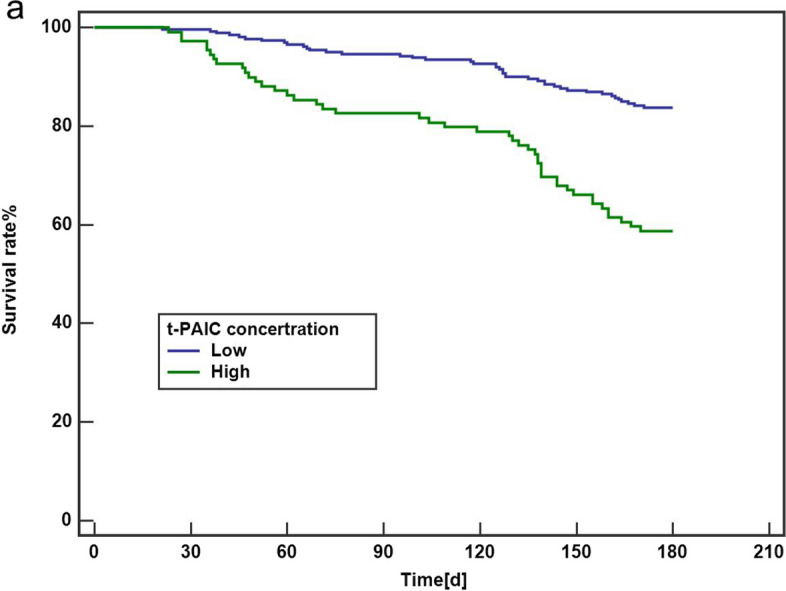
Kaplan–Meier curves for MACE in survivors stratified by t-PAIC concentration above and below the mean
Discussion
The pathogenesis of coronary heart disease (CHD) is intricately linked to thrombotic events, a process marked by considerable complexity. The balance of coagulation, fibrinolysis, and vascular endothelial integrity are pivotal in this context [13]. Currently, standard coagulation assays in laboratory settings include prothrombin time (PT), thrombin time (TT), activated partial thromboplastin time (APTT), coagulation factors, antithrombin, D-dimer, fibrinogen, and fibrin degradation products (FDP), encompassing both coagulation and fibrinolytic systems. However, these markers are typically identified post-thrombotic and exhibit limited sensitivity for monitoring pre-thrombotic states. Consequently, prothrombotic markers such as TAT, PIC, t-PAIC, and TM are increasingly being recognized and utilized. This study revealed two key findings regarding the correlation between TAT, PIC, t-PAIC, and TM with respect to AMI prognosis: (I) t-PAIC is associated with mortality in AMI, with higher levels being associated with increased fatality rates among patients. (II) t-PAIC is also associated with MACE in survivors, with higher levels predicting poorer MACE outcomes.
Research indicates that diabetes, prior AMI, prior PCI, and Killip grade are significantly associated with AMI prognosis. Ritsinger et al. [14] identified in their analysis of 74,000 AMI patients in the SWEDEHEART registry that rates of heart failure and overall mortality were significantly higher in diabetics compared to non-diabetics. Similarly, Marta and colleagues’ study on diabetic patients with AMI revealed a correlation between diabetes duration and post-AMI mortality [15]. This suggests that diabetes mellitus significantly influences AMI prognosis, aligning with our findings. Additionally, our study found that a history of AMI and PCI could elevate mortality rates among AMI patients.
Several studies have explored the relationship between PIC, TAT, TM, t-PAIC, and AMI, primarily focusing on their dynamics during the acute phase of AMI [8, 11, 16]. The predictive value of these markers for AMI prognosis remains inconclusive.
Zunker et al. observed a significant increase in t-PAIC levels in individuals with acute cerebral infarction, with higher levels in major vascular infarction groups compared to microvascular infarction groups, peaking in cardiogenic embolization cases [17]. Jood et al. [18] found that t-PAIC gene polymorphisms were closely associated with acute stroke, and t-PAIC levels remained elevated in stroke patients compared to controls even after three months of follow-up. In the context of AMI, studies have shown that plasma t-PAIC levels are higher in AMI patients than in controls, with particularly high levels in those with poor prognoses [8]. Our study also demonstrated that t-PAIC levels in the AMI fatality group were significantly higher than in the survival group (Table 1), and even after multivariate analysis, high t-PAIC levels continued to predict an increased risk of death in AMI patients (Table 2). However, some earlier studies did not report elevated t-PAIC in AMI patients [16], possibly due to blood sample collection during stable rather than acute stages, typically between 7AM-10AM. Our survival analysis indicated that t-PAIC has predictive power for six-month MACE event probability, as depicted in Figs. 2 and 3.
t-PAIC, an indicator of endothelial cell damage, serves as a molecular marker for the activation of the fibrinolytic system [19]. It is implicated in disseminated intravascular coagulation (DIC) and arteriovenous thrombosis and is a risk factor for venous thromboembolism (VTE) and myocardial infarction [5, 20–22]. AMI often results from coronary artery blockage due to the rupture of an unstable plaque [23]. Cytokine release subsequent to plaque rupture can exacerbate coronary artery spasm, thereby aggravating coronary endothelial injury [24, 25]. Endothelial injury, in turn, increases the formation of intra-coronary thrombosis, and previous literature has shown that endothelial cell injury is closely related to poor cardiovascular disease prognosis [26]. This suggests that elevated t-PAIC levels are associated with higher post-AMI mortality rates.
Endothelial cells play a crucial role in the pathogenesis of ischemic heart disease and its progression [26]. Damage to endothelial cells has been shown to directly contribute to the development of unstable angina pectoris [27]. Nitric oxide, an endothelial cell product, may serve as a biomarker for predicting coronary artery complexity in patients with unstable angina [28]. Coronary endothelial damage is closely linked to acute coronary vascular events. Our study found that t-PAIC, a marker of endothelial injury, was strongly associated with subsequent MACE in AMI survivors.
It is essential to emphasize that the correlation observed between t-PAIC levels and adverse outcomes in AMI does not inherently suggest causation. The pathophysiology of AMI is complex, involving a multitude of interacting factors. Although t-PAIC may act as a sensitive indicator of disease severity, our study does not establish a definitive causal relationship. Future research, ideally with a larger cohort, a longitudinal design, and potentially interventional studies, may be necessary to clarify the causal dynamics between t-PAIC and AMI prognosis.
Conclusions
In conclusion, our study demonstrates that t-PAIC, as a prothrombotic marker, exhibits predictive value for both mortality in AMI patients and the incidence of MACE in survivors within a six-month period. These findings offer significant implications for the clinical management of AMI. And further investigation is required to comprehensively understand the mechanistic role of t-PAIC within the context of AMI and its correlation with clinical outcomes.
Acknowledgements
The authors thank all subjects for participating in this study.
Clinical trial number
Not applicable.
Authors’ contributions
H.C. and Y.M. designed the study; Y.F., M.S., H.X., and S.Z. participated in collecting the data; Y.F. and H.C. carried out total data analysis and drafted the manuscript; Y.M. guided and reviewed this manuscript. All the authors approved the final version of the article.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was conducted following the Declaration of Helsinki and was approved by the Human Research Ethics Committee of The Affiliated Hospital of Xuzhou Medical University. All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi-fan Feng and Ming-yu Su contributed equally to the work.
Contributor Information
Yan-feng Ma, Email: mayanfeng8998@163.com.
Hong-ping Chen, Email: xiaomi0129@163.com.
References
- 1.Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389(10065):197–210. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhry SI, Khan RF, Chen J, et al. National trends in recurrent AMI hospitalizations 1 year after acute myocardial infarction in Medicare beneficiaries: 1999–2010. J Am Heart Assoc. 2014;3(5):e001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shabana, Shahid SU, Sarwar S. The abnormal lipid profile in obesity and coronary heart disease (CHD) in Pakistani subjects. Lipids Health Dis. 2020;19(1):73. [DOI] [PMC free article] [PubMed]
- 4.Chen Q, Shou W, Wu W, Wang G, Cui W. Performance evaluation of thrombomodulin, thrombin-antithrombin complex, plasmin-α2-antiplasmin complex, and t-PA: PAI-1 complex. J Clin Lab Anal. 2019;33(6):e22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada S, Asakura H. Management of disseminated intravascular coagulation associated with aortic aneurysm and vascular malformations. Int J Hematol. 2021;113(1):15–23. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Tang ZY, Fan J, Wu ZQ, Ji Y, Ye SL. The potential of plasma thrombomodulin as a biomarker of portal vein tumor thrombus in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2001;127(9):559–64. [DOI] [PubMed] [Google Scholar]
- 7.Seki Y, Koike T, Yano M, et al. Bone marrow necrosis with dyspnea in a patient with malignant lymphoma and plasma levels of thrombomodulin, tumor necrosis factor-alpha, and D-dimer. Am J Hematol. 2002;70(3):250–3. [DOI] [PubMed] [Google Scholar]
- 8.Van Dreden P, Rousseau A, Savoure A, Lenormand B, Fontaine S, Vasse M. Plasma thrombomodulin activity, tissue factor activity and high levels of circulating procoagulant phospholipid as prognostic factors for acute myocardial infarction. Blood Coagul Fibrinolysis. 2009;20(8):635–41. [DOI] [PubMed] [Google Scholar]
- 9.Liu KL, Lee KT, Chang CH, Chen YC, Lin SM, Chu PH. Elevated plasma thrombomodulin and angiopoietin-2 predict the development of acute kidney injury in patients with acute myocardial infarction. Crit Care. 2014;18(3):R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YH, Chen JH, Tsai WC, et al. Synergistic effect of thrombomodulin promoter -33G/A polymorphism and smoking on the onset of acute myocardial infarction. Thromb Haemost. 2002;87(1):86–91. [PubMed] [Google Scholar]
- 11.Dogra R, Das R, Ahluwalia J, Kumar RM, Talwar KK. Association of thrombomodulin gene polymorphisms and plasma thrombomodulin levels with acute myocardial infarction in north Indian patients. Clin Appl Thromb Hemost. 2013;19(6):637–43. [DOI] [PubMed] [Google Scholar]
- 12.Chao TH, Li YH, Chen JH, et al. Relation of thrombomodulin gene polymorphisms to acute myocardial infarction in patients <or =50 years of age. Am J Cardiol. 2004;93(2):204–7. [DOI] [PubMed] [Google Scholar]
- 13.Papandreou C, Sala-Vila A, Galié S, et al. Association between fatty acids of blood cell membranes and incidence of coronary heart disease. Arterioscler Thromb Vasc Biol. 2019;39(4):819–25. [DOI] [PubMed] [Google Scholar]
- 14.Ritsinger V, Nyström T, Saleh N, Lagerqvist B, Norhammar A. Heart failure is a common complication after acute myocardial infarction in patients with diabetes: a nationwide study in the SWEDEHEART registry. Eur J Prev Cardiol. 2020;27(17):1890–901. [DOI] [PubMed] [Google Scholar]
- 15.Baviera M, Genovese S, Colacioppo P, et al. Diabetes mellitus duration and mortality in patients hospitalized with acute myocardial infarction. Cardiovasc Diabetol. 2022;21(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pineda J, Marín F, Marco P, et al. The prognostic value of biomarkers after a premature myocardial infarction. Int J Cardiol. 2010;143(3):249–54. [DOI] [PubMed] [Google Scholar]
- 17.Zunker P, Schick A, Padró T, Kienast J, Phillips A, Ringelstein EB. Tissue plasminogen activator and plasminogen activator inhibitor in patients with acute ischemic stroke: relation to stroke etiology. Neurol Res. 1999;21(8):727–32. [DOI] [PubMed] [Google Scholar]
- 18.Jood K, Ladenvall P, Tjärnlund-Wolf A, et al. Fibrinolytic gene polymorphism and ischemic stroke. Stroke. 2005;36(10):2077–81. [DOI] [PubMed] [Google Scholar]
- 19.Mei H, Jiang Y, Luo L, et al. Evaluation the combined diagnostic value of TAT, PIC, tPAIC, and sTM in disseminated intravascular coagulation: a multi-center prospective observational study. Thromb Res. 2019;173:20–6. [DOI] [PubMed] [Google Scholar]
- 20.Iba T, Thachil J. Clinical significance of measuring plasminogen activator inhibitor-1 in sepsis. J Intensive Care. 2017;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tipoe TL, Wu W, Chung L, et al. Plasminogen activator inhibitor 1 for predicting sepsis severity and mortality outcomes: a systematic review and meta-analysis. Front Immunol. 2018;9:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iba T, Thachil J. Present and future of anticoagulant therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation: a perspective from Japan. Int J Hematol. 2016;103(3):253–61. [DOI] [PubMed] [Google Scholar]
- 23.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262–75. [DOI] [PubMed] [Google Scholar]
- 24.Itoh T, Mizuno Y, Harada E, Yoshimura M, Ogawa H, Yasue H. Coronary spasm is associated with chronic low-grade inflammation. Circ J. 2007;71(7):1074–8. [DOI] [PubMed] [Google Scholar]
- 25.Keaney JF Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–9. [DOI] [PubMed] [Google Scholar]
- 26.Xu S, Ilyas I, Little PJ, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73(3):924–67. [DOI] [PubMed] [Google Scholar]
- 27.Mutin M, Canavy I, Blann A, Bory M, Sampol J, Dignat-George F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood. 1999;93(9):2951–8. [PubMed] [Google Scholar]
- 28.Acara AC, Bolatkale M. Endothelial nitric oxide level as a predictor of coronary complexity in patients with unstable angina pectoris. Am J Med Sci. 2019;357(6):453–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.