Abstract
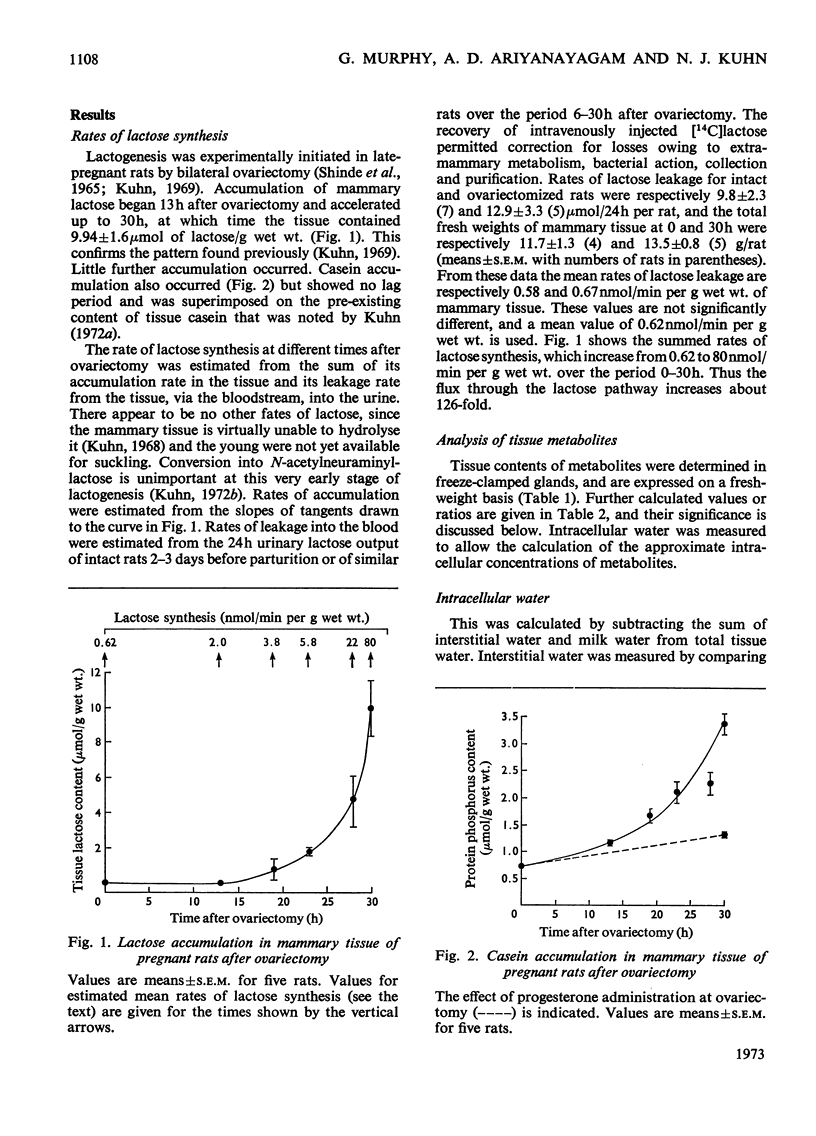
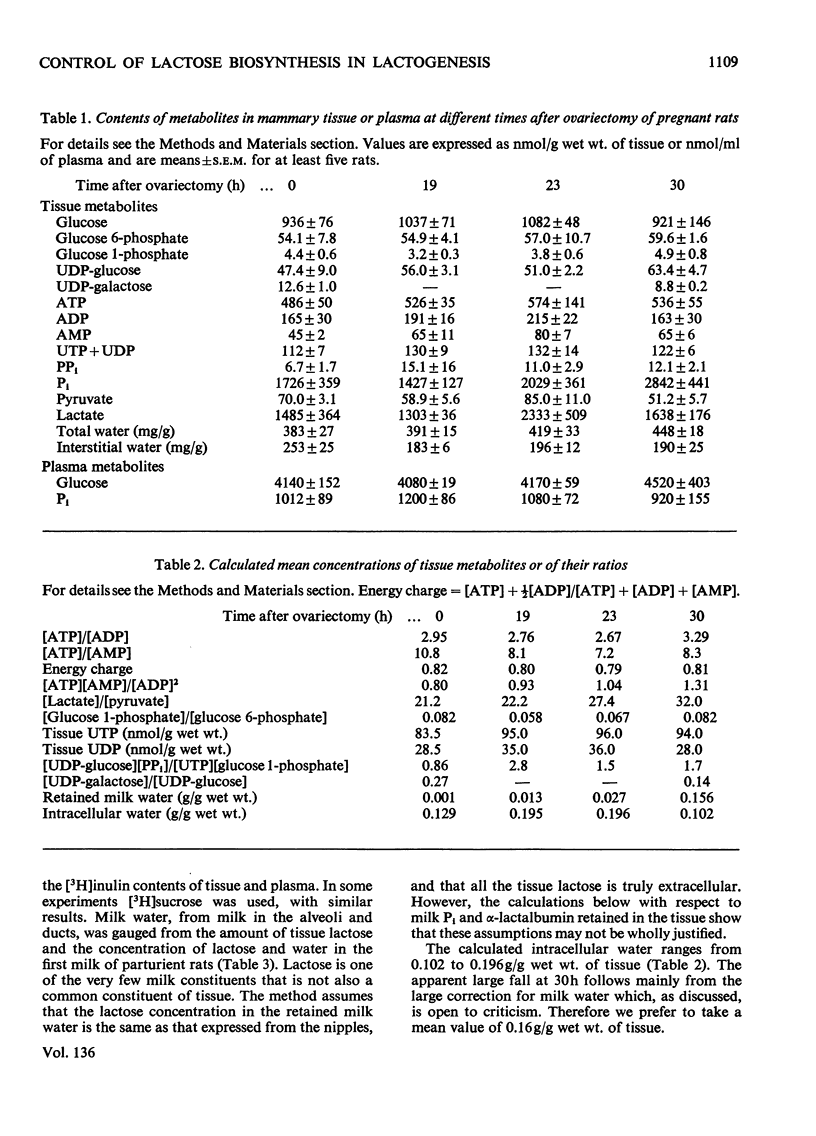
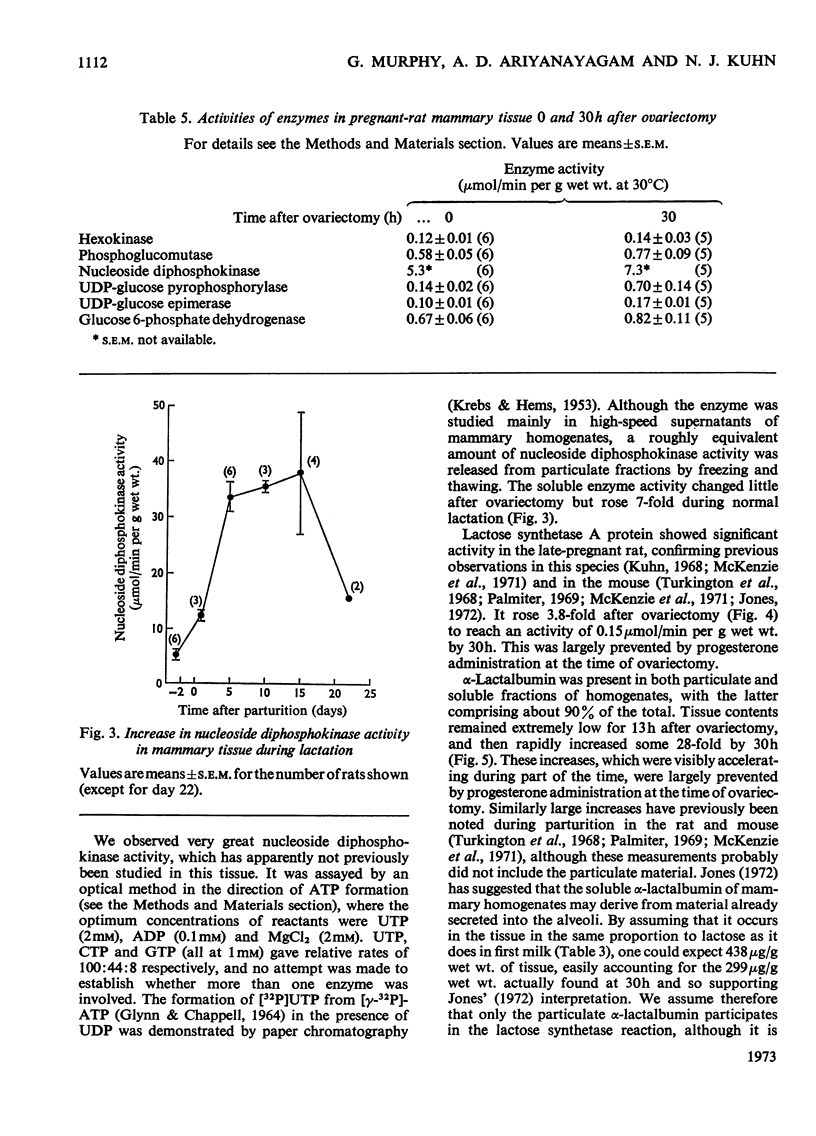
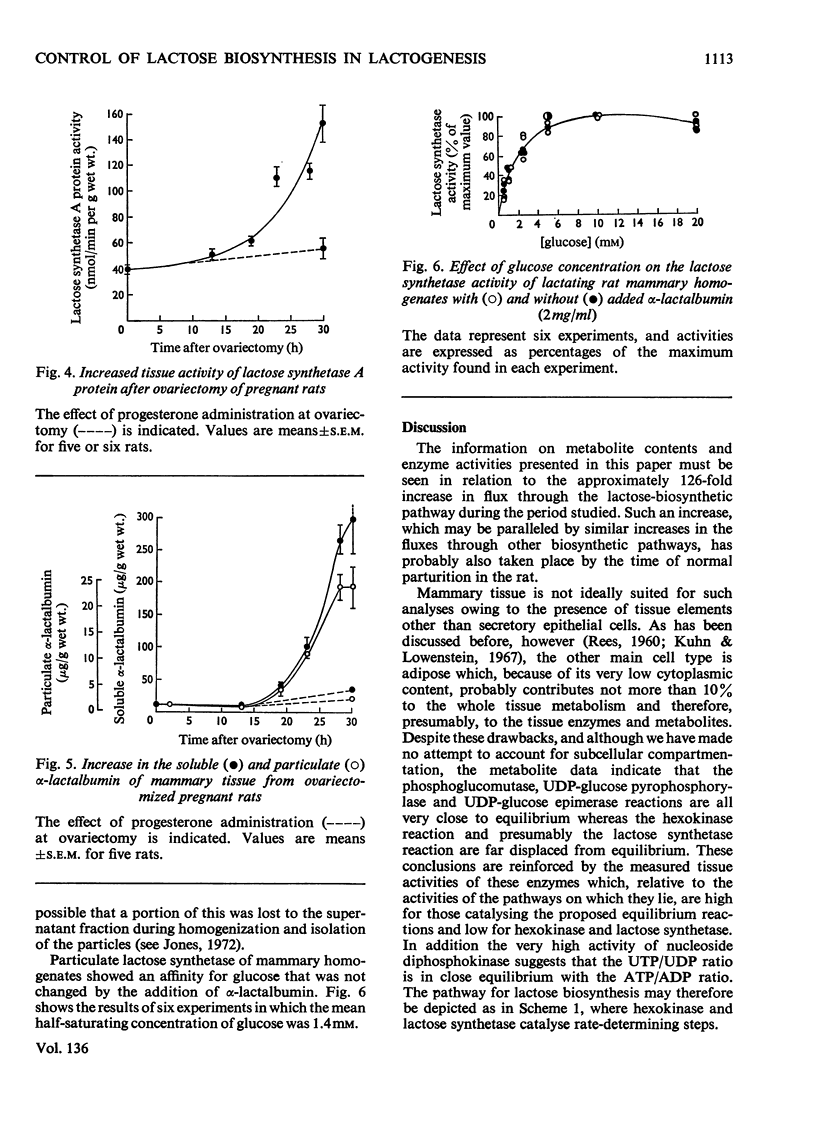
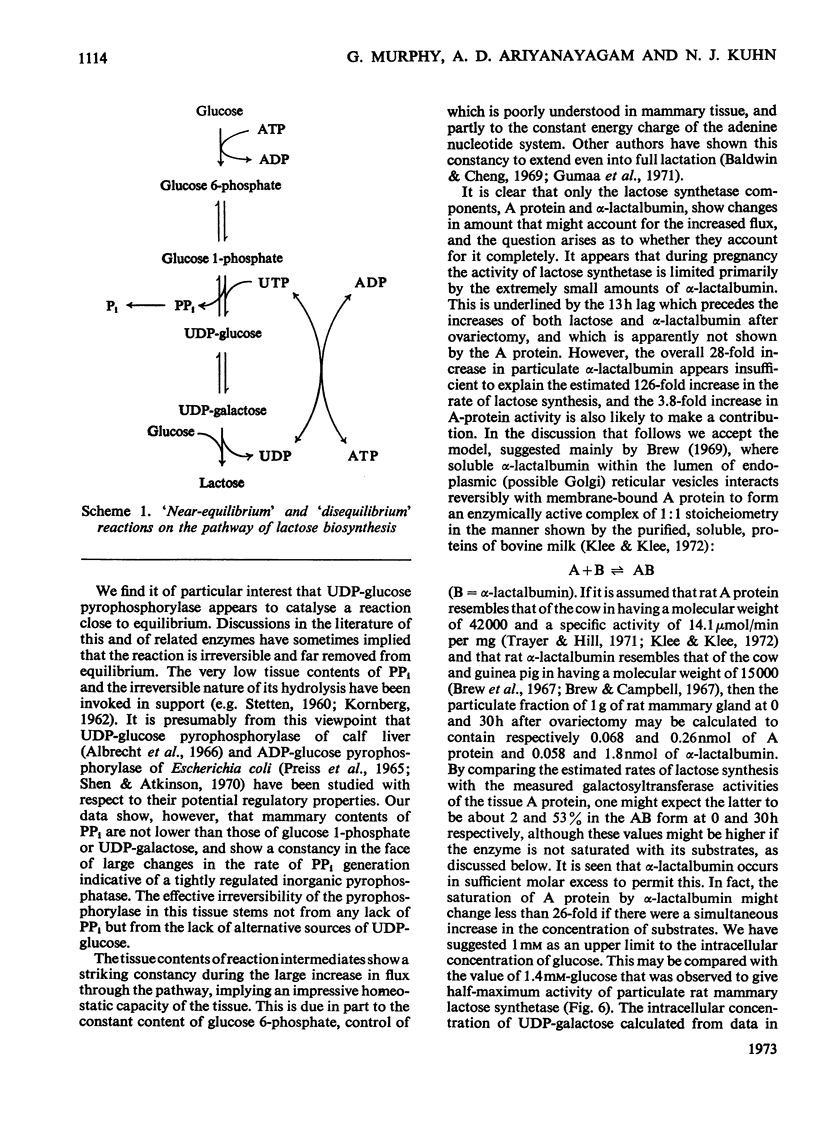
1. Lactogenesis was initiated in pregnant rats by ovariectomy, thereby causing progesterone withdrawal, after which the mammary tissue was analysed for contents of enzymes and metabolites concerned with the biosynthesis of lactose. 2. Lactose synthesis increased about 126-fold with little or no accompanying change in the contents of most metabolic intermediates or in the adenine nucleotide energy charge. 3. Comparison of mass-action ratios with equilibrium constants showed that phosphoglucomutase (EC 2.7.5.1), UDP-glucose pyrophosphorylase (EC 2.7.7.9) and UDP-glucose epimerase (EC 5.1.3.2.) catalysed reactions close to equilibrium. Nucleoside diphosphokinase (EC 2.7.4.6.) activity was very high and probably equilibrates the UTP–UDP and ATP–ADP couples. Lactose synthetase and hexokinase (EC 2.7.1.1) appeared to catalyse rate-limiting reactions. 4. Large increases were seen of UDP-glucose pyrophosphorylase (5-fold), lactose synthetase A protein (3.8-fold) and α-lactalbumin (28-fold), but not of hexokinase, phosphoglucomutase, UDP-glucose epimerase, nucleoside diphosphokinase or glucose 6-phosphate dehydrogenase (EC 1.1.1.49) activities. 5. It appeared that the increased lactose synthesis was largely accounted for by the increased lactose synthetase A protein activity and α-lactalbumin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht G. J., Bass S. T., Seifert L. L., Hansen R. G. Crystallization and properties of uridine diphosphate glucose pyrophosphorylase from liver. J Biol Chem. 1966 Jun 25;241(12):2968–2975. [PubMed] [Google Scholar]
- BAAR S., BULL J. P. Salt interference in sugar chromatography of urine. Nature. 1953 Aug 29;172(4374):414–415. doi: 10.1038/172414a0. [DOI] [PubMed] [Google Scholar]
- BOYER P. D., ROBBINS E. A. Determination of the equilibrium of the hexokinase reaction and the free energy of hydrolysis of adenosine triphosphate. J Biol Chem. 1957 Jan;224(1):121–135. [PubMed] [Google Scholar]
- Baldwin R. L., Cheng W. Metabolite changes associated with initiation and maintenance of lactation in rats and cows. J Dairy Sci. 1969 Apr;52(4):523–528. doi: 10.3168/jds.S0022-0302(69)86598-7. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L., Milligan L. P. Enzymatic changes associated with the initiation and maintenance of lactation in the rat. J Biol Chem. 1966 May 10;241(9):2058–2066. [PubMed] [Google Scholar]
- Ballard F. J. Adenine nucleotides and the adenylate kinase equilibrium in livers of foetal and newborn rats. Biochem J. 1970 Apr;117(2):231–235. doi: 10.1042/bj1170231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Campbell P. N. The characterization of the whey proteins of guinea-pig milk. The isolation and properties of alpha-lactalbumin. Biochem J. 1967 Jan;102(1):258–264. doi: 10.1042/bj1020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K. Secretion of alpha-lactalbumin into milk and its relevance to the organization and control of lactose synthetase. Nature. 1969 May 17;222(5194):671–672. doi: 10.1038/222671a0. [DOI] [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. Comparison of the amino acid sequence of bovine alpha-lactalbumin and hens egg white lysozyme. J Biol Chem. 1967 Aug 25;242(16):3747–3749. [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denamur R. Reviews of the progress of dairy science. Section A. Physiology. Hormonal control of lactogenesis. J Dairy Res. 1971 Jun;38(2):237–264. doi: 10.1017/s0022029900019348. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. K., Colvin B., Mawal R., Ebner K. E. Enzymic assay for galactosyl transferase activity of lactose synthetase and alpha-lactalbumin in purified and crude systems. Anal Biochem. 1970 Jul;36(1):43–61. doi: 10.1016/0003-2697(70)90330-1. [DOI] [PubMed] [Google Scholar]
- Flodgaard H. Isotope derivative method for determination of microquantities of inorganic pyrophosphate in biological material. Eur J Biochem. 1970 Aug;15(2):273–279. doi: 10.1111/j.1432-1033.1970.tb01004.x. [DOI] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Levels of enzymes of the direct oxidative pathway of carbohydrate metabolism in mammalian tissues and tumours. Biochem J. 1954 Jan;56(1):171–175. doi: 10.1042/bj0560171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul B., Dils R. Enzymic changes in rabbit and rat mammary gland during the lactation cycle. Biochem J. 1969 Apr;112(3):293–301. doi: 10.1042/bj1120293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Wolpert E. B., Giger K. E., Morgan H. E. Regulation of protein synthesis in heart muscle. 3. Effect of anoxia on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2171–2178. [PubMed] [Google Scholar]
- Jones E. A. Studies on the particulate lactose synthetase of mouse mammary gland and the role of -lactalbumin in the initiation of lactose synthesis. Biochem J. 1972 Jan;126(1):67–78. doi: 10.1042/bj1260067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R. Some reactions of adenosine and inosine phosphates in animal tissues. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):172–180. doi: 10.1016/0006-3002(53)90136-x. [DOI] [PubMed] [Google Scholar]
- Keppler D., Rudigier J., Decker K. Enzymic determination of uracil nucleotides in tissues. Anal Biochem. 1970 Nov;38(1):105–114. doi: 10.1016/0003-2697(70)90160-0. [DOI] [PubMed] [Google Scholar]
- Klee W. A., Klee C. B. The interaction of -lactalbumin and the A protein of lactose synthetase. J Biol Chem. 1972 Apr 25;247(8):2336–2344. [PubMed] [Google Scholar]
- Kuhn N. J. Changes in the protein phosphorus content of rat mammary tissue during lactogenesis. J Endocrinol. 1972 Oct;55(1):219–220. doi: 10.1677/joe.0.0550219. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J. Lactogenesis in the rat. Metabolism of uridine diphosphate galactose by mammary gland. Biochem J. 1968 Feb;106(3):743–748. doi: 10.1042/bj1060743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., Lowenstein J. M. Lactogenesis in the rat. Changes in metabolic parameters at parturition. Biochem J. 1967 Dec;105(3):995–1002. doi: 10.1042/bj1050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J. Progesterone withdrawal as the lactogenic trigger in the rat. J Endocrinol. 1969 May;44(1):39–54. doi: 10.1677/joe.0.0440039. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J. The lactose and neuraminlactose content of rat milk and mammary tissue. Biochem J. 1972 Nov;130(1):177–180. doi: 10.1042/bj1300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- MCILWAIN H., BUDDLE H. L. Techniques in tissue metabolism. I. A mechanical chopper. Biochem J. 1953 Feb;53(3):412–420. doi: 10.1042/bj0530412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J., Baldwin R. L. Effects of insulin and anti-insulin serum treatments on levels of metabolites in rat mammary glands. Endocrinology. 1971 Apr;88(4):868–871. doi: 10.1210/endo-88-4-868. [DOI] [PubMed] [Google Scholar]
- McKenzie L., Fitzgerald D. K., Ebner K. E. Lactose synthetase activities in rat and mouse mammary glands. Biochim Biophys Acta. 1971;230(3):526–530. doi: 10.1016/0304-4165(71)90183-8. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J. M., Ross B. D., Krebs H. A. Metabolic activities of the isolated perfused rat kidney. Biochem J. 1967 Jun;103(3):852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Kellie A. E. The effects of oestradiol on the acid-soluble nucleotides of rat uterus. Biochem J. 1970 Sep;119(2):187–191. doi: 10.1042/bj1190187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Hormonal induction and regulation of lactose synthetase in mouse mammary gland. Biochem J. 1969 Jun;113(2):409–417. doi: 10.1042/bj1130409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES E. D. Respiration of epithelial component of mammary gland slices. Am J Physiol. 1960 Dec;199:1067–1069. doi: 10.1152/ajplegacy.1960.199.6.1067. [DOI] [PubMed] [Google Scholar]
- Rose I. A. The state of magnesium in cells as estimated from the adenylate kinase equilibrium. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1079–1086. doi: 10.1073/pnas.61.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINDE Y., OTA K., YOKOYAMA A. LACTOSE CONTENT OF MAMMARY GLANDS OF PREGNANT RATS NEAR TERM: EFFECT OF REMOVAL OF OVARY, PLACENTA AND FOETUS. J Endocrinol. 1965 Jan;31:105–114. doi: 10.1677/joe.0.0310105. [DOI] [PubMed] [Google Scholar]
- STETTEN D., Jr Biosynthesis and pyrophosphate. Am J Med. 1960 Jun;28:867–870. doi: 10.1016/0002-9343(60)90195-9. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970 Sep;119(2):193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz D. W., Passonneau J. V., Lowry O. H. An enzymic method for the measurement of inorganic phosphate. Anal Biochem. 1967 May;19(2):300–314. doi: 10.1016/0003-2697(67)90166-2. [DOI] [PubMed] [Google Scholar]
- Shen L. C., Atkinson D. E. Regulation of adenosine diphosphate glucose synthase from Escherichia coli. Interactions of adenylate energy charge and modifier concentrations. J Biol Chem. 1970 Aug 10;245(15):3996–4000. [PubMed] [Google Scholar]
- Start C., Newsholme E. A. The effects of starvation and alloxan-diabetes on the contents of citrate and other metabolic intermediates in rat liver. Biochem J. 1968 Apr;107(3):411–415. doi: 10.1042/bj1070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUBOI K. K., PRICE T. D. Isolation, detection and measure of microgram quantities of labeled tissue nucleotides. Arch Biochem Biophys. 1959 Mar;81(1):223–237. doi: 10.1016/0003-9861(59)90192-4. [DOI] [PubMed] [Google Scholar]
- Trayer I. P., Hill R. L. The purification and properties of the A protein of lactose synthetase. J Biol Chem. 1971 Nov;246(21):6666–6675. [PubMed] [Google Scholar]
- Turkington R. W., Brew K., Vanaman T. C., Hill R. L. The hormonal control of lactose synthetase in the developing mouse mammary gland. J Biol Chem. 1968 Jun 25;243(12):3382–3387. [PubMed] [Google Scholar]
- Turkington R. W., Hill R. L. Lactose synthetase: progesterone inhibition of the induction of alpha-lactalbumin. Science. 1969 Mar 28;163(3874):1458–1460. doi: 10.1126/science.163.3874.1458. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Raijman L., Krebs H. A. Equilibrium relations between the cytoplasmic adenine nucleotide system and nicotinamide-adenine nucleotide system in rat liver. Biochem J. 1970 Apr;117(3):499–503. doi: 10.1042/bj1170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON D. B., HOGNESS D. S. THE ENZYMES OF THE GALACTOSE OPERON IN ESCHERICHIA COLI. I. PURIFICATION AND CHARACTERIZATION OF URIDINE DIPHOSPHOGALACTOSE 4-EPIMERASE. J Biol Chem. 1964 Aug;239:2469–2481. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Walters E., McLean P. Effect of alloxan-diabetes and treatment with anti-insulin serum on pathways of glucose metabolism in lactating rat mammary gland. Biochem J. 1968 Sep;109(3):407–417. doi: 10.1042/bj1090407. [DOI] [PMC free article] [PubMed] [Google Scholar]


