Abstract
Pancreatic diseases pose considerable health challenges due to their complex etiology and limited therapeutic options. Mitochondrial uncoupling protein 2 (UCP2), highly expressed in pancreatic tissue, participates in numerous physiological processes and signaling pathways, indicating its potential relevance in these diseases. Despite this, UCP2’s role in acute pancreatitis (AP) remains underexplored, and its functions in chronic pancreatitis (CP) and pancreatic steatosis are largely unknown. Additionally, the mechanisms connecting various pancreatic diseases are intricate and not yet fully elucidated. Given UCP2’s diverse functionality, broad expression in pancreatic tissue, and the distinct pathophysiological features of pancreatic diseases, this review offers a comprehensive analysis of current findings on UCP2’s involvement in these conditions. We discuss recent insights into UCP2’s complex regulatory mechanisms, propose that UCP2 may serve as a central regulatory factor in pancreatic disease progression, and hypothesize that UCP2 dysfunction could significantly contribute to disease pathogenesis. Understanding UCP2’s role and mechanisms in pancreatic diseases may pave the way for innovative therapeutic and diagnostic approaches.
Graphical Abstracts
Keywords: Mitochondrial uncoupling protein 2, Pancreatic diseases, Pancreatitis, Pancreatic cancer, Diabetes mellitus, Reactive oxygen species
Introduction
Mitochondria were traditionally regarded as the primary energy centers of eukaryotic cells (Zheng et al. 2023). However, a deeper understanding of mitochondria has revealed increasing evidence that they function as multifunctional, dynamic organelles engaged in genetic information processing, energy conversion, biosynthesis, and signal transduction. These organelles are essential components of the mitochondrial information processing system (MIPS) and play a crucial role in biological regulation through three primary steps: sensing, integration, and signal transduction (Picard and Shirihai 2022). Uncoupling proteins (UCPs), a class of mitochondrial carriers, primarily regulate reactive oxygen species (ROS) production during mitochondrial oxidative phosphorylation and also participate in mitochondria-related signal transduction (Cadenas 2018).
UCP2 is the most widely distributed uncoupling protein in the human body, with broad expression across the brain, liver, pancreas, muscle, and immune cells, where it plays a pivotal role in energy homeostasis and the regulation of ROS (Luby and Alves-Guerra 2022). Its critical functions in oxidative stress management and metabolic regulation, particularly its impact on insulin secretion and glucose and lipid metabolism, have drawn significant research interest (Diano and Horvath 2012). Importantly, UCP2 exhibits over 95% homology between humans and mice, a much higher similarity compared to other UCPs (Caggiano and Taniguchi 2024). Further underscoring its research relevance.
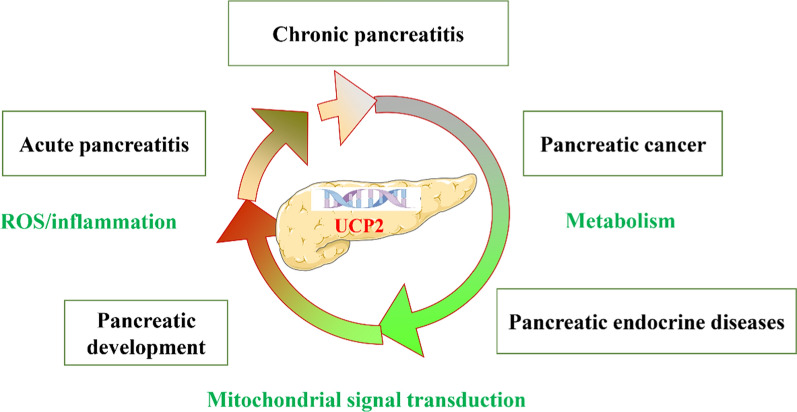
The pancreas, a digestive gland with both endocrine and exocrine functions, plays a vital role in regulating various metabolic processes. Pancreatic dysfunction can result in conditions such as pancreatitis, diabetes, and pancreatic cancer (Guillaumond et al. 2014; Schlünder et al. 2024). Rising incidence and prevalence of pancreatic diseases contribute to a substantial healthcare burden (Ouyang et al. 2020; Chen et al. 2020). UCP2 has attracted significant research interest due to its extensive expression in pancreatic tissue and its crucial roles in oxidative stress regulation and metabolic processes (Zhang et al. 2001; Galetti et al. 2009). Studies suggest that UCP2 influences the proliferation of pancreatic islet α and β cells, as well as the secretion of insulin and glucagon, thereby affecting glucose and lipid metabolism (Luo et al. 2022). Additionally, UCP2 may be involved in pancreatic development via the ROS-AKT signaling pathway (Broche et al. 2018). In models of acute pancreatitis (AP), UCP2 knockdown inhibits the proliferation of pancreatic stellate cells (Muller et al. 2016), and modulates macrophage redox responses, impacting the progression of KRAS-associated pancreatic cancer (Raho et al. 2020).
The role of UCP2 in acinar cell injury and macrophage regulation during AP remains unclear, and studies on UCP2 in chronic pancreatitis (CP) are limited. It is also unknown whether UCP2 influences pancreatic fat infiltration or fatty pancreas development, and by what mechanisms this may occur (Petrov 2023). Additionally, the mechanisms underlying recurrent AP, fibrosis in CP, and progression to pancreatic cancer are poorly understood, with few effective clinical targets available. The impact of pancreatitis episodes on glucose regulation and the development of diabetes also requires further investigation. Given UCP2’s diverse functions, widespread expression in pancreatic tissue, and the interconnected pathophysiology of pancreatic diseases, this review examines current findings on UCP2’s regulatory role, proposing that UCP2 dysfunction may play a central role in pancreatic disease pathogenesis. Understanding UCP2’s mechanisms could offer novel therapeutic and diagnostic insights.
Regulation of UCP2 in pancreatic diseases
The regulation of UCP2 in pancreatic diseases encompasses several mechanisms, including gene mutations, transcription factors influencing UCP2 expression in pancreatic diseases, and UCP2-related epigenetic modifications. Investigating UCP2 regulation is essential for understanding its role in pancreatic diseases and underscores its potential as a central therapeutic target.
Mutations of UCP2 in pancreatic diseases
Genetic polymorphism, defined as the presence of two or more allelic variants of a gene at the same locus with a variation frequency generally exceeding 1%, can affect gene expression and function, leading to biological differences between individuals (Krauss et al. 2005). Genetic polymorphism is a crucial source of biodiversity and serves as the basis for evolution and natural selection. Major types of genetic polymorphisms include single nucleotide polymorphisms (SNPs), insertion/deletion polymorphisms (Indels), repetitive sequence polymorphisms (RSPs), and structural variants (SV) (Hayashi et al. 2021).
The SNPs of UCP2 primarily include the 866G/A polymorphism in the promoter region and the Ala55Val polymorphism in the exon region. The Indels mainly involve the insertion of a 45 bp sequence in exon 8 of the 3′ untranslated region of the UCP2 gene (Jia et al. 2009; Donadelli et al. 2014). The relative mean mutation frequencies of 866G/A and Ala55Val were similar, at approximately 37% and 39.6%, respectively (Dalgaard 2011). Additionally, the 866G/A and Ala55Val polymorphisms may have a combinatorial effect; for example, individuals carrying both the 866G/A and Val55 alleles may exhibit higher UCP2 activity and stronger antioxidant capacity (Nicoletti et al. 2017).
The distribution and frequency of these UCP2 gene polymorphisms may vary among different populations and can affect susceptibility to pancreas-related diseases, such as insulin secretion and type 2 diabetes mellitus, differently in various individuals and genders (Andersen et al. 2013; Souza et al. 2013). By studying these polymorphisms and their functional significance, the role of UCP2 in pancreatic diseases can be better understood. Two studies have comprehensively summarized the impact of UCP2 gene polymorphisms on metabolic diseases (Jia et al. 2009; Donadelli et al. 2014). We have built upon these studies to summarize and update our understanding of the role of UCP2 gene polymorphisms in pancreatic diseases in recent years (Table 1).
Table 1.
Summary and update of the role of UCP2 gene polymorphisms in pancreatic diseases in recent years
| Years | Race | UCP2 Genetic polymorphism | Biological effect | Refs. |
|---|---|---|---|---|
| 2006 | Caucasians | 866G/A | Type 2 diabetes susceptibility | Gable et al. (2006) |
| 2002 | Austrian Caucasians | 866G/A | Inhibits insulin secretion | Krempler et al. (2002) |
| 2004 | Japanese | 866G/A | Inhibits insulin secretion | Sasahara et al. (2004) |
| 2004 | Italian Caucasian | 866G/A | Increased risk of type 2 diabetes | D'Adamo et al. (2004) |
| 2005 | Americans | Ala55Val | Increased risk of type 2 diabetes | Yu et al. (2005) |
| 2010 | Northern Indians | 866G/A | Increased risk of Hyperinsulinemia | Srivastava et al. (2010) |
| 2013 | Danes | 866G/A | Increased risk of type 2 diabetes | Andersen et al. (2013) |
| 2011 | Asian descent | Ala55Val | Increased risk of type 2 diabetes | Xu et al. (2011) |
| 2009 | European American women | Ala55Val | Increased risk of type 2 diabetes | Willig et al. (2009) |
| 2011 | Asian Indians | Ala55Val and −55C/T | Decreased risk of type 2 diabetes | Vimaleswaran et al. (2011) |
| 2008 | Koreans | UCP2 −5331G > A and UCP3 −2078C > T | Increased risk of type 2 diabetes | Lee et al. (2008) |
| 2008 | patients form Necker-Enfants Malades Hospital | Ucp2 variants (G174D and A268G) | Promotes insulin secretion | González-Barroso et al. (2008) |
| 2023 | Kashmiri population of Northern India | 866G/A | Increased risk of type 2 diabetes | Din et al. (2023) |
| 2021 | Asians | 866G/A | Decreased risk of type 2 diabetes | Huang et al. (2021) |
| 2021 | Asians | Ala55Val | Increased risk of type 2 diabetes | Huang et al. (2021) |
| 2021 | Russians | Ucp2 T/T variant | Increased risk of type 2 diabetes | Lapik et al. (2021) |
| 2021 | North-west of Iran | 45 bp I/D polymorphism in 3'UTR of UCP2 | Increased risk of type 2 diabetes | Rezapour et al. (2021) |
| 2021 | Asians | 866G/A | Increased risk of type 2 diabetes | Xu et al. (2021a) |
| 2020 | Northern Chinese population | 866G/A | Increased risk of type 2 diabetes | Hou et al. (2020) |
| 2019 | South Indian population | 866G/A | Increased risk of type 2 diabetes | Gomathi et al. (2019) |
| 2013 | Asians | UCP2 Ala55Val and UCP3 −55C/T | Increased risk of type 2 diabetes | Souza et al. (2013) |
Of the 21 studies we summarized, 11 focused on the −866G/A polymorphism of UCP2. Except for one study that indicated the −866G/A polymorphism reduces the risk of type 2 diabetes in Asian populations (Huang et al. 2021), the remaining studies showed that the −866G/A polymorphism predisposes individuals to an increased risk of developing type 2 diabetes. Similarly, 6 out of seven studies on the Ala55Val polymorphism associated it with an increased risk of type 2 diabetes, with the single study showing a negative association also based on an Asian population (Vimaleswaran et al. 2011). This may be related to selection bias in the studies. Additionally, one study showed that UCP2 variants (G174D and A268G) promoted insulin secretion (Lee et al. 2008), while another indicated that the UCP2 T/T variant increased the risk of type 2 diabetes (Lapik et al. 2021). Collectively, we conclude that genetic polymorphisms in UCP2 increase the risk of type 2 diabetes mellitus.
Transcription factor of UCP2 in Pancreatic Diseases
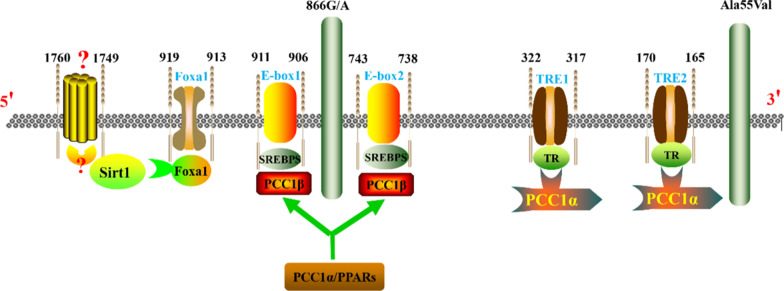
The transcriptional regulation of the UCP2 gene encompasses various mechanisms, such as transcription factors, cis-acting elements, epigenetic modifications, and environmental influences. These intricate regulatory mechanisms precisely control UCP2 gene expression under diverse physiological and pathological conditions, thereby fully elucidating the role of UCP2 in pancreatic diseases. The mouse and human UCP2 genes are located on chromosomes 7 and 11, respectively. Both genes comprise eight exons (six coding and two non-coding) and seven introns (Donadelli et al. 2014). The human UCP2 gene transcription and mutations are detailed schematically in Fig. 1. Transcription factors that bind to the UCP2 promoter include forkhead box protein O1(Foxa1), silent mating type information regulation 2 homolog-1, (SIRT1), sterol regulatory element binding protein isoforms (SREBP), thyroid hormone response elements (TRE), and helix-loop-helix protein binding sites (E-box).
Fig. 1.
The human UCP2 gene transcription and mutations
Initial interest in the role of Foxa1 in pancreatic disease arose from observations of hypoglycemia and abnormal changes in glucose metabolism in Foxa1 knockout mice (Shih et al. 1999). Subsequent studies in β-cells revealed reduced ATP synthesis following Foxa1 knockdown, accompanied by increased expression of UCP2. Chromatin immunoprecipitation assays further confirmed UCP2 as a direct transcriptional target of Foxa1 in vivo (Vatamaniuk et al. 2006). More importantly, Foxa1 has been shown to repress UCP2 gene transcription by binding to the − 919 to − 913 elements (Song et al. 2014). Controversially, Foxa1 is suggested to bind to the Ucp2 promoter at a preferred site located between − 1760 and − 1749 bp relative to the gene’s transcription start site, yet conclusive direct evidence is lacking (Donadelli et al. 2014). Additionally, a recent study demonstrated that silencing Foxa1 promotes UCP2 expression (Bao et al. 2022).
It was reported that peroxisome proliferator-activated receptor-γ coactivator-1 α (PGC-1α) promotes thyroid hormone-mediated transcriptional activation of the UCP2 gene in INS-1E cells (Oberkofler et al. 2009). Hannes Oberkofler et al. (Oberkofler et al. 2006) identified two TREs at positions − 322/− 317 (TRE1) and − 170/− 165 (TRE2). Mutations in TRE1 or TRE2 attenuated the stimulatory effects of thyroid hormone treatment. Additionally, two E-box motifs at positions − 911/− 906 (E1) and − 743/− 738 (E2) regulate UCP2 gene expression through SREBP-1a, SREBP-1c, and SREBP-2. Mutational analyses indicate that the presence of E1 or E2 alone is sufficient for the nuclear active SREBP-mediated activation of UCP2 gene transcription (Oberkofler et al. 2006). Moreover, miR-23a induces the expression of PGC-1α and also enhances the expression levels of UCP2 (Wang et al. 2015).
Elevated levels of long-chain fatty acids stimulate UCP2 expression, primarily mediated via peroxisome proliferator-activated receptors (PPARs) and SREBPs (Zhou et al. 2016; Chen et al. 2014). The PPAR family includes three principal genes: PPAR-α, PPAR-β, and PPAR-γ, while SREBP exists in three main isoforms: SREBP-1a, SREBP-1c, and SREBP-2 (Shimano 2009). Several studies have confirmed the potentially critical role of PPARs in pancreatic diseases, including protecting pancreatic islet β-cells from metabolic stress, enhancing insulin secretion, and mitigating lipotoxicity (Chen et al. 2015; Hogh et al. 2014; Jiang et al. 2010). Unlike Foxa1, SREBP, TRE, and E-box, which possess binding sites on the UCP2 promoter, no binding sites for PPAR have been identified within or near the Ucp2 gene. Therefore, the regulation of UCP2 by PPARs appears to be indirect (Donadelli et al. 2014). However, it has been documented that PPARs bind the direct repeat sequence 5′-AGGTCA-3′ as a specialized heterodimer with the retinoid X-like receptor (RXR) (IJpenberg et al. 1997; Gearing et al. 1993). Additionally, PPARs require a double E-box motif in their proximal promoter for their biological functions. Further investigation is necessary to confirm the regulatory role of PPARs in UCP2 gene transcription in future studies (Medvedev et al. 2001).
In β-cells, SIRT1 inhibits UCP2 transcription by directly binding to its promoter, thereby affecting insulin secretion (Bordone et al. 2006; Moynihan et al. 2005). SIRT1 also interacts with various transcription factors of UCP2. For example, it suppresses PPARγ, thereby regulating white adipose tissue function (Zu et al. 2020). The SIRT1-Ppargc1a-Ucp2 pathway is associated with insulin resistance and obesity (Kettunen et al. 2024). Additionally, SIRT1 modulates Foxa1, influencing cellular metabolic levels possibly due to its proximity to the Foxa1 binding site on the UCP2 promoter (Bordone et al. 2006). Moreover, SIRT1 synergizes with peroxisome proliferator-activated receptor coactivator PGC-1α (Xu et al. 2021b).
TGFβ signaling negatively regulates UCP2, as demonstrated in tumor cells where low malignancy levels suppress gene transcription by recruiting TGFβ-induced SMAD4 to six repressive SMAD-binding elements (RSBEs, − 100 to − 354) on the UCP2 promoter (Sayeed et al. 2010). Conversely, highly malignant tumor cells promote UCP2 expression. Additionally, glutamine induces UCP2 protein translation in a concentration-dependent manner. Insufficient glutamine inhibits UCP2 protein translation due to a short upstream open reading frame (uORF) consisting of 36 amino acids in the 5' untranslated region. In the presence of glutamine, the inhibitory effect of uORF on translation is alleviated (Hurtaud et al. 2007).
Epigenetic mechanisms of UCP2 in pancreatic diseases
The epigenetic regulation of UCP2 involves DNA methylation, histone modifications, non-coding RNAs (ncRNAs), and chromatin remodeling. These mechanisms are not independent; rather, they frequently interact synergistically, with transcription factors also contributing to their regulation. Consequently, the epigenetic regulation of UCP2 must be understood holistically.
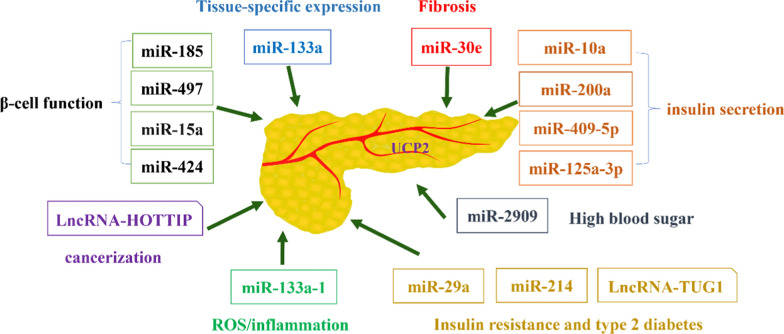
AMPK has been shown to enhance histone acetylation by phosphorylating DNMT1, RBBP7, and HAT1, which in turn reduces DNA methylation and chromatin remodeling at the UCP2 promoter (Marin et al. 2017). UCP2 also regulates acetyl-CoA levels, histone acetylation, and chromatin remodeling within the metabolic microenvironment (Rigaud et al. 2022). ncRNAs play a crucial role in the epigenetic regulation of UCP2 and have potential as biomarkers for diagnosing and prognosing pancreatic diseases (Liu et al. 2019). Specific microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) can serve as non-invasive biomarkers for the early detection and monitoring of CP, diabetes, and other pancreatic disorders. Furthermore, ncRNAs are vital regulators of pancreatic diseases, influencing inflammation, fibrosis, insulin secretion, and cell survival (Xiong et al. 2019).In this section, we summarize the ncRNAs involved in the epigenetic regulation of UCP2 (Fig. 2).
Fig. 2.
The relationship between ncRNAs and UCP2
MiR-133a plays a role in targeting and regulating the tissue-specific expression of UCP2 (Chen et al. 2009). However, miRNAs with analogous roles have not been identified in pancreatic tissue. miR-15a inhibition of endogenous UCP-2 protein levels is a critical regulator of β-cell function and insulin biosynthesis (Sun et al. 2011). Conversely, miR-15a, miR-424, miR-497, and miR-185 directly target the 3'UTR of UCP2 mRNA to suppress its expression, forming a regulatory network that influences β-cell function (Lang et al. 2018). Some researchers have explored the potential association between hypothalamic miRNA expression profiles and insulin responsiveness, identifying 34 up-regulated miRNAs and 4 down-regulated miRNAs. They specifically investigated the expression of miR-10a, miR-200a, miR-409-5p, and miR-125a-3p (Benoit et al. 2013). Another study highlighted the involvement of miR-2909 in regulating UCP2 expression, particularly in hyperglycemic conditions (Kaul et al. 2015). miR-29a impacts glucose and lipid metabolism, presenting as a potential target for managing insulin resistance and type 2 diabetes (Wu et al. 2018). Additionally, miR-214 and lncRNA TUG1 regulate UCP2 expression levels and play pivotal roles in insulin resistance and type 2 diabetes (Wei et al. 2022; Yang et al. 2019).
MiR-133a-1 inhibits the activation of NLRP3 inflammasomes by suppressing UCP2 (Bandyopadhyay et al. 2013). Interestingly, miR-133a-3p exhibits a positive correlation with UCP2 expression and a negative correlation with IL-18 (Bandyopadhyay et al. 2013). Additionally, the miR-133a/UCP2 signaling axis regulates downstream inflammation, oxidative stress, and energy metabolism (Jin et al. 2017). These findings suggest that miR-133a may hold potential value in the pathogenesis of AP, although no studies have yet been reported in this area. Notably, the miR-30e/UCP2 axis demonstrates significant relevance in renal fibrosis, implying potential applicability in fibrosis-characterized CP (Jiang et al. 2013). Furthermore, lncRNA HOTTIP regulates UCP2 to promote PDAC progression (Wong et al. 2020). However, there are no reports of circRNA regulating UCP2, with circRNA UCP2 involvement only documented in lung cancer (Du et al. 2023).
Pathological implications of UCP2 in pancreatic diseases
Typically, the primary function of UCP2 is to regulate cellular energy transduction and mitochondrial ROS generation. This makes it an attractive therapeutic target for addressing metabolic imbalance in pancreatic cancer and oxidative damage in pancreatitis (Caggiano and Taniguchi 2024; Jin et al. 2023). As research on UCP2 progresses, a clue to this discrepancy may differ in other organs, the unique role of UCP2 in the pancreas was demonstrated increasingly, with its impact on pancreatic biological functions gradually being uncovered. Significant changes in insulin and blood glucose levels have been observed in UCP2 knockout mice (González-Barroso et al. 2008; Zhou et al. 2009). Detailed studies have elucidated the biological mechanisms by which UCP2 regulates the functions of pancreatic alpha and beta cells as well as blood glucose control (Gomathi et al. 2019; Allister et al. 2013; Mizusawa et al. 2022). Additionally, the function of UCP2 in the development, transplantation, and autoimmune regulation of the pancreas, particularly the islets, has been confirmed (Zhang et al. 2011; Pi et al. 2009; Emre et al. 2007). Given the significant role of UCP2 in the pancreas and pancreatic diseases, this review focuses on the recent research progress regarding the involvement of UCP2 in pancreatic development, pancreatitis, pancreatic endocrine diseases, and pancreatic cancer.
Pancreatic development
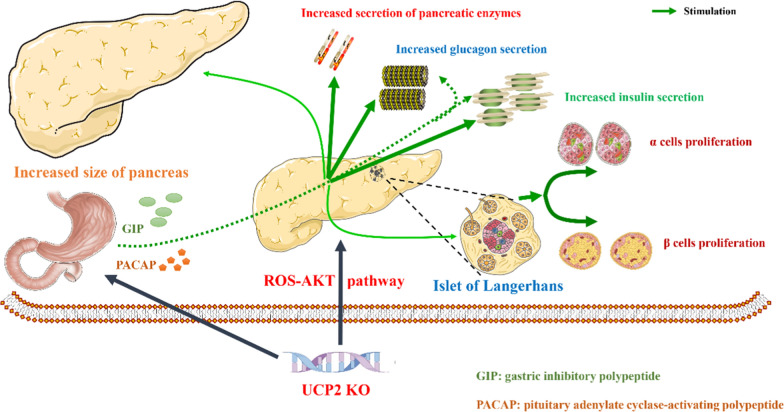
This section examines the physiological functions of UCP2 in pancreatic development, islet transplantation, and the two major islet cell types (alpha and beta cells), along with its role in regulating somatostatin, pancreatic polypeptides, and ghrelin. Figure 3 summarizes the potential roles of UCP2.
Fig. 3.
The potential roles of UCP2 in pancreatic development
Over the past decade, the effect of mitochondrial dysfunction on pancreatic islet development has been extensively investigated. Mutations in the human UCP2 gene are related to congenital hyperinsulinism (González-Barroso et al. 2008). The deletion of UCP2 in mice is associated with increased insulin secretion and elevated proliferation of endocrine cells (Zhang et al. 2001) a phenomenon that is more pronounced in mice on a high-fat diet (Joseph et al. 2002; Lee et al. 2009). To clarify the impact of UCP2 on pancreatic development, Benjamin Broche et al. (Broche et al. 2018) generated UCP2 whole-body knockout mice at various stages (from embryonic day 9.5 to 19.5) to observe the effects of UCP2 deficiency on pancreatic growth and development. Their results indicated that UCP2 is primarily expressed in pancreatic endocrine cells rather than stromal, epithelial, or other cell types. The absence of UCP2 resulted in significantly larger pancreatic volumes in late-stage embryos compared to controls, and the expression levels of insulin, glucagon, and amylase were significantly increased in fetal and neonatal mice compared to the control group. This phenotype may be related to the proliferation of pancreatic progenitor cells and the activation of the ROS-AKT signaling pathway (Broche et al. 2018).
Islet transplantation is an effective method for treating uncontrollable diabetes, such as recurrent hypoglycemia and insulin desensitization. However, the challenge of restoring pancreatic β-cell function after transplantation limits the clinical application of this technique (Rickels and Robertson 2019). Studies indicate that downregulating UCP2 may mitigate brain death post-islet transplantation and enhance the recovery of pancreatic β-cell function. This may be related to the high expression of UCP2 mediating systemic inflammation and pancreatic β-cell apoptosis (Brondani et al. 2017). The exact biological role of UCP2 in pancreatic islet cells remains controversial due to the mutual influence between α-cells and β-cells, making it difficult to distinguish causal from concomitant effects (Diao et al. 2008). The successful construction of islet α- and β-cell-specific UCP2 knockout mouse models has, fortunately, provided a clearer understanding of the physiological functions of UCP2 in pancreatic islet cells (Allister et al. 2013; Hardy et al. 2011).
Similarly, in a β-cell-specific UCP2 overexpression mouse model, increased levels of UCP2 are associated with glucose intolerance, inadequate insulin secretion, and pancreatic β-cell failure in mice (Inoue et al. 2022). Unlike pancreatic β-cells, the function of UCP2 in pancreatic α-cells is likely more comprehensive. This may be due to the significantly higher expression of UCP2 in pancreatic islet α-cells compared to β-cells (Diao et al. 2008).In islet β-cells, UCP2 knockdown primarily regulates blood glucose levels by increasing ROS production and promoting insulin secretion, with minimal effects on mitochondrial membrane potential and ATP production (Lee et al. 2009). In pancreatic islet α-cells, UCP2 not only functions similarly to β-cells in endocrine regulation at low glucose concentrations but also plays an electroactive regulatory role. UCP2 regulates glucagon secretion to maintain blood glucose levels by modulating ATP generation, plasma membrane potential, and ROS levels (Allister et al. 2013; Robson-Doucette et al. 2011).
While no studies have reported that UCP2 directly affects δ-cells, PP-cells, and ε-cells, UCP2 may be involved in regulating the hormones secreted by these pancreatic islet cells (somatostatin, pancreatic polypeptide, and ghrelin, respectively). The modulation of these hormone levels is primarily influenced by blood glucose levels, with the balance of insulin and glucagon acting as the key regulatory mechanism (Lewandowski et al. 2024; Hoffman et al. 2023; Arafat et al. 2013). The direct role of the UCP2 gene in regulating somatostatin, pancreatic polypeptide, and ghrelin remains unclear. However, it is hypothesized that UCP2 may influence the secretion of these hormones by modulating the metabolic state and ROS levels within δ-cells, PP-cells, and ε-cells of the pancreatic islets (Coskun et al. 2013). Somatostatin plays a crucial role in inhibiting the release of other hormones such as insulin and glucagon (Henquin et al. 2017). The primary function of pancreatic polypeptide is to regulate pancreatic secretion and intestinal activity, while ghrelin primarily promotes appetite. Additionally, UCP2 can influence the release and regulation of pancreatic hormone levels by modulating gut hormone gastric inhibitory polypeptide (GIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) (Zhou et al. 2005; Nakata et al. 2010). These hormones interact to form a complex regulatory network that sustains various biological functions in the body (Brink 2003; Röder et al. 2016; Müller et al. 2017). Collectively, UCP2 is a crucial target for pancreatic growth, development, and the maintenance of normal physiological function.
Acute pancreatitis
AP arises primarily from the abnormal activation of pancreatic enzymes within acinar cells, initiating an inflammatory response and amplifying oxidative stress in a cascade effect. This process induces cell death, aggravates tissue damage, and can progress to systemic inflammatory response syndrome (SIRS), making it a life-threatening acute abdominal condition (He et al. 2024). UCP2 has been implicated in the pathophysiology of AP and its more severe form, SAP (Müller et al. 2014). This involvement occurs through various mechanisms, primarily due to the function of UCP2 in regulating mitochondrial function and modulating oxidative stress (Geng et al. 2024). In AP, mitochondrial dysfunction and oxidative stress are key factors in cellular injury. By regulating mitochondrial membrane potential and ROS levels, UCP2 may help maintain mitochondrial integrity and function, thereby reducing the severity of mitochondrial injury during AP (Hu et al. 2023).
Significantly higher transcript levels of UCP2 were observed in two classic animal models of pancreatitis—the continuous cerulein-injected mouse model and the taurocholic acid-injected rat model—compared to the control group (Segersvärd et al. 2005). The high expression of UCP2 suggested increased pancreatic follicular cell damage and a higher degree of pancreatitis. Interestingly, a greater degree of pancreatitis due to UCP2 knockout was observed only in aged UCP2-deficient mice (12 months old) and was more pronounced in the late stages of pancreatitis induction by sequential cerulein injections (24 h and 7 days after AP) (Segersvärd et al. 2005). In contrast, the degree of AP inflammation induced by UCP2 knockout in young mice did not differ from that in wild-type mice. Moreover, pancreatic enzymes were not significantly activated in UCP2 knockout pancreatitis mice, suggesting that the onset of pancreatitis in aged UCP2 knockout mice is not significantly related to pancreatic acinar cell activation (Müller et al. 2014).
Based on this, primary pancreatic stellate cells (PSCs) were extracted from aged UCP2 knockout mice and wild-type (WT) mice for further study. The results showed that the proliferation rate of PSCs from UCP2 knockout mice was lower than that of WT mice. However, there were no significant differences in aging rate, ROS levels, fat droplet loss, or fibrosis degree compared to the corresponding WT cells (Muller et al. 2016). These findings suggest that UCP2 knockout delays pancreatic repair by affecting PSCs proliferation. Persistent activation of PSCs is the main cause of CP (Wang et al. 2023), indicating that targeting UCP2 may have significant translational potential for its diagnosis and treatment (Yang et al. 2022a). Currently, there are no studies on the role of UCP2 in CP. However, our team has conducted in-depth research in this area and discovered some interesting findings, which we will report in due course.
Additionally, studies have confirmed that UCP2 knockout counteracts the inhibitory effects of marine on SAP-induced lung injury and ferroptosis, highlighting the important role of UCP2 in SAP progression (Jin et al. 2023). Low SIRT1 expression decreases intracellular NAD+ levels and inhibits the deacetylation of critical downstream molecules, promoting the development and progression of AP (Shen et al. 2017). Targeting SIRT1 has shown promise as an effective strategy to suppress AP progression. (Wang et al. 2021a; Bansod and Godugu 2021; Abdelzaher et al. 2021) Additionally, obesity—an escalating global health challenge—is linked to a rising incidence of obesity-related AP. PGC-1α plays a pivotal role in obesity-related AP; in obese states, pancreatic PGC-1α levels are suppressed, which prevents its binding to the NF-κB subunit p65, thereby promoting oxidative damage and amplifying IL-6-mediated inflammation, worsening AP severity (Pérez et al. 2019). Importantly, SIRT1, PPARγ, PGC-1α, and UCP2 constitute an interconnected regulatory network that jointly governs cellular energy metabolism, oxidative stress response, and inflammation (Oberkofler et al. 2009) SIRT1 modulates the activities of PGC-1α and PPARγ, both of which subsequently influence UCP2 expression levels, helping cells maintain stability and an anti-inflammatory state during metabolic stress or disease conditions. This regulatory interplay among these factors plays a crucial role in the pathogenesis of AP. In conclusion, UCP2 may be an important therapeutic target for pancreatitis and a key focus for future research.
Pancreatic endocrine diseases
Endocrine diseases of the pancreas involve disorders of the hormone-producing cells in the pancreas. These primarily include Diabetes Mellitus, Insulinoma, Gastrinoma, Glucagonoma, Multiple Endocrine Neoplasia Type 1 (MEN1), Somatostatinoma, VIPoma, and Congenital Hyperinsulinism (CHI).
Diabetes Mellitus, the most common endocrine disease of the pancreas, is categorized into Type 1 and Type 2. Type 1 diabetes results from the autoimmune destruction of pancreatic beta-cells, leading to insufficient insulin secretion. In contrast, Type 2 diabetes is characterized by insulin resistance and inadequate insulin secretion. Table 2 summarizes studies related to UCP2 in pancreatic endocrine diseases, particularly diabetes mellitus, highlighting its role in glucose metabolism, insulin secretion, and oxidative stress. These studies collectively suggest that UCP2 plays a significant role in the pathogenesis of pancreatic endocrine diseases (González-Barroso et al. 2008; Gomathi et al. 2019; Mizusawa et al. 2022; Inoue et al. 2022; Giri et al. 2022; Yang et al. 2022b; Liu et al. 2022, 2014; Grubelnik et al. 2022; Buckels et al. 2021; Li et al. 2021; Odei-Addo et al. 2021; Naderi et al. 2020; Tavoosi et al. 2020; Sankaranarayanan and Kalaivani 2020; Yoo et al. 2020; Wade et al. 2019; Plecitá-Hlavatá et al. 2019; Wang et al. 2019; Maiztegui et al. 2018; Demirbilek and Hussain 2017; Matsunaga et al. 2014; Hals et al. 2012; Han et al. 2004).
Table 2.
The studies related to UCP2 in pancreatic endocrine diseases
| Years | Type | UCP2 level | Main findings | Refs. |
|---|---|---|---|---|
| 2021 | Congenital Hyperinsulinism | Down-regulation | UCP2, as one of the 16 key genes, is involved in regulating insulin secretion by pancreatic β-cells | Giri et al. (2022) |
| 2022 | Type 2 diabetes | Up-regulation | All-trans retinoic acid modulates the RXR/SREBP-1c/UCP2 signaling axis, thereby inhibiting insulin secretion and promoting the progression of diabetes | Yang et al. (2022b) |
| 2022 | pancreatic islet after severe burns | Up-regulation | Nicotinamide mononucleotide could maintain mitochondrial function through the SIRT1-UCP2 axis | Liu et al. (2022) |
| 2022 | Prss53 knockdown murine MIN6 β-cells | Up-regulation | The inhibition of UCP2 by mitochondrial Prss53 plays an auxiliary role in maintaining beta cell health | Mizusawa et al. (2022) |
| 2022 | Type 2 diabetes | Up-regulation | UCP2 upregulation is associated with β-cell failure, and the UCP2/AldB axis is a potential target for restoring β-cell function | Inoue et al. (2022) |
| 2022 | Pre- type 2diabetic hyperlipidemia | Up-regulation | Chronic high levels of free fatty acids upregulate UCP2, leading to β-cell dysfunction. This dysfunction is characterized by β-cells remaining highly active during hypoglycemia but becoming functionally quiescent during hyperglycemia | Grubelnik et al. (2022) |
| 2021 | Fetal growth restriction | Up-regulation | UCP2 may mediate IGF-I in a sex-specific manner to alter pancreatic endocrine function in adult children with fetal growth restriction | Buckels et al. (2021) |
| 2021 | Chronic adrenergic-stimulated beta cells | Down-regulation | Persistently low levels of UCP2 mediate the long-term adaptation of beta cells to adrenergic signaling | Li et al. (2021) |
| 2021 | Type 2 diabetes | Adipose tissue upregulated, liver tissue downregulated | High expression of UCP2 in adipose tissue may mediate the inhibitory effects of Leonurus extract and marrubium on type 2 diabetes | Odei-Addo et al. (2021) |
| 2021 | STZ-induced type 1 diabetic rats | Up-regulation | The effects of Tropisetron in type 1 diabetes are associated with modulation of the UCP2/ZnT8 signaling pathway and amelioration of oxidative stress | Naderi et al. (2020) |
| 2020 | Type 1 diabetes cell model | Down-regulation | Protective effects of cerium and yttrium oxide nitrogen oxides on CRI-D2 β cell lines exposed to H2O2 are associated with the regulation of UCP2 | Tavoosi et al. (2020) |
| 2020 | HFD/STZ-induced type 2 diabetic rats | Up-regulation | Down-regulation of UCP2 expression by isoproterenol attenuates oxidative and ER stress responses in high-fat combined with STZ-induced diabetic rats | Sankaranarayanan and Kalaivani (2020) |
| 2020 | Type 2diabetes cell model | Up-regulation | Chebulic acid downregulates UCP2 to prevent MG-induced development of insulin sensitivity and oxidative stress-induced β-cell dysfunction | Yoo et al. (2020) |
| 2019 | Diabetes | Up-regulation | RNF20 and RNF40 regulate β-cell gene expression and insulin secretion associated with the regulation of UCP2 | Wade et al. (2019) |
| 2019 | Type 2diabetes cell model | / | UCP2 promotes an antioxidant mechanism based on SkQ1+ fatty acid anion pairing | Plecitá-Hlavatá et al. (2019) |
| 2019 | Type 2diabetes | Polymorphism | UCP2 polymorphism affects insulin secretion leading to type 2 diabetes mellitus | Gomathi et al. (2019) |
| 2019 | Type 2diabetes cell model | Up-regulation | RP3-SeNPs down-regulate UCP2 to exert anti-oxidative stress effects | Wang et al. (2019) |
| 2018 | Type 2diabetes | Up-regulation | Upregulation of UCP2 affects pancreatic β-cell function | Maiztegui et al. (2018) |
| 2017 | Hyperinsulinaemic hypoglycaemia | / | UCP2 mutations affect the regulation of insulin secretion in pancreatic β-cells as a potential molecular mechanism leading to Hyperinsulinaemic hypoglycemia | Demirbilek and Hussain (2017) |
| 2014 | Type 2diabetes | Down-regulation | Up-regulation of UCP2 expression after berberine treatment is an important mechanism of its antidiabetic action | Liu et al. (2014) |
| 2014 | Chronic high glucose | Down-regulation | Glucotoxicity leading to beta-cell hypoxia is associated with down-regulation of UCP2 | Matsunaga et al. (2014) |
| 2013 | Alpha cell-specific UCP2 knockout mice | Down-regulation | UCP2 is an essential gene for glucose sensing and maintenance of normal function in normal alpha cells | Allister et al. (2013) |
| 2012 | Type 2diabetes | Up-regulation | Effects on mitochondrial metabolism were possible only after a fourfold increase in UCP2 expression levels | Hals et al. (2012) |
| 2011 | βcell-specific UCP2 knockout mice | Up-regulation | UCP2 regulates ROS levels more significantly in β-cells | Robson-Doucette et al. (2011) |
| 2009 | Type 2diabetes | Down-regulation | UCP2 inhibition leads to enhanced insulin secretion and impaired α-cell function | Lee et al. (2009) |
| 2008 | Congenital Hyperinsulinism | Down-regulation | UCP2 knockout affects mitochondrial function and insulin secretion leading to hyperinsulinemic hypoglycemia | González-Barroso et al. (2008) |
| 2007 | Autoimmune diabetes | Down-regulation | Ucp2-KO mouse model of autoimmune diabetes has more severe symptoms | Emre et al. (2007) |
| 2004 | Type 2diabetes | Up-regulation | Inhibition of glucose sensitivity by taurine in UCP2 overexpressing β-cells was associated with an increased ATP/ADP ratio | Han et al. (2004) |
Although the exact role of high and low UCP2 expression levels in these diseases is controversial, most studies indicate that increased UCP2 expression is generally associated with impaired insulin secretion and reduced β-cell function, contributing to hyperglycemia. Glucose-stimulated insulin secretion (GSIS) is essential for the endocrine regulation of the pancreas (Seshadri et al. 2017). Impaired GSIS is a significant contributor to insulin resistance and β-cell failure in type 2 diabetes mellitus. Furthermore, the upregulation of UCP2 is believed to be a contributing factor to impaired GSIS (Affourtit et al. 2011; Brand et al. 2010). Additionally, UCP2 influences mitochondrial function and reactive oxygen species production, further impacting cellular metabolism and insulin resistance. The main evidence supporting this view includes: (1) increased insulin secretion in UCP2 knockout mice (Zhang et al. 2001; Patanè et al. 2002), (2) elevated UCP2 expression levels strongly associated with high blood glucose levels (Brown et al. 2002), and (3) the therapeutic effect observed upon UCP2 knockout in mice modeling Type 2 diabetes (Zhang et al. 2001). However, contrary results were observed in another in vivo study (Pi et al. 2009), where UCP2 overexpression showed conflicting findings, with inhibitory, promotional, or no effects on β-cell function (Hong et al. 2001; Produit-Zengaffinen et al. 2007; Wang et al. 1999).
To verify this paradoxical phenomenon, Ingrid K. et al. (Hals et al. 2012). elevated UCP2 expression levels in β-cells in vitro to assess effects on parameters related to mitochondrial metabolism, including cell viability, apoptosis, insulin secretion, glucose oxidation, glutamine metabolism, mitochondrial membrane potential, mitochondrial mass, mitochondrial uncoupling, and ROS levels. Their results indicated that effects on β-cell metabolic levels were observed only when UCP2 levels were elevated more than four-fold. This study suggests that the role of UCP2 in blood glucose regulation and diabetes may not be concentration-dependent. Instead, a complex regulatory network centered on UCP2 likely exists, where high UCP2 levels may exert a protective effect in the pre-diabetic phase, but inhibit β-cell function under prolonged hyperglycemia. Since this study was conducted only in vitro and did not evaluate the effect of UCP2 expression levels on pancreatic islets, it may not fully elucidate the exact role of UCP2, but it is worthwhile to pursue further investigation.
Pancreatic cancer
Pancreatic cancer encompasses a group of malignant tumors primarily arising from the pancreatic ductal epithelium and follicular cells. It is characterized by an insidious onset, challenging early diagnosis, rapid progression, short survival time, and poor prognosis (He et al. 2020). Pancreatic ductal adenocarcinoma (PDAC), the most prevalent pathological type, accounts for over 90% of cases (Wang et al. 2021b). The metabolic profile of PDAC is unique and complex, reflecting a high degree of metabolic flexibility to meet its growth and survival needs. The metabolic reprogramming features of PDAC include the Warburg effect, glutamine dependence, alterations in cholesterol and fatty acid metabolism, and resistance to oxidative stress (Santis et al. 2024). The significant reliance of pancreatic cancer on mitochondrial metabolism can lead to oxidative phosphorylation to produce ATP, driving malignant phenotypes such as metastasis and treatment resistance. Therefore, targeting mitochondrial metabolism is a promising therapeutic approach for pancreatic cancer. However, specifically targeting mitochondria without off-target effects in normal tissues remains a significant challenge (Yin et al. 2022).
Although precise targeting of mitochondrial function is still a distant goal, oxidative phosphorylation regulated by these organelles is indispensable in the metabolic reprogramming of PDAC. Specifically, the metabolic homeostasis of glutamine and aspartate is critical in this process (Caggiano and Taniguchi 2024). However, the key molecules involved in these energy metabolic pathways in PDAC tumorigenesis and progression cannot traverse the mitochondria alone; they require carriers to transport them to the inner mitochondrial membrane. Thus, UCP2, a member of the SLC25 family acting as a transmembrane anion carrier, may play a role in PDAC progression (Li et al. 2013). Numerous studies have detailed how UCP2 regulates glutamine and aspartate metabolism, particularly its role in mitochondrial energy regulation via the tricarboxylic acid (TCA) cycle and ROS management (Caggiano and Taniguchi 2024; Lauria et al. 2023). KRAS mutations, the most prevalent mutations in PDAC, impact not only cancer cells but also the tumor microenvironment. These mutations promote the tumor mesenchymal response and angiogenesis by secreting various cytokines and growth factors, thus creating a more favorable growth environment for tumor cells (Buscail et al. 2020). Notably, recent research indicates that UCP2-mediated aspartate transport is a crucial step in KRAS-regulated glutamine metabolism (Raho et al. 2020).
It is widely recognized that UCP2 expression is upregulated in PDAC (Caggiano and Taniguchi 2024). Table 3 summarizes studies related to UCP2 in Pancreatic cancer. UCP2 is downregulated before the tumor is fully formed to promote ROS accumulation and genomic instability (Lauria et al. 2023). In the later stages of tumorigenesis, UCP2 expression levels are upregulated to meet the metabolic needs of the tumor tissue, such as maintaining high ATP production, providing ROS protection, promoting therapeutic resistance, and facilitating immune evasion (Donadelli et al. 2015). Collectively, these results demonstrate the specificity and significance of UCP2 in PDAC progression, suggesting that UCP2 could serve as a potential therapeutic target for PDAC.
Table 3.
Studies related to UCP2 in Pancreatic cancer
| Years | Type | UCP2 role | Signaling pathway | Potential value | Refs. |
|---|---|---|---|---|---|
| 2012 | PDAC cell lines PaCa44, PaCa3, Panc1, CFPAC1, T3M4, and MiaPaCa2 | Mitochondrial uncoupling of UCP2 mediates the mechanism of PDAC resistance to gemcitabine | ROS-mediated apoptosis pathway | UCP2 mediates PDAC gemcitabine chemotherapy drug resistance | Dalla et al. (2012) |
| 2015 | PDAC cell lines Panc1 and PaCa44 | Onconase induces mitochondrial ROS production by inhibiting UCP2 expression levels | ROS/Akt/mTOR axis | UCP2 mediates the chemosensitivity of PDAC to gemcitabine | Fiorini et al. (2015) |
| 2016 | PDAC cell lines Panc-1 | UCP2 inhibits ROS levels to induce pancreatic cancer cell death | ROS-mediated apoptosis pathway | UCP2 as a potential target for pancreatic cancer therapy | Yang et al. (2016) |
| 2016 | PDAC cell lines PaCa44 and Panc1 | UCP2 mediates the metabolic transition of PDAC from mitochondrial oxidative phosphorylation to glycolysis | Induction of hnRNPA2/B1 and stimulation of GLUT1, PKM2 expression and L-lactate secretion | Inhibition of the UCP2-mediated glycolytic pathway promises a new approach to cancer therapy | Brandi et al. (2016) |
| 2017 | PDAC cell lines PaCa44, PaCa3, Panc1, MiaPaCa2, and T3M4 | UCP2 overexpression promotes chemoresistance in PDAC | ROS/Akt/mTOR axis and GAPDH nuclear translocation | Combined inhibition of UCP2 and Akt/mTOR pathways is a novel therapeutic strategy for pancreatic cancer | Dando et al. (2017) |
| 2020 | PDAC cell lines PANC–1, SW1990, CAPAN–2, CFPAC–1, PANC0403, and BxPC–3 | The HOTTIP-HOXA13 pathway promotes PDAC progression by upregulating UCP2 expression levels | HOTTIP–HOXA13 axis and HOTTIP–WDR5–MLL1–H3K4me3 pathway | Targeting downstream effector molecules of the HOTTIP pathway, including UCP2, could lead to the development of new PDAC therapies | Wong et al. (2020) |
| 2020 | PDAC cell lines Patu8988T, Panc1 and BxPC3 | UCP2 connects the mitochondrial and cytoplasmic responses required for KRAS in PDAC rewired for glutamine metabolism | UCP2/ROS axis | UCP2 is a key metabolic target for the treatment of refractory tumors like PDAC | Raho et al. (2020) |
| 2023 | Murine cell line 6606PDA and Panc02 | Ucp2 regulates the tumor microenvironment in favor of PDAC progression | Tumor stroma-related pathways | UCP2 promises to be a therapeutic target for PDAC | Revskij et al. (2023) |
UCP2-regulated macrophage phenotypic transformation in the pathogenesis of pancreatic diseases
Macrophages, a type of immune cell within the pancreatic microenvironment, play a pivotal role in the progression and pathogenesis of AP, CP, and pancreatic cancer (Wu et al. 2020). Their phenotypic transformation primarily involves macrophage polarization and macrophage-to-myofibroblast transition (MMT). Traditionally, M1 macrophage polarization is considered a key driver in the progression of AP and SAP (Peng et al. 2023), while M2 macrophage polarization, which exerts anti-inflammatory and pro-fibrotic effects, contributes to fibrosis in CP (Xue et al. 2015). In pancreatic cancer, M2 macrophages primarily mediate tissue repair and immune suppression, thereby promoting a microenvironment conducive to tumor progression (He et al. 2022). UCP2 is notably involved in the regulation of macrophage function, particularly in macrophage polarization. Studies indicate that UCP2 modulates the polarization of human primary macrophages (Lang et al. 2023). In AP, especially in obesity-associated AP, FABP4 upregulates UCP2, which in turn reduces oxidative stress to modulate macrophage signaling and inflammatory responses (Dierendonck et al. 2020; Steen et al. 2017). UCP2-regulated mitochondrial respiration acts as a crucial regulatory mechanism for IL-33-induced M2 macrophage polarization, facilitating the progression of CP (Faas et al. 2021). Additionally, macrophages are essential mediators in tissue repair following AP and contribute to the progression of pancreatic cancer (Wu et al. 2020). Furthermore, UCP2 regulation of macrophage-mediated NO/ROS damage is implicated in the progression of type 1 diabetes (Emre et al. 2007).
More recently, it has been discovered that certain macrophages can directly differentiate into myofibroblasts through a process known as MMT (Vierhout et al. 2021). While no studies to date have reported MMT in pancreatitis or pancreatic cancer, MMT is known to contribute to the progression of fibrotic diseases, such as kidney fibrosis, and cancers, including lung cancer (Wang et al. 2017; Tang et al. 2024). Indirect evidence suggests that STAT6-PPARα interactions regulate MMT, mediating kidney fibrosis progression (Yuan et al. 2023). This evidence supports the reasonable hypothesis that MMT may also play a role in pancreatic diseases, particularly in CP and pancreatic cancer, with UCP2 likely influencing this process to some extent. Overall, UCP2-regulated macrophage phenotypic transformation appears to significantly impact the progression of pancreatic diseases, lending further support to the hypothesis that UCP2 is a central regulatory factor in these conditions.
Signaling pathways related to UCP2 regulation
Given the significant role of the UCP2 gene in regulating energy homeostasis, ROS, insulin secretion, and overall metabolism, as well as its critical regulatory role in pancreatic diseases.ROS generated by metabolic stress in the mitochondria of β-cells activates several ROS-related signaling pathways, such as the AMP-activated protein kinase (AMPK), Wnt, and nuclear factor kappa B (NF-κB) (Beall et al. 2013; Wang et al. 2014; Yu et al. 2020). These pathways, on the one hand, activate UCP2, causing proton leakage across the inner mitochondrial membrane and reducing ATP synthesis. On the other hand, they disrupt membrane integrity by oxidizing polyunsaturated fatty acids in the mitochondrial membrane, leading to the release of cytochrome c into the cytoplasm and inducing cellular apoptosis and autophagy (Ma et al. 2012; Dando et al. 2013). The regulatory relationship between UCP2 and ROS-related pathways not only influences pancreatic endocrine diseases by affecting the insulin secretory function of β-cells but also contributes to the progression of AP and PDAC.
The AMPK signaling pathway significantly affects UCP2 expression. During cellular energy stress, AMPK is activated to restore energy homeostasis and upregulate UCP2 expression by enhancing catabolism and inhibiting anabolism (Luo et al. 2022). Activated AMPK directly affects transcription factors like PPAR and SIRT1 to promote UCP2 transcription and enhances mitochondrial biogenesis by regulating coactivators like PGC-1α, further upregulating UCP2 (Xu et al. 2021b). AMPK activation also promotes fatty acid oxidation, regulates ROS levels, reduces oxidative stress and mitochondrial membrane potential, prevents oxidative damage, and maintains cellular function (Tripathi et al. 2023; Zhao et al. 2022). Additionally, AMPK influences glucose metabolism and insulin sensitivity (Entezari et al. 2022).
No studies have reported NF-κB binding to the κB site in the UCP2 gene promoter region. Like AMPK, NF-κB can regulate UCP2 expression in concert with coactivators (Wei et al. 2021). Inflammatory cytokines activate NF-κB, increasing UCP2 expression as part of the cellular response to inflammation and oxidative stress. UCP2 helps attenuate mitochondrial damage and maintain cellular homeostasis (Pan et al. 2021). NF-κB activation is often accompanied by elevated ROS levels. UCP2 reduces oxidative stress by lowering mitochondrial membrane potential and ROS production, providing feedback to control inflammation and oxidative damage (Adelakun et al. 2022). By regulating UCP2, NF-κB affects cellular energy metabolism. UCP2 uncouples oxidative phosphorylation, decreasing ATP production and increasing thermogenesis, impacting energy homeostasis during inflammation and stress responses (Zhang et al. 2020).
GSIS and the renin-angiotensin system (RAS) play crucial roles in pancreatic endocrinology. Palmitate-induced oxidative stress in β-cell mitochondria serves as a primary cellular model for GSIS impairment (Shaheen and Aljebali 2016), and several studies have shown that while UCP2 is not involved in palmitate-induced ROS generation, its upregulation protects against this damage (Li et al. 2017; Barlow et al. 2015; Hirschberg and Affourtit 2015). Blockade of RAS has been found to inhibit inflammation, oxidative stress in organelles, and apoptosis in pancreatic islet cells in a long-term high-fat diet rat model (Yuan et al. 2013). Accumulation of free fatty acids (FAs) induces oxidative stress, impairing pancreatic β-cell function (Ježek et al. 2015), with more pronounced damage from polyunsaturated FAs and their lipid peroxidation products compared to saturated FAs and their metabolites, possibly due to more extensive regulatory pathways mediating proton leakage, ATP synthesis, and ROS generation (Beck et al. 2007; Hu et al. 2017). Sustained ROS stimulation has been shown to directly damage β-cells by upregulating the JNK/P38 signaling pathway and activating UCP2 (Bo et al. 2016), with the glutathionylated state of UCP2 contributing to the regulation of GSIS levels in pancreatic islet cells (Mailloux et al. 2012). Collectively, UCP2 plays a crucial role in regulating energy homeostasis, ROS, insulin secretion, and overall metabolism, influencing the progression of pancreatic diseases and β-cell function via pathways including AMPK, Wnt, and NF-κB.
Prospects and challenges
Consumption of foods rich in long-chain fatty acids, such as black soybeans and raw donkey's milk, has been shown to modestly increase UCP2 expression, potentially mitigating oxidative stress-related diseases. (Lionetti et al. 2012; Kanamoto et al. 2011) This offers a potential preventive strategy against the progression from AP, PDAC, and CP to pancreatic cancer. Earlier, we discussed the regulation of UCP2 via the AMPK signaling pathway (Beall et al. 2013). Metformin, a classic drug for type 2 diabetes, exerts hypoglycemic effects by activating the AMPK-mediated catabolic pathway, influencing blood glucose levels. Recently, its therapeutic potential in pancreatic cancer and other inflammatory conditions has gained considerable attention (Xu et al. 2022; Eibl and Rozengurt 2021; Gong et al. 2014). Therefore, metformin and other AMPK modulators show promise in pancreatic diseases and warrant further investigation as potential novel therapies. Additionally, traditional Chinese medicine, with its millennia-long foundation, also exhibits regulatory effects on UCP2 (Sun et al. 2024; Yang et al. 2011). Combining UCP2 with chemotherapeutic agents as an adjuvant strategy shows potential application value, enhancing effectiveness in inhibiting pancreatic cancer (Dalla et al. 2012; Fiorini et al. 2015). In conclusion, while the theoretical foundation supports the potential application of UCP2 in pancreatic diseases, clinical validation is necessary.
Inevitably, there are challenges for UCP2 as a therapeutic target for pancreatic diseases. Firstly, tissue specificity and selectivity pose significant challenges, as UCP2 is widely distributed across various tissues, making it difficult to design inhibitors or activators that are highly specific to pancreatic tissue. Secondly, systemic modulation of UCP2 may cause side effects, given its diverse roles in different tissues. For instance, excessive inhibition of UCP2 could lead to abnormal energy metabolism and dysfunction in other tissues. Additionally, the precise mechanisms of UCP2's action in pancreatic diseases remain inadequately understood. UCP2's multiple roles in energy metabolism, oxidative stress, apoptosis, and immune responses complicate targeting strategies, preventing the focus on a single specific role. Finally, extensive studies and validations are required to determine the efficacy and safety of UCP2-targeted therapies, transitioning from basic research to clinical applications, and design rational clinical trial protocols.
Conclusions
UCP2 is broadly expressed in numerous tissues, including the pancreas, and demonstrates the highest homology between humans and mice. UCP2 is involved in various physiological functions, such as cellular energy metabolism, oxidative stress management, insulin secretion, lipid regulation, metabolic reprogramming, and immune modulation. UCP2 plays a role in regulating both endocrine and exocrine pancreatic functions. Epidemiological data on pancreatic diseases, such as acute AP, CP, pancreatic cancer, and diabetes, indicate concerning trends, with evidence suggesting frequent interconversion among these conditions. However, the understanding of these diseases’ pathogenesis and interrelationships remains limited, particularly in identifying and validating key molecules that may connect or transform these conditions. UCP2 is expected to serve as such a key target. This review presents a comprehensive analysis of current research on UCP2’s role in pancreatic diseases. We discuss recent findings on UCP2’s complex regulatory mechanisms, propose UCP2 as a central regulatory factor in pancreatic disease progression, and hypothesize that UCP2 dysfunction could significantly contribute to disease pathogenesis and interconversion. Clarifying UCP2’s role and mechanisms in pancreatic diseases could provide new directions for therapeutic and diagnostic innovation.
Acknowledgements
Not applicable.
Abbreviations
- UCP2
Mitochondrial uncoupling protein 2
- MIPS
Mitochondrial information processing system
- UCPs
Uncoupling proteins
- ROS
Reactive oxygen species
- PACAP
Pituitary adenylate cyclase-activating polypeptide
- GIP
Gastric inhibitory polypeptide
- AP
Acute pancreatitis
- SAP
Severe acute pancreatitis
- PSCs
Pancreatic stellate cells
- WT
Wild-type
- CP
Chronic pancreatitis
- MEN1
Multiple Endocrine Neoplasia Type 1
- CHI
Congenital hyperinsulinism
- GSIS
Glucose-stimulated insulin secretion
- PDAC
Pancreatic ductal adenocarcinoma
- TCA
Tricarboxylic acid
- SNPs
Single nucleotide polymorphisms
- Indels
Insertion/deletion polymorphisms
- RSPs
Repetitive sequence polymorphisms
- SV
Structural variants
- Foxa1
Forkhead box protein O1
- SIRT1
Silent mating type information regulation 2 homolog-1
- SREBP
Sterol regulatory element binding protein isoforms
- TRE
Thyroid hormone response elements
- E-box
Helix-loop-helix protein binding sites
- PGC-1α
Peroxisome proliferator-activated receptor-γ coactivator-1 α
- PPARs
Peroxisome proliferator-activated receptors
- RXR
Retinoid X-like receptor
- Uorf
Upstream open reading frame
- NcRNAs
Non-coding RNAs
- miRNAs
MicroRNAs
- LncRNAs
Long non-coding RNAs
- AMPK
AMP-activated protein kinase
- NF-κB
Nuclear factor kappa B
- RAS
Renin-angiotensin system
- FAs
Fatty acids
- MMT
Macrophage-to-myofibroblast transition
- SIRS
Systemic inflammatory response syndrome
Author contributions
Kunpeng Wang: conceived and designed the study, performed the article searching, extracted the data, and wrote the manuscript. Lilong Zhang: conceived and designed the study, performed the article searching, extracted the data, and wrote the manuscript. Beiying Deng: conceived and designed the study, performed the article searching, and extracted the data. Kailiang Zhao: performed the article searching, and extracted the data. Chen Chen: performed the article searching, and supervised the manuscript. Weixing Wang: extracted the data and supervised the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No: 82172855, and 82370654), the Natural Science Foundation of Hubei Province (Grant No: 2023AFB734), and the Hubei Microcirculation Society Research Grant Fund (Grant No: 2024HX0036).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kunpeng Wang and Lilong Zhang have contributed equally to this work.
Contributor Information
Chen Chen, Email: appreciation@whu.edu.cn.
Weixing Wang, Email: wangwx@whu.edu.cn.
References
- Abdelzaher WY, Ahmed SM, Welson NN, et al. Vinpocetine ameliorates L-arginine induced acute pancreatitis via Sirt1/Nrf2/TNF pathway and inhibition of oxidative stress, inflammation, and apoptosis. Biomed Pharmacother. 2021;133:110976. [DOI] [PubMed] [Google Scholar]
- Adelakun SA, Ukwenya VO, Akintunde OW. Vitamin B(12) ameliorate tramadol-induced oxidative stress, endocrine imbalance, apoptosis and NO/iNOS/NF-κB expression in sprague dawley rats through regulatory mechanism in the pituitary-gonadal axis. Tissue Cell. 2022;74:101697. [DOI] [PubMed] [Google Scholar]
- Affourtit C, Jastroch M, Brand MD. Uncoupling protein-2 attenuates glucose-stimulated insulin secretion in INS-1E insulinoma cells by lowering mitochondrial reactive oxygen species. Free Radic Biol Med. 2011;50(5):609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allister EM, Robson-Doucette CA, Prentice KJ, et al. UCP2 regulates the glucagon response to fasting and starvation. Diabetes. 2013;62(5):1623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen G, Dalgaard LT, Justesen JM, et al. The frequent UCP2 -866G>A polymorphism protects against insulin resistance and is associated with obesity: a study of obesity and related metabolic traits among 17 636 Danes. Int J Obes. 2013;37(2):175–81. [DOI] [PubMed] [Google Scholar]
- Arafat AM, Weickert MO, Adamidou A, et al. The impact of insulin-independent, glucagon-induced suppression of total ghrelin on satiety in obesity and type 1 diabetes mellitus. J Clin Endocrinol Metab. 2013;98(10):4133–42. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Lane T, Venugopal R, et al. MicroRNA-133a-1 regulates inflammasome activation through uncoupling protein-2. Biochem Biophys Res Commun. 2013;439(3):407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansod S, Godugu C. Nimbolide ameliorates pancreatic inflammation and apoptosis by modulating NF-κB/SIRT1 and apoptosis signaling in acute pancreatitis model. Int Immunopharmacol. 2021;90:107246. [DOI] [PubMed] [Google Scholar]
- Bao T, Zhu H, Zheng Y, et al. Expression of long noncoding RNA uc.375 in bronchopulmonary dysplasia and its function in the proliferation and apoptosis of mouse alveolar epithelial cell line MLE 12. Front Physiol. 2022;13:971732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow J, Hirschberg JV, Affourtit C. Uncoupling protein-2 attenuates palmitoleate protection against the cytotoxic production of mitochondrial reactive oxygen species in INS-1E insulinoma cells. Redox Biol. 2015;4:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall C, Watterson KR, McCrimmon RJ, et al. AMPK modulates glucose-sensing in insulin-secreting cells by altered phosphotransfer to KATP channels. J Bioenerg Biomembr. 2013;45(3):229–41. [DOI] [PubMed] [Google Scholar]
- Beck V, Jabůrek M, Demina T, et al. Polyunsaturated fatty acids activate human uncoupling proteins 1 and 2 in planar lipid bilayers. FASEB J. 2007;21(4):1137–44. [DOI] [PubMed] [Google Scholar]
- Benoit C, Ould-Hamouda H, Crepin D, et al. Early leptin blockade predisposes fat-fed rats to overweight and modifies hypothalamic microRNAs. J Endocrinol. 2013;218(1):35–47. [DOI] [PubMed] [Google Scholar]
- Bo J, Xie S, Guo Y, et al. Methylglyoxal impairs insulin secretion of pancreatic β-cells through increased production of ROS and mitochondrial dysfunction mediated by upregulation of UCP2 and MAPKs. J Diabetes Res. 2016;2016:2029854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4(2): e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Parker N, Affourtit C, et al. Mitochondrial uncoupling protein 2 in pancreatic β-cells. Diabetes Obes Metab. 2010;12(Suppl 2):134–40. [DOI] [PubMed] [Google Scholar]
- Brandi J, Cecconi D, Cordani M, et al. The antioxidant uncoupling protein 2 stimulates hnRNPA2/B1, GLUT1 and PKM2 expression and sensitizes pancreas cancer cells to glycolysis inhibition. Free Radic Biol Med. 2016;101:305–16. [DOI] [PubMed] [Google Scholar]
- Brink C. Promoter elements in endocrine pancreas development and hormone regulation. Cell Mol Life Sci. 2003;60(6):1033–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broche B, Ben FS, Aguilar E, et al. Mitochondrial protein UCP2 controls pancreas development. Diabetes. 2018;67(1):78–84. [DOI] [PubMed] [Google Scholar]
- Brondani LA, Rech TH, Boelter G, et al. UCP2 expression is increased in pancreas from brain-dead donors and involved in cytokine-induced β cells apoptosis. Transplantation. 2017;101(3):e59–67. [DOI] [PubMed] [Google Scholar]
- Brown JE, Thomas S, Digby JE, et al. Glucose induces and leptin decreases expression of uncoupling protein-2 mRNA in human islets. FEBS Lett. 2002;513(2–3):189–92. [DOI] [PubMed] [Google Scholar]
- Buckels EJ, Bloomfield FH, Oliver MH, et al. Sexually dimorphic changes in the endocrine pancreas and skeletal muscle in young adulthood following intra-amniotic IGF-I treatment of growth-restricted fetal sheep. Am J Physiol Endocrinol Metab. 2021;321(4):E530–42. [DOI] [PubMed] [Google Scholar]
- Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17(3):153–68. [DOI] [PubMed] [Google Scholar]
- Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg. 2018;1859(9):940–50. [DOI] [PubMed] [Google Scholar]
- Caggiano EG, Taniguchi CM. UCP2 and pancreatic cancer: conscious uncoupling for therapeutic effect. Cancer Metastasis Rev. 2024. 10.1007/s10555-023-10157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang K, Chen J, et al. In vitro evidence suggests that miR-133a-mediated regulation of uncoupling protein 2 (UCP2) is an indispensable step in myogenic differentiation. J Biol Chem. 2009;284(8):5362–9. [DOI] [PubMed] [Google Scholar]
- Chen K, Zhao L, He H, et al. Silibinin protects β cells from glucotoxicity through regulation of the Insig-1/SREBP-1c pathway. Int J Mol Med. 2014;34(4):1073–80. [DOI] [PubMed] [Google Scholar]
- Chen L, So WY, Li SY, et al. Niacin-induced hyperglycemia is partially mediated via niacin receptor GPR109a in pancreatic islets. Mol Cell Endocrinol. 2015;404:56–66. [DOI] [PubMed] [Google Scholar]
- Chen X, Yi B, Liu Z, et al. Global, regional and national burden of pancreatic cancer, 1990 to 2017: results from the global burden of disease study 2017. Pancreatology. 2020;20(3):462–9. [DOI] [PubMed] [Google Scholar]
- Coskun ZM, Sacan O, Karatug A, et al. Regulation of oxidative stress and somatostatin, cholecystokinin, apelin gene expressions by ghrelin in stomach of newborn diabetic rats. Acta Histochem. 2013;115(7):740–7. [DOI] [PubMed] [Google Scholar]
- D’Adamo M, Perego L, Cardellini M, et al. The -866A/A genotype in the promoter of the human uncoupling protein 2 gene is associated with insulin resistance and increased risk of type 2 diabetes. Diabetes. 2004;53(7):1905–10. [DOI] [PubMed] [Google Scholar]
- Dalgaard LT. Genetic variance in uncoupling protein 2 in relation to obesity, type 2 diabetes, and related metabolic traits: focus on the functional -866G>A promoter variant (rs659366). J Obes. 2011;2011:340241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla PE, Fiorini C, Dando I, et al. Role of mitochondrial uncoupling protein 2 in cancer cell resistance to gemcitabine. Biochim Biophys Acta. 2012;1823(10):1856–63. [DOI] [PubMed] [Google Scholar]
- Dando I, Fiorini C, Pozza ED, et al. UCP2 inhibition triggers ROS-dependent nuclear translocation of GAPDH and autophagic cell death in pancreatic adenocarcinoma cells. Biochim Biophys Acta. 2013;1833(3):672–9. [DOI] [PubMed] [Google Scholar]
- Dando I, Pacchiana R, Pozza ED, et al. UCP2 inhibition induces ROS/Akt/mTOR axis: role of GAPDH nuclear translocation in genipin/everolimus anticancer synergism. Free Radic Biol Med. 2017;113:176–89. [DOI] [PubMed] [Google Scholar]
- De Santis MC, Bockorny B, Hirsch E, et al. Exploiting pancreatic cancer metabolism: challenges and opportunities. Trends Mol Med. 2024;30(6):592–604. [DOI] [PubMed] [Google Scholar]
- de Souza BM, Brondani LA, Bouças AP, et al. Associations between UCP1 -3826A/G, UCP2 -866G/A, Ala55Val and Ins/Del, and UCP3 -55C/T polymorphisms and susceptibility to type 2 diabetes mellitus: case-control study and meta-analysis. PLoS ONE. 2013;8(1): e54259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirbilek H, Hussain K. Congenital hyperinsulinism: diagnosis and treatment update. J Clin Res Pediatr Endocrinol. 2017;9(Suppl 2):69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med. 2012;18(1):52–8. [DOI] [PubMed] [Google Scholar]
- Diao J, Allister EM, Koshkin V, et al. UCP2 is highly expressed in pancreatic alpha-cells and influences secretion and survival. Proc Natl Acad Sci U S A. 2008;105(33):12057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din I, Majid S, Rashid F, et al. Mitochondrial uncoupling protein 2 (UCP2) gene polymorphism—866 G/A in the promoter region is associated with type 2 diabetes mellitus among Kashmiri population of Northern India. Mol Biol Rep. 2023;50(1):475–83. [DOI] [PubMed] [Google Scholar]
- Donadelli M, Dando I, Fiorini C, et al. UCP2, a mitochondrial protein regulated at multiple levels. Cell Mol Life Sci. 2014;71(7):1171–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadelli M, Dando I, Dalla PE, et al. Mitochondrial uncoupling protein 2 and pancreatic cancer: a new potential target therapy. World J Gastroenterol. 2015;21(11):3232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Yin F, Zhong Y, et al. CircUCP2 promotes the tumor progression of non-small cell lung cancer through the miR-149/UCP2 pathway. Oncol Res. 2023;31(6):929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibl G, Rozengurt E. Metformin: review of epidemiology and mechanisms of action in pancreatic cancer. Cancer Metastasis Rev. 2021;40(3):865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre Y, Hurtaud C, Karaca M, et al. Role of uncoupling protein UCP2 in cell-mediated immunity: how macrophage-mediated insulitis is accelerated in a model of autoimmune diabetes. Proc Natl Acad Sci U S A. 2007;104(48):19085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entezari M, Hashemi D, Taheriazam A, et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: a pre-clinical and clinical investigation. Biomed Pharmacother. 2022;146:112563. [DOI] [PubMed] [Google Scholar]
- Faas M, Ipseiz N, Ackermann J, et al. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity. 2021;54(11):2531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini C, Cordani M, Gotte G, et al. Onconase induces autophagy sensitizing pancreatic cancer cells to gemcitabine and activates Akt/mTOR pathway in a ROS-dependent manner. Biochim Biophys Acta. 2015;1853(3):549–60. [DOI] [PubMed] [Google Scholar]
- Gable DR, Stephens JW, Cooper JA, et al. Variation in the UCP2-UCP3 gene cluster predicts the development of type 2 diabetes in healthy middle-aged men. Diabetes. 2006;55(5):1504–11. [DOI] [PubMed] [Google Scholar]
- Galetti S, Sarre A, Perreten H, et al. Fatty acids do not activate UCP2 in pancreatic beta cells: comparison with UCP1. Pflugers Arch. 2009;457(4):931–40. [DOI] [PubMed] [Google Scholar]
- Gearing KL, Göttlicher M, Teboul M, et al. Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci U S A. 1993;90(4):1440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Z, Chen W, Lu Q, et al. UCP2 overexpression activates SIRT3 to regulate oxidative stress and mitochondrial dynamics induced by myocardial injury. Arch Biochem Biophys. 2024;753:109918. [DOI] [PubMed] [Google Scholar]
- Giri D, Hawton K, Senniappan S. Congenital hyperinsulinism: recent updates on molecular mechanisms, diagnosis and management. J Pediatr Endocrinol Metab. 2022;35(3):279–96. [DOI] [PubMed] [Google Scholar]
- Gomathi P, Samarth AP, Raj N, et al. The -866G/A polymorphism in the promoter of the UCP2 gene is associated with risk for type 2 diabetes and with decreased insulin levels. Gene. 2019;701:125–30. [DOI] [PubMed] [Google Scholar]
- Gong J, Robbins LA, Lugea A, et al. Diabetes, pancreatic cancer, and metformin therapy. Front Physiol. 2014;5:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Barroso MM, Giurgea I, Bouillaud F, et al. Mutations in UCP2 in congenital hyperinsulinism reveal a role for regulation of insulin secretion. PLoS ONE. 2008;3(12): e3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubelnik V, Zmazek J, Završnik M, et al. Lipotoxicity in a vicious cycle of pancreatic beta cell exhaustion. Biomedicines. 2022;10(7):1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaumond F, Iovanna JL, Vasseur S. Pancreatic tumor cell metabolism: focus on glycolysis and its connected metabolic pathways. Arch Biochem Biophys. 2014;545:69–73. [DOI] [PubMed] [Google Scholar]
- Hals IK, Ogata H, Pettersen E, et al. Marked over expression of uncoupling protein-2 in beta cells exerts minor effects on mitochondrial metabolism. Biochem Biophys Res Commun. 2012;423(2):259–64. [DOI] [PubMed] [Google Scholar]
- Han J, Bae JH, Kim SY, et al. Taurine increases glucose sensitivity of UCP2-overexpressing beta-cells by ameliorating mitochondrial metabolism. Am J Physiol Endocrinol Metab. 2004;287(5):E1008–18. [DOI] [PubMed] [Google Scholar]
- Hardy AB, Serino AS, Wijesekara N, et al. Regulation of glucagon secretion by zinc: lessons from the β cell-specific Znt8 knockout mouse model. Diabetes Obes Metab. 2011;13(Suppl 1):112–7. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Hong J, Iacobuzio-Donahue CA. The pancreatic cancer genome revisited. Nat Rev Gastroenterol Hepatol. 2021;18(7):469–81. [DOI] [PubMed] [Google Scholar]
- He J, Li F, Zhou Y, et al. LncRNA XLOC_006390 promotes pancreatic carcinogenesis and glutamate metabolism by stabilizing c-Myc. Cancer Lett. 2020;469:419–28. [DOI] [PubMed] [Google Scholar]
- He Z, Wang J, Zhu C, et al. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer Lett. 2022;548:215751. [DOI] [PubMed] [Google Scholar]
- He J, Hou X, Wu J, et al. Hspb1 protects against severe acute pancreatitis by attenuating apoptosis and ferroptosis via interacting with Anxa2 to restore the antioxidative activity of Prdx1. Int J Biol Sci. 2024;20(5):1707–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin JC, Ibrahim MM, Rahier J. Insulin, glucagon and somatostatin stores in the pancreas of subjects with type-2 diabetes and their lean and obese non-diabetic controls. Sci Rep. 2017;7(1):11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg JV, Affourtit C. Mitochondrial uncoupling protein-2 is not involved in palmitate-induced impairment of glucose-stimulated insulin secretion in INS-1E insulinoma cells and is not needed for the amplification of insulin release. Biochem Biophys Rep. 2015;1:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EG, D’Souza NC, Aiken J, et al. Effects of somatostatin receptor type 2 antagonism during insulin-induced hypoglycaemia in male rats with prediabetes. Diabetes Obes Metab. 2023;25(6):1547–56. [DOI] [PubMed] [Google Scholar]
- Hogh KL, Craig MN, Uy CE, et al. Overexpression of PPARγ specifically in pancreatic β-cells exacerbates obesity-induced glucose intolerance, reduces β-cell mass, and alters islet lipid metabolism in male mice. Endocrinology. 2014;155(10):3843–52. [DOI] [PubMed] [Google Scholar]
- Hong Y, Fink BD, Dillon JS, et al. Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology. 2001;142(1):249–56. [DOI] [PubMed] [Google Scholar]
- Hou G, Jin Y, Liu M, et al. UCP2-866G/A polymorphism is associated with prediabetes and type 2 diabetes. Arch Med Res. 2020;51(6):556–63. [DOI] [PubMed] [Google Scholar]
- Hu M, Lin H, Yang L, et al. Interleukin-22 restored mitochondrial damage and impaired glucose-stimulated insulin secretion through down-regulation of uncoupling protein-2 in INS-1 cells. J Biochem. 2017;161(5):433–9. [DOI] [PubMed] [Google Scholar]
- Hu Z, Wang D, Gong J, et al. MSCs deliver hypoxia-treated mitochondria reprogramming acinar metabolism to alleviate severe acute pancreatitis injury. Adv Sci. 2023;10(25): e2207691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Cai T, Zhou Y, et al. Ethnicity differences in the association of UCP1-3826A/G, UCP2-866G/A and Ala55Val, and UCP3-55C/T polymorphisms with type 2 diabetes mellitus susceptibility: an updated meta-analysis. Biomed Res Int. 2021;2021:3482879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtaud C, Gelly C, Chen Z, et al. Glutamine stimulates translation of uncoupling protein 2mRNA. Cell Mol Life Sci. 2007;64(14):1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJpenberg A, Jeannin E, Wahli W, et al. Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element. J Biol Chem. 1997;272(32):20108–17. [DOI] [PubMed] [Google Scholar]
- Inoue R, Tsuno T, Togashi Y, et al. Uncoupling protein 2 and aldolase B impact insulin release by modulating mitochondrial function and Ca(2+) release from the ER. iScience. 2022;25(7):104603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ježek J, Dlasková A, Zelenka J, et al. H₂O₂-activated mitochondrial phospholipase iPLA₂γ prevents lipotoxic oxidative stress in synergy with UCP2, amplifies signaling via G-protein-coupled receptor GPR40, and regulates insulin secretion in pancreatic β-cells. Antioxid Redox Signal. 2015;23(12):958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia JJ, Zhang X, Ge CR, et al. The polymorphisms of UCP2 and UCP3 genes associated with fat metabolism, obesity and diabetes. Obes Rev. 2009;10(5):519–26. [DOI] [PubMed] [Google Scholar]
- Jiang L, Wan J, Ke LQ, et al. Activation of PPARδ promotes mitochondrial energy metabolism and decreases basal insulin secretion in palmitate-treated β-cells. Mol Cell Biochem. 2010;343(1–2):249–56. [DOI] [PubMed] [Google Scholar]
- Jiang L, Qiu W, Zhou Y, et al. A microRNA-30e/mitochondrial uncoupling protein 2 axis mediates TGF-β1-induced tubular epithelial cell extracellular matrix production and kidney fibrosis. Kidney Int. 2013;84(2):285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Chen D, Zheng RH, et al. miRNA-133a-UCP2 pathway regulates inflammatory bowel disease progress by influencing inflammation, oxidative stress and energy metabolism. World J Gastroenterol. 2017;23(1):76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Zhao K, Li J, et al. Matrine alleviates oxidative stress and ferroptosis in severe acute pancreatitis-induced acute lung injury by activating the UCP2/SIRT3/PGC1α pathway. Int Immunopharmacol. 2023;117:109981. [DOI] [PubMed] [Google Scholar]
- Joseph JW, Koshkin V, Zhang CY, et al. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51(11):3211–9. [DOI] [PubMed] [Google Scholar]
- Kanamoto Y, Yamashita Y, Nanba F, et al. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J Agric Food Chem. 2011;59(16):8985–93. [DOI] [PubMed] [Google Scholar]
- Kaul D, Sharma S, Garg A. Mitochondrial uncoupling protein (UCP2) gene expression is regulated by miR-2909. Blood Cells Mol Dis. 2015;55(1):89–93. [DOI] [PubMed] [Google Scholar]
- Kettunen S, Suoranta T, Beikverdi S, et al. Deletion of the murine ortholog of the human 9p21.3 locus leads to insulin resistance and obesity in hypercholesterolemic mice. Cells. 2024;13(11):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6(3):248–61. [DOI] [PubMed] [Google Scholar]
- Krempler F, Esterbauer H, Weitgasser R, et al. A functional polymorphism in the promoter of UCP2 enhances obesity risk but reduces type 2 diabetes risk in obese middle-aged humans. Diabetes. 2002;51(11):3331–5. [DOI] [PubMed] [Google Scholar]
- Lang H, Xiang Y, Lin N, et al. Identification of a panel of MiRNAs as positive regulators of insulin release in pancreatic Β-cells. Cell Physiol Biochem. 2018;48(1):185–93. [DOI] [PubMed] [Google Scholar]
- Lang L, Zheng D, Jiang Q, et al. Uncoupling protein 2 modulates polarization and metabolism of human primary macrophages via glycolysis and the NF-κB pathway. Exp Ther Med. 2023;26(6):583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapik IA, Ranjit R, Galchenko AV. Impact of KCNJ11 rs5219, UCP2 rs659366, and MTHFR rs1801133 polymorphisms on type 2 diabetes: a cross-sectional study. Rev Diabet Stud. 2021;17(1):21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauria G, Curcio R, Lunetti P, et al. Role of mitochondrial transporters on metabolic rewiring of pancreatic adenocarcinoma: a comprehensive review. Cancers. 2023;15(2):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Ryu HJ, Shin HD, et al. Associations between polymorphisms in the mitochondrial uncoupling proteins (UCPs) with T2DM. Clin Chim Acta. 2008;398(1–2):27–33. [DOI] [PubMed] [Google Scholar]
- Lee SC, Robson-Doucette CA, Wheeler MB. Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin. J Endocrinol. 2009;203(1):33–43. [DOI] [PubMed] [Google Scholar]
- Lewandowski SL, El K, Campbell JE. Evaluating GIP and glucagon as key regulators of insulin secretion in the pancreatic islet. Am J Physiol Endocrinol Metab. 2024. 10.1152/ajpendo.00360.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Nichols K, Nathan CA, et al. Mitochondrial uncoupling protein 2 is up-regulated in human head and neck, skin, pancreatic, and prostate tumors. Cancer Biomark. 2013;13(5):377–83. [DOI] [PubMed] [Google Scholar]
- Li N, Karaca M, Maechler P. Upregulation of UCP2 in beta-cells confers partial protection against both oxidative stress and glucotoxicity. Redox Biol. 2017;13:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Huang H, Limesand SW, et al. Pancreatic islets exhibit dysregulated adaptation of insulin secretion after chronic epinephrine exposure. Curr Issues Mol Biol. 2021;43(1):240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti L, Cavaliere G, Bergamo P, et al. Diet supplementation with donkey milk upregulates liver mitochondrial uncoupling, reduces energy efficiency and improves antioxidant and antiinflammatory defences in rats. Mol Nutr Food Res. 2012;56(10):1596–600. [DOI] [PubMed] [Google Scholar]
- Liu L, Liu J, Gao Y, et al. Uncoupling protein-2 mediates the protective action of berberine against oxidative stress in rat insulinoma INS-1E cells and in diabetic mouse islets. Br J Pharmacol. 2014;171(13):3246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yu K, Ma P, et al. Long noncoding RNA myocardial infarction-associated transcript regulated the pancreatic stellate cell activation to promote the fibrosis process of chronic pancreatitis. J Cell Biochem. 2019;120(6):9547–55. [DOI] [PubMed] [Google Scholar]
- Liu X, Li D, Liu Z, et al. Nicotinamide mononucleotide promotes pancreatic islet function through the SIRT1 pathway in mice after severe burns. Burns. 2022;48(8):1922–32. [DOI] [PubMed] [Google Scholar]
- Luby A, Alves-Guerra MC. UCP2 as a cancer target through energy metabolism and oxidative stress control. Int J Mol Sci. 2022. 10.3390/ijms232315077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JY, Cheng CK, He L, et al. Endothelial UCP2 is a mechanosensitive suppressor of atherosclerosis. Circ Res. 2022;131(5):424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZA, Zhao Z, Turk J. Mitochondrial dysfunction and β-cell failure in type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux RJ, Fu A, Robson-Doucette C, et al. Glutathionylation state of uncoupling protein-2 and the control of glucose-stimulated insulin secretion. J Biol Chem. 2012;287(47):39673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiztegui B, Román CL, Gagliardino JJ, et al. Impaired endocrine-metabolic homeostasis: underlying mechanism of its induction by unbalanced diet. Clin Sci. 2018;132(8):869–81. [DOI] [PubMed] [Google Scholar]
- Marin TL, Gongol B, Zhang F, et al. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci Signal. 2017;10(464):7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T, Li S, Adachi T, et al. Hyperoxia reverses glucotoxicity-induced inhibition of insulin secretion in rat INS-1 β cells. Biosci Biotechnol Biochem. 2014;78(5):843–50. [DOI] [PubMed] [Google Scholar]
- Medvedev AV, Snedden SK, Raimbault S, et al. Transcriptional regulation of the mouse uncoupling protein-2 gene. Double E-box motif is required for peroxisome proliferator-activated receptor-gamma-dependent activation. J Biol Chem. 2001;276(14):10817–23. [DOI] [PubMed] [Google Scholar]
- Mizusawa N, Harada N, Iwata T, et al. Identification of protease serine S1 family member 53 as a mitochondrial protein in murine islet beta cells. Islets. 2022;14(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2(2):105–17. [DOI] [PubMed] [Google Scholar]
- Müller S, Kaiser H, Krüger B, et al. Age-dependent effects of UCP2 deficiency on experimental acute pancreatitis in mice. PLoS ONE. 2014;9(4): e94494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller TD, Finan B, Clemmensen C, et al. The new biology and pharmacology of glucagon. Physiol Rev. 2017;97(2):721–66. [DOI] [PubMed] [Google Scholar]
- Muller S, Klingbeil SM, Sandica A, et al. Uncoupling protein 2 deficiency reduces proliferative capacity of murine pancreatic stellate cells. Hepatobiliary Pancreat Dis Int. 2016;15(6):647–54. [DOI] [PubMed] [Google Scholar]
- Naderi R, Shirpoor A, Samadi M, et al. Tropisetron improves pancreas function and increases insulin synthesis and secretion in the STZ-induced diabetic rats: involvement of UCP2/ZnT8 pathway. J Pharm Pharmacol. 2020;72(8):1082–91. [DOI] [PubMed] [Google Scholar]
- Nakata M, Shintani N, Hashimoto H, et al. Intra-islet PACAP protects pancreatic β-cells against glucotoxicity and lipotoxicity. J Mol Neurosci. 2010;42(3):404–10. [DOI] [PubMed] [Google Scholar]
- Nicoletti CF, de Oliveira AP, Brochado MJ, et al. The Ala55Val and -866G>A polymorphisms of the UCP2 gene could be biomarkers for weight loss in patients who had Roux-en-Y gastric bypass. Nutrition. 2017;33:326–30. [DOI] [PubMed] [Google Scholar]
- Oberkofler H, Klein K, Felder TK, et al. Role of peroxisome proliferator-activated receptor-gamma coactivator-1alpha in the transcriptional regulation of the human uncoupling protein 2 gene in INS-1E cells. Endocrinology. 2006;147(2):966–76. [DOI] [PubMed] [Google Scholar]
- Oberkofler H, Hafner M, Felder T, et al. Transcriptional co-activator peroxisome proliferator-activated receptor (PPAR)gamma co-activator-1beta is involved in the regulation of glucose-stimulated insulin secretion in INS-1E cells. J Mol Med (Berl). 2009;87(3):299–306. [DOI] [PubMed] [Google Scholar]
- Odei-Addo F, Ramlugon S, Levendal RA, et al. Leonotis Leonurus improves the crosstalk between peripheral tissues both in vivo and in vitro. J Ethnopharmacol. 2021;267:113609. [DOI] [PubMed] [Google Scholar]
- Ouyang G, Pan G, Liu Q, et al. The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. BMC Med. 2020;18(1):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JA, Zhang H, Lin H, et al. Irisin ameliorates doxorubicin-induced cardiac perivascular fibrosis through inhibiting endothelial-to-mesenchymal transition by regulating ROS accumulation and autophagy disorder in endothelial cells. Redox Biol. 2021;46:102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanè G, Anello M, Piro S, et al. Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-gamma inhibition. Diabetes. 2002;51(9):2749–56. [DOI] [PubMed] [Google Scholar]
- Peng C, Tu G, Wang J, et al. MLKL signaling regulates macrophage polarization in acute pancreatitis through CXCL10. Cell Death Dis. 2023;14(2):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez S, Rius-Pérez S, Finamor I, et al. Obesity causes PGC-1α deficiency in the pancreas leading to marked IL-6 upregulation via NF-κB in acute pancreatitis. J Pathol. 2019;247(1):48–59. [DOI] [PubMed] [Google Scholar]
- Petrov MS. Fatty change of the pancreas: the Pandora’s box of pancreatology. Lancet Gastroenterol Hepatol. 2023;8(7):671–82. [DOI] [PubMed] [Google Scholar]
- Pi J, Bai Y, Daniel KW, et al. Persistent oxidative stress due to absence of uncoupling protein 2 associated with impaired pancreatic beta-cell function. Endocrinology. 2009;150(7):3040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Shirihai OS. Mitochondrial signal transduction. Cell Metab. 2022;34(11):1620–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plecitá-Hlavatá L, Engstová H, Ježek J, et al. Potential of mitochondria-targeted antioxidants to prevent oxidative stress in pancreatic β-cells. Oxid Med Cell Longev. 2019;2019:1826303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Produit-Zengaffinen N, Davis-Lameloise N, Perreten H, et al. Increasing uncoupling protein-2 in pancreatic beta cells does not alter glucose-induced insulin secretion but decreases production of reactive oxygen species. Diabetologia. 2007;50(1):84–93. [DOI] [PubMed] [Google Scholar]
- Raho S, Capobianco L, Malivindi R, et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth. Nat Metab. 2020;2(12):1373–81. [DOI] [PubMed] [Google Scholar]
- Revskij D, Runst J, Umstätter C, et al. Uncoupling protein 2 deficiency of non-cancerous tissues inhibits the progression of pancreatic cancer in mice. Hepatobiliary Pancreat Dis Int. 2023;22(2):190–9. [DOI] [PubMed] [Google Scholar]
- Rezapour S, Khosroshahi SA, Farajnia H, et al. Association of 45-bp ins/del polymorphism of uncoupling protein 2 (UCP2) and susceptibility to nonalcoholic fatty liver and type 2 diabetes mellitus in North-west of Iran. BMC Res Notes. 2021;14(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickels MR, Robertson RP. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr Rev. 2019;40(2):631–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud VO, Zarka C, Kurian J, et al. UCP2 modulates cardiomyocyte cell cycle activity, acetyl-CoA, and histone acetylation in response to moderate hypoxia. JCI Insight. 2022;7(15): e155475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson-Doucette CA, Sultan S, Allister EM, et al. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes. 2011;60(11):2710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röder PV, Wu B, Liu Y, et al. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48(3): e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan C, Kalaivani K. Isopulegol mitigates hyperglycemia mediated oxidative and endoplasmic reticulum stress in HFD/STZ induced diabetic rats. Arch Med Res. 2020;51(3):204–14. [DOI] [PubMed] [Google Scholar]
- Sasahara M, Nishi M, Kawashima H, et al. Uncoupling protein 2 promoter polymorphism -866G/A affects its expression in beta-cells and modulates clinical profiles of Japanese type 2 diabetic patients. Diabetes. 2004;53(2):482–5. [DOI] [PubMed] [Google Scholar]
- Sayeed A, Meng Z, Luciani G, et al. Negative regulation of UCP2 by TGFβ signaling characterizes low and intermediate-grade primary breast cancer. Cell Death Dis. 2010;1(7): e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlünder K, Cipriano M, Zbinden A, et al. Microphysiological pancreas-on-chip platform with integrated sensors to model endocrine function and metabolism. Lab Chip. 2024;24(7):2080–93. [DOI] [PubMed] [Google Scholar]
- Segersvärd R, Rippe C, Duplantier M, et al. mRNA for pancreatic uncoupling protein 2 increases in two models of acute experimental pancreatitis in rats and mice. Cell Tissue Res. 2005;320(2):251–8. [DOI] [PubMed] [Google Scholar]
- Seshadri N, Jonasson ME, Hunt KL, et al. Uncoupling protein 2 regulates daily rhythms of insulin secretion capacity in MIN6 cells and isolated islets from male mice. Mol Metab. 2017;6(7):760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen A, Aljebali AM. A hypothetical model to solve the controversy over the involvement of UCP2 in palmitate-induced β-cell dysfunction. Endocrine. 2016;54(2):276–83. [DOI] [PubMed] [Google Scholar]
- Shen A, Kim HJ, Oh GS, et al. NAD(+) augmentation ameliorates acute pancreatitis through regulation of inflammasome signalling. Sci Rep. 2017;7(1):3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih DQ, Navas MA, Kuwajima S, et al. Impaired glucose homeostasis and neonatal mortality in hepatocyte nuclear factor 3alpha-deficient mice. Proc Natl Acad Sci U S A. 1999;96(18):10152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H. SREBPs: physiology and pathophysiology of the SREBP family. FEBS J. 2009;276(3):616–21. [DOI] [PubMed] [Google Scholar]
- Song L, Xu Z, Li L, et al. Forkhead box protein A1 inhibits the expression of uncoupling protein 2 in hydrogen peroxide-induced A549 cell line. Cell Stress Chaperones. 2014;19(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava N, Prakash J, Lakhan R, et al. A common polymorphism in the promoter of UCP2 is associated with obesity and hyperinsulenemia in northern Indians. Mol Cell Biochem. 2010;337(1–2):293–8. [DOI] [PubMed] [Google Scholar]
- Steen KA, Xu H, Bernlohr DA. FABP4/aP2 regulates macrophage redox signaling and inflammasome activation via control of UCP2. Mol Cell Biol. 2017. 10.1128/MCB.00282-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LL, Jiang BG, Li WT, et al. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract. 2011;91(1):94–100. [DOI] [PubMed] [Google Scholar]
- Sun K, Chen Y, Zheng S, et al. Genipin ameliorates diabetic retinopathy via the HIF-1α and AGEs-RAGE pathways. Phytomedicine. 2024;129:155596. [DOI] [PubMed] [Google Scholar]
- Tang PC, Chan MK, Chung JY, et al. Hematopoietic transcription factor RUNX1 is essential for promoting macrophage-Myofibroblast transition in non-small-cell lung carcinoma. Adv Sci. 2024;11(1): e2302203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavoosi S, Baghsheikhi AH, Shetab-Boushehri SV, et al. Cerium and yttrium oxide nanoparticles and nano-selenium produce protective effects against H2O2-induced oxidative stress in pancreatic beta cells by modulating mitochondrial dysfunction. Pharm Nanotechnol. 2020;8(1):63–75. [DOI] [PubMed] [Google Scholar]
- Tripathi R, Banerjee SK, Nirala JP, et al. Exposure to electromagnetic fields from mobile phones and fructose consumption coalesce to perturb metabolic regulators AMPK/SIRT1-UCP2/FOXO1 in growing rats. Biomed Environ Sci. 2023;36(11):1045–58. [DOI] [PubMed] [Google Scholar]
- van Dierendonck X, Sancerni T, Alves-Guerra MC, et al. The role of uncoupling protein 2 in macrophages and its impact on obesity-induced adipose tissue inflammation and insulin resistance. J Biol Chem. 2020;295(51):17535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamaniuk MZ, Gupta RK, Lantz KA, et al. Foxa1-deficient mice exhibit impaired insulin secretion due to uncoupled oxidative phosphorylation. Diabetes. 2006;55(10):2730–6. [DOI] [PubMed] [Google Scholar]
- Vierhout M, Ayoub A, Naiel S, et al. Monocyte and macrophage derived myofibroblasts: Is it fate? A review of the current evidence. Wound Repair Regen. 2021;29(4):548–62. [DOI] [PubMed] [Google Scholar]
- Vimaleswaran KS, Radha V, Ghosh S, et al. Uncoupling protein 2 and 3 gene polymorphisms and their association with type 2 diabetes in asian indians. Diabetes Technol Ther. 2011;13(1):19–25. [DOI] [PubMed] [Google Scholar]
- Wade AK, Liu Y, Bethea MM, et al. LIM-domain transcription complexes interact with ring-finger ubiquitin ligases and thereby impact islet β-cell function. J Biol Chem. 2019;294(31):11728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Shimabukuro M, Lee Y, et al. Adenovirus-mediated overexpression of uncoupling protein-2 in pancreatic islets of Zucker diabetic rats increases oxidative activity and improves beta-cell function. Diabetes. 1999;48(5):1020–5. [DOI] [PubMed] [Google Scholar]
- Wang X, Lei XG, Wang J. Malondialdehyde regulates glucose-stimulated insulin secretion in murine islets via TCF7L2-dependent Wnt signaling pathway. Mol Cell Endocrinol. 2014;382(1):8–16. [DOI] [PubMed] [Google Scholar]
- Wang C, Li Q, Wang W, et al. GLP-1 contributes to increases in PGC-1α expression by downregulating miR-23a to reduce apoptosis. Biochem Biophys Res Commun. 2015;466(1):33–9. [DOI] [PubMed] [Google Scholar]
- Wang YY, Jiang H, Pan J, et al. Macrophage-to-Myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J Am Soc Nephrol. 2017;28(7):2053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li C, Huang Q, et al. Biofunctionalization of selenium nanoparticles with a polysaccharide from Rosa roxburghii fruit and their protective effect against H(2)O(2)-induced apoptosis in INS-1 cells. Food Funct. 2019;10(2):539–53. [DOI] [PubMed] [Google Scholar]
- Wang XD, Yu WL, Sun Y. Activation of AMPK restored impaired autophagy and inhibited inflammation reaction by up-regulating SIRT1 in acute pancreatitis. Life Sci. 2021a;277:119435. [DOI] [PubMed] [Google Scholar]
- Wang K, Miao X, Kong F, et al. Integrating network pharmacology and experimental verification to explore the mechanism of effect of Zuojin pills in pancreatic cancer treatment. Drug des Devel Ther. 2021b;15:3749–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Han S, Lv G, et al. Pancreatic acinar cells-derived sphingosine-1-phosphate contributes to fibrosis of chronic pancreatitis via inducing autophagy and activation of pancreatic stellate cells. Gastroenterology. 2023;165(6):1488–504. [DOI] [PubMed] [Google Scholar]
- Wei J, Deng X, Li Y, et al. PP2 ameliorates renal fibrosis by regulating the NF-κB/COX-2 and PPARγ/UCP2 pathway in diabetic mice. Oxid Med Cell Longev. 2021;2021:7394344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Wang X, Wei Y, et al. lncRNA TUG1 protects intestinal epithelial cells from damage induced by high glucose and high fat via AMPK/SIRT1. Mol Med Rep. 2022;25(4):1–9. [DOI] [PubMed] [Google Scholar]
- Willig AL, Casazza KR, Divers J, et al. Uncoupling protein 2 Ala55Val polymorphism is associated with a higher acute insulin response to glucose. Metabolism. 2009;58(6):877–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Li CH, He Q, et al. Ectopic HOTTIP expression induces noncanonical transactivation pathways to promote growth and invasiveness in pancreatic ductal adenocarcinoma. Cancer Lett. 2020;477:1–9. [DOI] [PubMed] [Google Scholar]
- Wu P, Wang Q, Jiang C, et al. MicroRNA-29a is involved lipid metabolism dysfunction and insulin resistance in C2C12 myotubes by targeting PPARδ. Mol Med Rep. 2018;17(6):8493–501. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang L, Shi J, et al. Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury. EBioMedicine. 2020;58:102920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Shi Q, Yang X, et al. LINC00052 functions as a tumor suppressor through negatively modulating miR-330-3p in pancreatic cancer. J Cell Physiol. 2019;234(9):15619–26. [DOI] [PubMed] [Google Scholar]
- Xu K, Zhang M, Cui D, et al. UCP2 -866G/A and Ala55Val, and UCP3 -55C/T polymorphisms in association with type 2 diabetes susceptibility: a meta-analysis study. Diabetologia. 2011;54(9):2315–24. [DOI] [PubMed] [Google Scholar]
- Xu L, Chen S, Zhan L. Association of uncoupling protein-2 -866G/A and Ala55Val polymorphisms with susceptibility to type 2 diabetes mellitus: a meta-analysis of case-control studies. Medicine. 2021a;100(6): e24464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yan J, Ocak U, et al. Melanocortin 1 receptor attenuates early brain injury following subarachnoid hemorrhage by controlling mitochondrial metabolism via AMPK/SIRT1/PGC-1α pathway in rats. Theranostics. 2021b;11(2):522–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Ye H, Xiao W, et al. Metformin attenuates inflammation and fibrosis in thyroid-associated ophthalmopathy. Int J Mol Sci. 2022;23(24):15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Sharma V, Hsieh MH, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QH, Hu SP, Zhang YP, et al. Effect of berberine on expressions of uncoupling protein-2 mRNA and protein in hepatic tissue of non-alcoholic fatty liver disease in rats. Chin J Integr Med. 2011;17(3):205–11. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yang Y, Hou J, et al. The hydroxyl at position C1 of genipin is the active inhibitory group that affects mitochondrial uncoupling protein 2 in panc-1 cells. PLoS ONE. 2016;11(1): e147026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Fei X, Lu Y, et al. miRNA-214 suppresses oxidative stress in diabetic nephropathy via the ROS/Akt/mTOR signaling pathway and uncoupling protein 2. Exp Ther Med. 2019;17(5):3530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chen J, Wang J, et al. Very-low-density lipoprotein receptor-enhanced lipid metabolism in pancreatic stellate cells promotes pancreatic fibrosis. Immunity. 2022a;55(7):1185–99. [DOI] [PubMed] [Google Scholar]
- Yang HY, Liu M, Sheng Y, et al. All-trans retinoic acid impairs glucose-stimulated insulin secretion by activating the RXR/SREBP-1c/UCP2 pathway. Acta Pharmacol Sin. 2022b;43(6):1441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Xu R, Song J, et al. Lipid metabolism in pancreatic cancer: emerging roles and potential targets. Cancer Commun. 2022;42(12):1234–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HJ, Hong CO, Ha SK, et al. Chebulic acid prevents methylglyoxal-induced mitochondrial dysfunction in INS-1 pancreatic β-cells. Antioxidants. 2020;9(9):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Jacobs DJ, Schreiner PJ, et al. The uncoupling protein 2 Ala55Val polymorphism is associated with diabetes mellitus: the CARDIA study. Clin Chem. 2005;51(8):1451–6. [DOI] [PubMed] [Google Scholar]
- Yu J, Shi L, Lin W, et al. UCP2 promotes proliferation and chemoresistance through regulating the NF-κB/β-catenin axis and mitochondrial ROS in gallbladder cancer. Biochem Pharmacol. 2020;172:113745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Li X, Li J, et al. Effects of renin-angiotensin system blockade on the islet morphology and function in rats with long-term high-fat diet. Acta Diabetol. 2013;50(4):479–88. [DOI] [PubMed] [Google Scholar]
- Yuan T, Xia Y, Pan S, et al. STAT6 promoting oxalate crystal deposition-induced renal fibrosis by mediating macrophage-to-myofibroblast transition via inhibiting fatty acid oxidation. Inflamm Res. 2023;72(12):2111–26. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105(6):745–55. [DOI] [PubMed] [Google Scholar]
- Zhang D, Shen M, Mikita A, et al. Targeting uncoupling protein-2 improves islet graft function. Cell Transpl. 2011;20(3):421–9. [DOI] [PubMed] [Google Scholar]
- Zhang S, Feng Z, Gao W, et al. Aucubin attenuates liver ischemia-reperfusion injury by inhibiting the HMGB1/TLR-4/NF-κB signaling pathway, oxidative stress, and apoptosis. Front Pharmacol. 2020;11:544124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Li X, Yang Q, et al. Malvidin alleviates mitochondrial dysfunction and ROS accumulation through activating AMPK-α/UCP2 axis, thereby resisting inflammation and apoptosis in SAE mice. Front Pharmacol. 2022;13:1038802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Xie J, Ma L, et al. Vitamin D receptor activation targets ROS-mediated crosstalk between autophagy and apoptosis in hepatocytes in cholestasic mice. Cell Mol Gastroenterol Hepatol. 2023;15(4):887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Yamada Y, Tsukiyama K, et al. Gastric inhibitory polypeptide modulates adiposity and fat oxidation under diminished insulin action. Biochem Biophys Res Commun. 2005;335(3):937–42. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhao J, Zhang X. Inhibition of uncoupling protein 2 by genipin reduces insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Arch Biochem Biophys. 2009;486(1):88–93. [DOI] [PubMed] [Google Scholar]
- Zhou MC, Yu P, Sun Q, et al. Expression profiling analysis: uncoupling protein 2 deficiency improves hepatic glucose, lipid profiles and insulin sensitivity in high-fat diet-fed mice by modulating expression of genes in peroxisome proliferator-activated receptor signaling pathway. J Diabetes Investig. 2016;7(2):179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu YX, Lu HY, Liu WW, et al. Jiang Gui Fang activated interscapular brown adipose tissue and induced epididymal white adipose tissue browning through the PPARγ/SIRT1-PGC1α pathway. J Ethnopharmacol. 2020;248:112271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.