Abstract
Background
Trabeculectomy (TRAB) traditionally has been the gold-standard surgical treatment for primary open-angle glaucoma (POAG), while gonioscopy-assisted transluminal trabeculotomy (GATT) is an emerging minimally invasive surgery used for the treatment of various open-angle glaucoma (OAG) types. In this study, we aimed to compare the efficacy and safety between GATT and TRAB for the treatment of POAG.
Methods
This cohort study included eyes with POAG that underwent a single GATT (30 eyes) or TRAB (34 eyes). Follow-up was conducted at 1 day, 1 week, and 1, 3, 6, and 12 months postoperatively. Intraocular pressure (IOP), the numbers of glaucoma medication, visual field mean deviation, peripapillary retinal nerve fiber layer thickness, surgical time, and complications were analyzed. Success criteria were defined as IOP ≤ 21 mmHg and ≥ 20% IOP reduction from baseline. Qualified and complete surgical success rates were also compared.
Results
IOP and antiglaucoma drug use decreased significantly at 12 months postoperatively in the both groups (P < 0.001), with no significant differences between the two groups pre- and postoperatively (P > 0.05). The success rates at 12 months were 70% (95% confidence interval [CI] = 52.6–87.4%) in the GATT group and 76.5% (95% CI = 61.4–91.5%) in the TRAB group (P = 0.559).Visual field loss remained unchanged at 12 months postoperatively compared with preoperative levels in both groups (P > 0.05); however, peripapillary retinal nerve fiber layer thickness decreased significantly at 12 months postoperatively compared with preoperative levels in the GATT group (P < 0.001). The most frequent complications after TRAB and GATT were bleb-related complications and hyphema, respectively.
Conclusions
GATT demonstrated an efficacy comparable to that of TRAB for the treatment of POAG with regards to lowering IOP, reducing medication use, and preserving visual fields. Thus, GATT is a minimally invasive technique that enables an effective and safe decrease in IOP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-024-03798-8.
Keywords: Gonioscopy-assisted transluminal trabeculotomy, Trabeculectomy, Primary open-angle glaucoma, Single procedure
Background
Glaucoma is the leading cause of irreversible blindness; it is projected to affect approximately 112 million people worldwide by 2040 [1–3]. Primary open-angle glaucoma (POAG) is the predominant subtype of the disease, and blindness occurred in 6 million people with POAG in 2020 [4–6]. Trabeculectomy (TRAB) has been the gold-standard surgical treatment for POAG for over 50 years [7]. However, TRAB is associated with numerous short- and long-term complications, many of which are vision-threatening [8]. In recent decades, ophthalmologists have attempted to identify new procedures for glaucoma treatment.
Among these alternative procedures, minimally invasive glaucoma surgery has become an increasingly available group of safe and less traumatic ab interno procedures [9, 10]. Gonioscopy-assisted transluminal trabeculotomy (GATT) was introduced by Grover et al. in 2014 to manage OAG [11]. Since its introduction, multiple studies have demonstrated the clinical effectiveness of GATT for the treatment of various OAG types [12–15], and the combination of GATT and cataract surgery is commonly used in clinical practice [16, 17]. However, trials comparing the outcomes of GATT and TRAB for treatment specifically addressing POAG are lacking; the only two relevant studies targeting OAG did not adjust for the confounding effect of combined cataract extraction [18, 19].
Therefore, in this study, we aimed to compare the efficacy and safety of GATT and TRAB alone in patients with POAG during a postoperative follow-up of 12 months. We compared the changes in intraocular pressure (IOP) and the administration of antiglaucoma medication. Visual field (VF), optical coherence tomography (OCT) of the optic disc, and gonioscopy were simultaneously used to observe structural and functional changes in the patients.
Methods
This retrospective comparative cohort study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Qilu Hospital of Shandong University (approval number: KYLL-202008-167). All patients signed an informed consent form, and no patient received a stipend.
Research participants
This retrospective cohort study included patients with POAG who underwent single TRAB or GATT at Qilu Hospital of Shandong University between November 2018 and March 2023. POAG was diagnosed based on the characteristic glaucomatous fundus, visual field damage, and open anterior chamber angle. Patients who failed to achieve the target IOP or those with unstable IOP control despite receiving the maximum tolerated dose of IOP-lowering medications were deemed to require surgical intervention and included in this study.
Patients with types of glaucoma other than POAG, including secondary glaucoma, angle-closure glaucoma, trauma-associated glaucoma, or syndrome-associated glaucoma; those < 18 years old; those who had undergone combined cataract extraction and GATT/TRAB; and those who had undergone previous ophthalmic surgery other than cataract and glaucoma surgery were excluded.
Research data
Data on sex, age, and history of cataract or glaucoma surgery were obtained from the patients’ charts. All the included patients underwent documented full ophthalmic examinations preoperatively and postoperatively, including IOP measurement (TX-20, CANON, Tokyo, Japan) and VF testing (HFAII, ZEISS, Jena, Germany) evaluated with the mean deviation (MD), OCT (CIRRUS HD-OCT 4000, ZEISS, Jena, Germany) of the optic disc to measure peripapillary retinal nerve fiber layer (RNFL) thickness, and gonioscopy (Ocular Instrument, Bellevue, WA, USA) to assess the angles of the anterior chamber. The episcleral venous fluid wave (EVFW) was examined in the GATT group before the end of the surgery. The morphology of the filtering bleb was assessed postoperatively using a slit-lamp microscope. The number of glaucoma medications and the incidence and types of complications were also determined. Follow-up data were collected at 1 day, 1 week, and 1, 2, 3, 6, and 12 months postoperatively. VF and OCT were performed 12 months postoperatively.
Surgical approaches
All surgeries were performed by the same experienced glaucoma surgeon (HG). For the GATT procedure, a corneal limbal incision was made, and a viscoelastic agent was injected into the anterior chamber. The structures of the anterior chamber angle on the nasal side were visualized using the goniolens, and the Schlemm’s canal was localized. A needle was used to make a 1–2 mm incision on the inner wall of the canal. A heat-blunted 6 − 0 prolene suture was then inserted into the broken end of Schlemm’s canal and passed through the canal until it exited the other broken end. The trabeculotomy was completed by holding the ends of the sutures with forceps and slowly pulling the sutures to incise the inner wall of Schlemm’s canal. Residual viscoelastic and anterior chamber blood were removed by balanced salt solution flushing, and the EVFW was examined at maximum irrigation (Supplemental File 1). At the end of the surgery, a watertight closure of the corneal incision was made to maintain a slightly elevated IOP.
For the TRAB procedure, a conjunctival flap based on the fornix and a scleral flap (3 × 4 mm) based on the corneal limbus were fabricated. Cotton swabs impregnated with mitomycin (0.4 mg/mL) were placed between the conjunctival flap, scleral flap, and scleral bed for 2.5 min, after which the mitomycin was fully rinsed away using lactated Ringer solution. The deep corneoscleral tissue (2 × 2 mm) was excised, followed by the removal of the iris tissue from the periphery. The free angle of the scleral flap was closed using 10 − 0 prolene sutures along with two releasable sutures on each side of the scleral flap.
Efficacy assessment
Procedural success was defined as postoperative IOP ≤ 21 mmHg and an IOP reduction of at least 20% from baseline. Eyes that met the abovementioned criteria without the use of antiglaucoma medications were defined as complete successes, whereas those that used glaucoma medications were defined as qualified successes.
In this study, 1% pilocarpine eye drops (miotic) were routinely administered for 3 months postoperatively to patients in the GATT group to prevent peripheral anterior iris adhesions; therefore, these drops were not considered glaucoma medications.
Statistical analysis
The sample size was determined to be 26 for each group to achieve adequate power (80%, alpha = 0.05), and non-inferiority was detected using a one-sided two-sample t-test. At the last follow-up, the GATT group constituted 29 participants and the TRAB group 31 participants, which exceeded the minimum sample requirements for each group.
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., USA). Continuous data such as age, IOP, number of glaucoma medications used, visual field MD, RNFL thickness, operation time, and extension of trabeculotomy achieved in the GATT group were expressed as means ± standard deviations. Categorical data such as sex, glaucoma type, lens status, history of antiglaucoma surgery, and surgical complications were expressed as frequencies and percentages.
The normality and homogeneity of variance of the continuous data were tested before comparison. Data that demonstrated normal distribution and homogeneity were analyzed using a t-test. Data that did not satisfy normal distribution or homogeneity were analyzed using a corrected t-test, Mann–Whitney U-test, or Wilcoxon rank-sum test, as appropriate. Pearson’s chi-squared and Fisher’s exact tests were used to compare the categorical data. Survival rate was analyzed with Kaplan-Meier survival analysis. Statistical significance was set at P < 0.05 for all the analyses.
Results
No statistically significant differences were observed in age, sex, lens status, or history of antiglaucoma surgery between the two groups (P > 0.05; Table 1). Regarding antiglaucoma surgical history, the GATT group included four eyes that had undergone TRAB and two eyes with Ahmed glaucoma valve implantation. The TRAB group included two eyes that had undergone GATT. All patients underwent regular follow-up until the 12-month mark.
Table 1.
Comparison of the general participant characteristics between the two treatment groups
| GATT group | TRAB group | P value | |
|---|---|---|---|
| Patients/eyes (n) | 30/30 | 34/34 | |
| Age (years) | 47.20 ± 10.46 | 47.65 ± 12.49 | 0.878 |
| Sex (n, %) | 0.885 | ||
| Male | 18 (60%) | 21 (61.8%) | |
| Female | 12 (40%) | 13 (38.2%) | |
| Lens status (n, %) | 0.748 | ||
| Phakic | 28 (93.33%) | 31 (91.18%) | |
| Pseudophakic | 2(6.67%) | 3 (8.82%) | |
| Previous antiglaucoma surgery (n, %) | 0.088 | ||
| No | 24 (80%) | 32 (94.12%) | |
| Yes | 6 (20%) | 2 (5.88%) | |
GATT, gonioscopy-assisted transluminal trabeculotomy; POAG, primary open-angle glaucoma; TRAB, trabeculectomy
IOP reduction
The average preoperative IOPs did not differ significantly between the GATT and TRAB groups (27.35 ± 10.02 vs. 30.00 ± 11.84 mmHg, P = 0.34). The IOPs of both groups at 12 months were significantly lower than those before surgery (P < 0.001). At postoperative day 1, the IOP in the TRAB group was significantly higher than that in the GATT group (14.83 ± 4.94 vs. 19.18 ± 9.33 mmHg, P = 0.026). No other follow-up time point showed significant differences in IOP between the two groups (P > 0.05). The reduction in IOP compared to preoperative levels did not differ between the groups at any follow-up time points (P > 0.05)(Table 2). IOP decreased by an average of 33.59% in the GATT group and 41.32% in the TRAB group at 12 months postoperatively. The range of IOP reduction (33.59% vs. 41.32%) did not differ significantly between the two groups (P = 0.363). History of previous glaucoma surgery did not affect preoperative IOP, 12-month postoperative IOP, IOP reduction, or range of IOP reduction in the GATT and TRAB groups (P > 0.05, Supplemental File 2).
Table 2.
Pre- and postoperative IOP values and reduction after TRAB and GATT during the follow-up period
| IOP (mmHg, Mean ± SD) | IOP reduction from baseline (mmHg, Mean ± SD) | |||||
|---|---|---|---|---|---|---|
| GATT Group | TRAB Group | P value | GATT Group | TRAB Group | P value | |
| Preoperative (baseline) | 27.35 ± 10.02 | 30.00 ± 11.84 | 0.342 | |||
| 1 day after surgery | 14.83 ± 4.94 | 19.18 ± 9.33 | 0.026 | 12.53 ± 10.42 | 10.79 ± 14.81 | 0.594 |
| 1 week after surgery | 15.78 ± 6.81 | 13.81 ± 3.97 | 0.161 | 11.60 ± 10.53 | 15.88 ± 13.19 | 0.163 |
| 1 month after surgery | 16.07 ± 3.53 | 15.54 ± 4.60 | 0.616 | 10.79 ± 10.50 | 14.15 ± 12.68 | 0.264 |
| 2 months after surgery | 14.94 ± 3.66 | 15.83 ± 4.63 | 0.415 | 11.93 ± 9.84 | 13.88 ± 13.08 | 0.515 |
| 3 months after surgery | 15.39 ± 3.50 | 14.62 ± 2.92 | 0.354 | 11.52 ± 10.03 | 15.41 ± 11.88 | 0.175 |
| 6 months after surgery | 15.94 ± 3.44 | 15.01 ± 4.19 | 0.202 | 10.93 ± 10.13 | 15.03 ± 12.95 | 0.177 |
| 12 months after surgery | 15.64 ± 4.80 | 15.15 ± 3.02 | 0.994 | 11.24 ± 10.77 | 15.19 ± 12.14 | 0.189 |
GATT, gonioscopy-assisted transluminal trabeculotomy; IOP, intraocular pressure; TRAB, trabeculectomy
Antiglaucoma medication
Preoperatively, 11 and 17 eyes in the GATT group received 3 and 2 types of antiglaucoma drugs, respectively (average: 2.43 ± 0.63 types). In the TRAB group, 19 and 9 eyes received 3 and 2 types of antiglaucoma drugs preoperatively, respectively (average: 2.29 ± 1.03 types). The number of types of drugs did not differ significantly between the two groups (P = 0.880).
In the GATT group, 2 eyes received 2 types of drugs, 9 eyes received 1 type of drug, and 18 eyes did not use antiglaucoma drugs (1 eye that underwent reoperation during the follow-up period was not included), with an average of 0.45 ± 0.63 types at 12 months postoperatively. In the TRAB group, 2 eyes received 2 types of drugs, 7 eyes received 1 type of drug, and 22 eyes received no antiglaucoma drugs at 12 months postoperatively (3 eyes that underwent reoperations during the follow-up period were not included), with an average of 0.35 ± 0.61 types. The number of types of drugs did not differ significantly between the two groups (P = 0.497). Compared with that before the procedure, the use of antiglaucoma medications in both groups was significantly lower at 12 months postoperatively (P < 0.001).
Visual field MD and RNFL thickness reduction
The visual field MD values did not substantially change at 12 months postoperatively compared with the preoperative level in both the GATT (P = 0.797) and TRAB (P = 0.639) groups (Table 3).
Table 3.
Changes in visual field MD values and RNFL thickness before and after operation
| GATT Group | TRAB Group | P Value | |
|---|---|---|---|
| Visual field MD value/dB | |||
| Preoperative | -16.18 ± 16.37 | -23.42 ± 8.42 | 0.011 |
| 12 months after Surgery | -18.74 ± 9.27 | -23.98 ± 8.85 | 0.019 |
| P Value | 0.797 | 0.639 | —— |
| RNFL thickness/µm | |||
| Preoperative | 60.23 ± 18.26 | 57.74 ± 9.97 | 0.128 |
| 12 months postoperatively | 57.79 ± 12.15 | 54.19 ± 8.32 | 0.495 |
| P Value | 0.034 | 0.077 | —— |
GATT, gonioscopy-assisted transluminal trabeculotomy; MD, mean deviation; RNFL, retinal nerve fiber layer; TRAB, trabeculectomy
However, the RNFL thickness decreased significantly at 12 months in the GATT group (P = 0.034), whereas the decrease was not statistically significant in the TRAB group (P = 0.077) when comparing with preoperative levels (Table 3).
Preoperative and postoperative angle status
Gonioscopy was performed on all the participants to assess the angle status preoperatively and during the postoperative follow-up. Supplemental File 3 reveals a gonioscopic photograph showing an open angle preoperatively. Different pigmentations could be observed covering the trabecular meshwork in different individuals. Multiple iris processes were commonly observed crossing the scleral spur. At the follow-up after the GATT surgery, gonioscopy showed a wide-open angle and a good visualization of the posterior wall of the Schlemm’s canal (Supplemental File 4). Several participants had blood in their Schlemm’s canal. During follow-up, different extents of peripheral anterior synechiae formation were observed in the angle after GATT surgery.
Operation time
The mean operation time in the GATT group was significantly shorter than that in the TRAB group (30.60 ± 11.67 vs. 37.26 ± 11.75 min, P = 0.020). The operation time was not affected by the history of antiglaucoma surgery in either the GATT group (31.44 ± 13.55 vs. 29.18 ± 7.80 min, P = 0.621) or TRAB group (37.40 ± 12.40 vs. 36.25 ± 5.56 min, P = 0.936). The mean operation times differed significantly between the first and last five patients in the GATT group (46.00 ± 10.25 vs. 27.00 ± 5.70 min, P = 0.018).
Trabecular meshwork incision range
In the GATT group, 25 eyes (83.33%) completed a 360º trabecular meshwork incision. Among the five eyes that did not complete the 360º incision, one eye had a history of antiglaucoma surgery; three, one, and one eye had 270º, 240º, and 180º incisions, respectively. The incision range of the trabecular meshwork did not differ significantly between patients with or without a history of antiglaucoma surgery (339.47 ± 41.30º vs. 343.64 ± 54.27º, P = 0.486).
Surgical complications and success rates
No intraoperative complications were observed in either group. Furthermore, the frequency of surgical complications did not differ significantly between the two groups at 12 months postoperatively (P = 0.986). The main surgical complications in the GATT and TRAB groups were hyphema and bleb-related complications (Supplemental File 5), respectively (Table 4). During the follow-up period, in the TRAB group, secondary surgical procedures were performed in three eyes (8.82%): one eye underwent anterior vitrectomy because of malignant glaucoma at 1 week postoperatively, one eye underwent GATT at 2 months postoperatively, and one eye underwent repeated TRAB at the 6-month follow-up owing to uncontrolled IOP despite medical treatment. In the GATT group, one eye (3.3%) underwent TRAB at the 1-month follow-up. These cases were considered treatment failures and were excluded from the analysis after subsequent intervention.
Table 4.
Incidence of postoperative complications in the two treatment groups
| Postoperative complications, n (%) | GATT | TRAB |
|---|---|---|
| Hyphema | 4 (13.33%) | 0 |
| Transient ocular hypertension | 2 (6.67%) | 0 |
| Filtration bleb-related complications | 0 | 5 (14.71%) |
| Hypotony | 1 (3.33%) | 1 (2.94%) |
| Malignant glaucoma | 0 | 1 (2.94%) |
| Uncontrolled IOP | 1 (3.3%) | 2 (5.88%) |
| Total | 8 (26.67%) | 9 (26.47%) |
GATT, gonioscopy-assisted transluminal trabeculotomy; IOP, intraocular pressure; TRAB, trabeculectomy
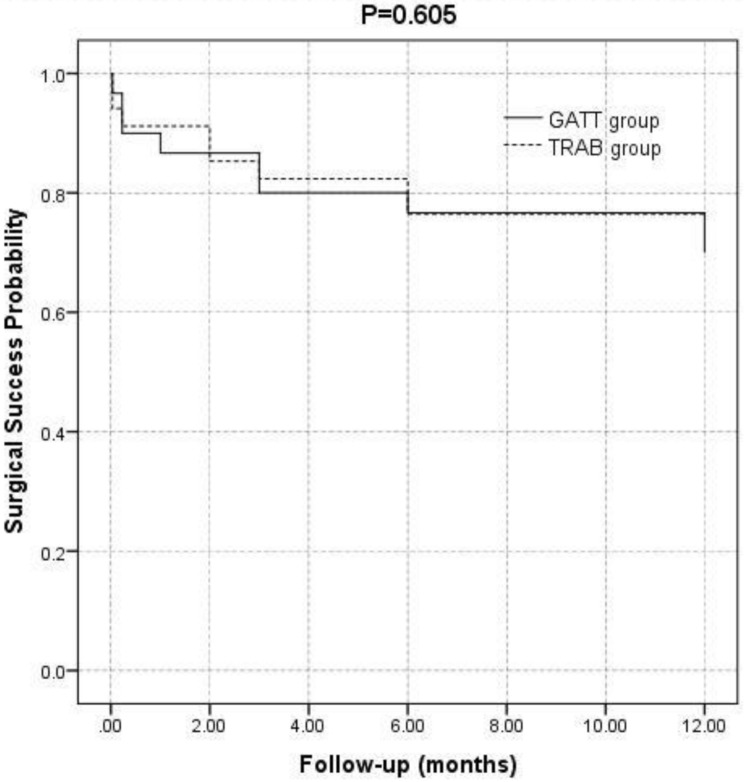
The results of the survival analysis demonstrated no significant difference in the probability of success between the two groups (P = 0.605). The probabilities of success after 12 months were 70% (95% CI = 52.6–87.4%) and 76.5% (95% CI = 61.4–91.5%) for TRAB and GATT, respectively (Fig. 1). The complete and qualified success rates in the GATT group were 53.3% (16 eyes) and 70% (21 eyes) at 12 months postoperatively, respectively, whereas those in the TRAB group were 55.9% (19 eyes) and 76.5% (26 eyes), respectively. The complete and qualified success rates did not differ significantly between the two groups (P = 0.838 and 0.559, respectively).
Fig. 1.
Kaplan–Meier curves showing the probabilities of surgical success versus months of follow-up. The Logrank statistical test was used. Eyes were classified as failures if IOP decreased >20% from baseline or the absolute IOP was <21 mm Hg. The solid line and dashed line represent patients treated with gonioscopy-assisted transluminal trabeculotomy (GATT) and trabeculectomy (TRAB), respectively
Discussion
The present study investigated and compared the clinical effects of GATT and TRAB for the treatment of POAG during a 12-month postoperative follow-up period. GATT and TRAB exhibited similar efficacies in lowering IOP, reducing medication use, and preserving visual fields. The occurrence of surgical complications and success rates at 12 months did not differ significantly between the two procedures. Specific complications varied, with the most common complications being hyphema in the GATT group and bleb-related complications in the TRAB group.
The primary target of antiglaucoma procedures in patients with POAG is reducing the IOP. Multiple studies have confirmed the ocular hypotensive effects of GATT [20, 21]. Fontana et al. reported that the IOP-lowering effect of TRAB for OAG in the late postoperative period (18 months) was better than that of GATT in some patients undergoing concomitant cataract surgery [18]. Another study demonstrated better IOP reduction for early postoperative TRAB combined with cataract surgery; however, GATT combined with cataract surgery had a better ocular hypotensive effect at 12 months postoperatively [19]. Thus, controversy exists regarding the efficacy of GATT and TRAB for the treatment of OAG, and no study specific to POAG patients has compared these two procedures. Combined cataract surgery may have an important influence on the outcomes, considering the additional IOP-lowering effect of cataract surgery [22]. In this study, we excluded patients who underwent TRAB or GATT combined with any other procedure, including cataract extraction, which may explain the younger age and phakic status of our patient population. To the best of our knowledge, this is the first study to compare the effects of GATT alone and TRAB alone for the treatment of POAG. Our results demonstrated similar postoperative IOP and its reduction between the two groups during the entire follow-up period, except for postoperative day 1, where the IOP of the TRAB group was higher than that of the GATT group. The two groups showed no significant differences in the number of IOP-lowering medications used pre- and post-operatively. Furthermore, both surgeries significantly reduced the number of medications used within 12 months postoperatively, consistent with previously reported clinical research findings [18].
Examination of the visual field is the most common functional assessment used in glaucoma, with a smaller visual field MD indicating greater damage. The visual field protective effect of both GATT and TRAB has been indicated in several studies [8, 23]. Fontana et al. reported no change in visual field loss in patients undergoing GATT and TRAB [18]. However, refractive interstitial clouding, such as cataracts, can affect the accuracy of visual field MD measurements, rendering combined cataract surgery a confounding factor in comparing changes in visual field MD before and after antiglaucoma surgery [24]. The present study excluded the influence of cataract extraction, and GATT and TRAB showed comparable visual field protective effects at 12 months postoperatively.
RNFL thickness is a sensitive indicator of glaucomatous optic nerve damage [25]. In contrast to the visual field results, our follow-up results showed that the RNFL thickness of the GATT group was lower at 12 months postoperatively than preoperatively, although the RNFL thickness was comparable between the GATT and TRAB groups pre- and post-operatively. Damage to the RNFL may precede measurable changes in the visual field after GATT; however, no previous studies have investigated visual field and RNFL thickness changes after GATT surgery. These inconsistent changes demonstrated for the first time in the present study suggest that RNFL thickness and visual field sensitivity should be carefully assessed and integratively analyzed during the follow-up after GATT or TRAB surgeries. Accordingly, adjusting the target IOP and identifying other glaucoma risk factors may reflect the different reasons for glaucoma progression and guide the selection of appropriate treatment interventions.
In the present study, the operation times of GATT were shorter than those of TRAB, suggesting that GATT is a relatively time-saving procedure. The operation time for the last five cases of GATT was significantly lower than that for the first five cases of GATT, indicating that while GATT requires a learning curve, it constitutes a relatively easy-to-learn technique. Patterson et al. also reported that senior residents could safely and effectively perform GATT [26].
Patients with prior glaucoma surgery were included in this study to evaluate the effect of previous antiglaucoma surgeries on the efficacy of the current procedures. GATT was proven to be a safe and effective treatment for patients with refractory OAG who have failed various previous glaucoma surgeries [12, 27]. GATT has since become our first choice for patients with refractory POAG. In the present study, no statistically significant difference was observed regarding the history of antiglaucoma surgery between the GATT and TRAB groups, and the previous antiglaucoma surgery did not significantly affect the operation time, trabecular mesh incision range, 12-month postoperative IOP, or IOP reduction in either the GATT or TRAB group (P > 0.05).
The complication rates were similar between GATT and TRAB (26.67% vs. 26.47%). Hyphema was the most common surgical complications after GATT [11, 28], whereas the most common surgical complications in the TRAB group were filtering bleb-related complications, including early excessive filtration and long-term hypofiltration, which often result from scarring of the filtration channel [29]. The results of the present study are consistent with those of previous reports, with hyphemas occurring in four eyes (13.33%) in the GATT group and filtration bleb-related complications occurring in five eyes (15.41%) in the TRAB group. In the GATT group, no vision-threatening complications occurred during the surgeries or follow-up period. Malignant glaucoma occurred in one eye in the TRAB group. Mydriasis and anti-inflammatory treatments were administered, and phacoemulsification combined with anterior vitrectomy resulted in the resolution of the condition. The reoperation rates of GATT are reportedly 9% after 6 months of follow-up [11] and 14.8% after 18 months of follow-up, whereas the reoperation rate of TRAB is reportedly 8.2% [18]. In the present study, one eye (3.3%) in the GATT group required repeat antiglaucoma surgery during the follow-up period, whereas three eyes (8.82%) in the TRAB group underwent repeat surgery. The difference was not statistically significant (P = 0.365), verifying the comparable safety of GATT and TRAB.
The complete and qualified surgical success rates were comparable between the two groups, with some factors significantly affecting the success rate. GATT is an outflow procedure designed to improve drainage into the patient’s natural drainage system, which was confirmed using gonioscopy to observe the opening status of the trabecular cleft [11]. The EVFW is reported to be a prognostic indicator for the success of GATT surgery since the trabeculotomy is circumferential, allowing access to all collecting channels [30, 31]. The EVFW was observed in most patients who underwent GATT surgery in this study. The long-term success of TRAB primarily depends on developing a functioning filtering bleb to achieve subconjunctival flow [8], which was recorded in the TRAB group.
This study had some limitations. Firstly, the sample size was limited due to the single-centre study design and the exclusion of patients who had undergone combined surgery. Second, the follow-up period was relatively short and participants were limited to patients with POAG. Thirdly, the non-randomised nature of the study limits the interpretation of the comparative data. Large randomised trials with longer follow-up to compare the therapeutic effects of GATT and TRAB in various OAG are our future research directions.
In conclusion, to the best of our knowledge, this is the first study to compare the effects of GATT alone and TRAB alone for the treatment of POAG, with the simultaneous detection of structural and functional changes using the visual field and RNFL thickness. GATT showed comparable efficacy to TRAB for the treatment of POAG during the 12-month postoperative follow-up period, including lowered IOP, reduced medication use, preserved visual fields, and safety. However, TRAB was superior at mitigating RNFL damage, whereas GATT was minimally invasive and had a shorter procedure time. The types of complications differed between the two procedures, with the complications of the GATT procedure being primarily hyphema-related and those of the TRAB primarily bleb-related. Our results suggest that GATT is a safe, effective, time-saving and minimally invasive surgical option for the treatment of POAG, which will be beneficial to ophthalmologists in making clinical decisions when treating patients with POAG.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental file 1: Representative images of the episcleral venous fluid wave examination during gonioscopy-assisted transluminal trabeculotomy (GATT) surgery (Description of data: A: Intraoperative appearance of episcleral veins when there is no flow in the anterior chamber. Blood (white arrow) refluxes into the anterior chamber with low IOP. Episcleral veins appeard dilated and twisted and merged with the conjunctival plexus (black arrows). B: The BSS fluid surge produced a marked fluid wave, and the episcleral venous plexus showed blanching (black arrows). The upper 4 black arrows designated thinning and stiffness of episcleral veins; the lowering 4 horizontal black arrows showed the invisible episcleral venous plexus due to the fluid wave of BSS flushing. C: The black arrows pointed to multiple slender superficial episcleral veins before BSS flushing. D: The black arrows indicated the location of the corresponding black arrows in C, however, the vessels were no longer visible at maximal irrigation of the anterior chamber)
Supplemental file 2: Pre- and postoperative IOP values and reduction with or without previous antiglaucoma surgery (Description of data: History of previous glaucoma surgery did not affect preoperative IOP, 12-month postoperative IOP, IOP reduction, or range of IOP reduction in the GATT and TRAB groups)
Supplemental file 3: Representative images of the angles by gonioscopy in primary open-angle glaucoma individuals before surgery (Description of data: A: Gonioscopic photograph showed a normal open angle, trace pigmentation in the posterior trabecular meshwork and normal insertion of the iris into a narrow ciliary body band. B: Open angle with brown pigment covering the trabecular meshwork. C: Multiple iris processes were observed in the angle. D: Gonioscopic photograph shows mild pigmentation of the trabecular meshwork)
Supplemental file 4: Representative images of the angle by gonioscopy in primary open-angle glaucoma individuals after GATT surgery (Description of data: A-C: gonioscopy showed a wide open angle and a good visualization of the posterior wall of the Schlemm’s canal. D: Blood was seen in Schlemm’s canal after trabeculectomy. E, F: Gonioscopy photograph showed localized peripheral anterior synechiae formation (arrow) in the angle after GATT surgery
Supplemental file 5: Representative images of the filtration blebs obtained by photographs (A, B, C) and slit-lamp examination (D, E, F) in individuals after TRAB surgery (Description of data: A, D: the filtration bleb was bulged and well diffused. B, E: the filtration bleb showed a multi-cavity thin-walled shape. C, F: scar-type filtration bleb showed scarring and a large number of blood vessels)
Acknowledgements
We thank Dr. Yuan Zhang of the Clinical Research Center of Shandong University Qilu Hospital for providing statistical guidance.
Abbreviations
- EVFW
Episcleral venous fluid wave
- GATT
Gonioscopy-assisted transluminal trabeculotomy
- IOP
Intraocular pressure
- MD
Mean deviation
- OCT
Optical coherence tomography
- POAG
Primary open-angle glaucoma
- RNFL
Retinal nerve fiber layer
- TRAB
Trabeculectomy
- VF
Visual field
Author contributions
Study conception and design: LYW, HG; Data collection: PYW, CW, RK, WZZ, JYW; Analysis and interpretation of results: LYW, CW, PYW, CYD, HG; Draft manuscript preparation: LYW, CW, RK, CYD, HG. All authors reviewed the results and approved the final version of the manuscript.
Funding
This work was supported by the Natural and Science Foundation of Shandong Province [grant number ZR2023LSW026] and the National Natural Science Foundation of China [grant number 82101088].
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Qilu Hospital of Shandong University (approval number: KYLL-202008-167). All patients provided informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–90. [DOI] [PubMed] [Google Scholar]
- 2.Stein JD, Khawaja AP, Weizer JS. Glaucoma in Adults-Screening, diagnosis, and management: a review. JAMA-J AM MED ASSOC. 2021;325(2):164–74. [DOI] [PubMed] [Google Scholar]
- 3.Borroni D, Gadhvi KA, Hristova R, McLean K, Rocha DLC, Romano V, Kaye S. Influence of corneal visualization Scheimpflug Technology Tonometry on intraocular pressure. Ophthalmol Sci. 2021;1(1):100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang N, Wang J, Li Y, Jiang B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. SCI REP-UK. 2021;11(1):13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreb RN, Leung CK, Crowston JG, Medeiros FA, Friedman DS, Wiggs JL, Martin KR. Primary open-angle glaucoma. NAT REV DIS PRIMERS. 2016;2:16067. [DOI] [PubMed] [Google Scholar]
- 6.Vallabh NA, Kennedy S, Vinciguerra R, McLean K, Levis H, Borroni D, Romano V, Willoughby CE. Corneal Endothelial Cell Loss in Glaucoma and Glaucoma Surgery and the Utility of Management with Descemet Membrane Endothelial Keratoplasty (DMEK). J OPHTHALMOL 2022, 2022:1315299. [DOI] [PMC free article] [PubMed]
- 7.Lim R. The surgical management of glaucoma: a review. CLIN EXP OPHTHALMOL. 2022;50(2):213–31. [DOI] [PubMed] [Google Scholar]
- 8.King AJ, Hudson J, Fernie G, Kernohan A, Azuara-Blanco A, Burr J, Homer T, Shabaninejad H, Sparrow JM, Garway-Heath D, et al. Primary trabeculectomy for advanced glaucoma: pragmatic multicentre randomised controlled trial (TAGS). BMJ-BRIT MED J. 2021;373:n1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowson AC, Hogarty DT, Maher D, Liu L. Minimally Invasive Glaucoma Surgery: Safety of Individual Devices. J CLIN MED 2022, 11(22). [DOI] [PMC free article] [PubMed]
- 10.Bicket AK, Le JT, Azuara-Blanco A, Gazzard G, Wormald R, Bunce C, Hu K, Jayaram H, King A, Otarola F, et al. Minimally invasive Glaucoma Surgical techniques for Open-Angle Glaucoma: an overview of Cochrane Systematic Reviews and Network Meta-analysis. JAMA OPHTHALMOL. 2021;139(9):983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes DOI, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. OPHTHALMOLOGY 2014, 121(4):855–861. [DOI] [PubMed]
- 12.Wang Y, Zhang W, Xin C, Sang J, Sun Y, Wang H. Gonioscopy-assisted transluminal trabeculotomy for open-angle glaucoma with failed incisional glaucoma surgery: two-year results. BMC OPHTHALMOL. 2023;23(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharkawi E, Lindegger DJ, Artes PH, Lehmann-Clarke L, El WM, Misteli M, Pasquier J, Guarnieri A. Outcomes of gonioscopy-assisted transluminal trabeculotomy in pseudoexfoliative glaucoma: 24-month follow-up. BRIT J OPHTHALMOL. 2021;105(7):977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan AV, Yannuzzi NA, Chen J, Wang YE, Townsend JH, Chang TC. Gonioscopy-assisted Transluminal Trabeculotomy (GATT) in patients with secondary Open-Angle Glaucoma following vitreoretinal surgery. J GLAUCOMA. 2020;29(4):e23–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aktas Z, Ozdemir ZE, Uysal BS, Yigiter A. Outcomes of Prolene Gonioscopy assisted Transluminal Trabeculotomy in Primary Open Angle Glaucoma and pseudoexfoliation Glaucoma: a comparative study. J GLAUCOMA. 2022;31(9):751–6. [DOI] [PubMed] [Google Scholar]
- 16.Wan Y, Cao K, Wang J, Sun Y, Du R, Wang Z, Zhang J, Wang H, Wang N. Gonioscopy-assisted Transluminal Trabeculotomy (GATT) combined phacoemulsification surgery: outcomes at a 2-year follow-up. EYE. 2023;37(6):1258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen RI, Purgert R, Eisengart J. Gonioscopy-assisted transluminal trabeculotomy and Goniotomy, with or without concomitant cataract extraction, in Steroid-Induced and Uveitic Glaucoma: 24-Month outcomes. J GLAUCOMA. 2023;32(6):501–10. [DOI] [PubMed] [Google Scholar]
- 18.Fontana L, De Maria M, Caristia A, Mastrofilippo V, Braglia L, Iannetta D, Scarale GP. Comparison of Gonioscopy-assisted Transluminal Trabeculotomy Versus Trabeculectomy with Mitomycin C in patients with Open-angle Glaucoma. J GLAUCOMA. 2021;30(1):101–8. [DOI] [PubMed] [Google Scholar]
- 19.Olgun A, Celik HU, Yenihayat F, Bozkurt E, Sahbaz I. Efficacy comparison of combined trabeculectomy with MMC and gonioscopy-assisted transluminal trabeculotomy. INT OPHTHALMOL. 2022;42(6):1711–8. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Wang H, Han Y, Shi Y, Xin C, Yin P, Li M, Cao K, Wang N. Outcomes of gonioscopy-assisted transluminal trabeculotomy in juvenile-onset primary open-angle glaucoma. EYE. 2021;35(10):2848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo CY, Qi XH, Qi JM. Systematic review and Meta-analysis of treating open angle glaucoma with gonioscopy-assisted transluminal trabeculotomy. INT J OPHTHALMOL-CHI. 2020;13(2):317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young C, Seibold LK, Kahook MY. Cataract surgery and intraocular pressure in glaucoma. CURR OPIN OPHTHALMOL. 2020;31(1):15–22. [DOI] [PubMed] [Google Scholar]
- 23.Cubuk MO, Ucgul AY, Unsal E. Gonioscopy-assisted transluminal trabeculotomy as an option after failed trabeculectomy. INT OPHTHALMOL. 2020;40(8):1923–30. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Rabiolo A, Morales E, Fatehi N, Lee WS, Yu F, Afifi AA, Nouri-Mahdavi K, Caprioli J. Cataract surgery and rate of Visual Field Progression in Primary Open-Angle Glaucoma. AM J OPHTHALMOL. 2019;201:19–30. [DOI] [PubMed] [Google Scholar]
- 25.Swaminathan SS, Jammal AA, Berchuck SI, Medeiros FA. Rapid initial OCT RNFL thinning is predictive of faster visual field loss during extended follow-up in glaucoma. AM J OPHTHALMOL. 2021;229:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson I, Avdagic E, Qiu M. Safety and Efficacy of Resident-Performed Gonioscopy-assisted transluminal trabeculotomy. J GLAUCOMA. 2023;32(4):313–9. [DOI] [PubMed] [Google Scholar]
- 27.Sigona M, Saravanan A, Pipis S, Masood I. Gonioscopy-assisted transluminal trabeculotomy following failed iStent surgery. J GLAUCOMA. 2022;31(9):e83–6. [DOI] [PubMed] [Google Scholar]
- 28.Sarkisian SR, Mathews B, Ding K, Patel A, Nicek Z. 360 degrees ab-interno trabeculotomy in refractory primary open-angle glaucoma. CLIN OPHTHALMOL. 2019;13:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rulli E, Biagioli E, Riva I, Gambirasio G, De Simone I, Floriani I, Quaranta L. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA OPHTHALMOL. 2013;131(12):1573–82. [DOI] [PubMed] [Google Scholar]
- 30.Zeng LZ, He Y, Wang XQ, Xian YP, Fan HY, Jing L, Shu J, Li Q, Wang NL. Clinical significance of episcleral venous fluid wave in gonioscopy-assisted transluminal trabeculotomy. INT J OPHTHALMOL-CHI. 2023;16(12):1971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aktas Z, Ozmen MC, Atalay HT, Ucgul AY. Evaluation of episcleral venous fluid wave during gonioscopy assisted transluminal trabeculotomy in patients with advanced glaucoma. EYE. 2019;33(4):668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental file 1: Representative images of the episcleral venous fluid wave examination during gonioscopy-assisted transluminal trabeculotomy (GATT) surgery (Description of data: A: Intraoperative appearance of episcleral veins when there is no flow in the anterior chamber. Blood (white arrow) refluxes into the anterior chamber with low IOP. Episcleral veins appeard dilated and twisted and merged with the conjunctival plexus (black arrows). B: The BSS fluid surge produced a marked fluid wave, and the episcleral venous plexus showed blanching (black arrows). The upper 4 black arrows designated thinning and stiffness of episcleral veins; the lowering 4 horizontal black arrows showed the invisible episcleral venous plexus due to the fluid wave of BSS flushing. C: The black arrows pointed to multiple slender superficial episcleral veins before BSS flushing. D: The black arrows indicated the location of the corresponding black arrows in C, however, the vessels were no longer visible at maximal irrigation of the anterior chamber)
Supplemental file 2: Pre- and postoperative IOP values and reduction with or without previous antiglaucoma surgery (Description of data: History of previous glaucoma surgery did not affect preoperative IOP, 12-month postoperative IOP, IOP reduction, or range of IOP reduction in the GATT and TRAB groups)
Supplemental file 3: Representative images of the angles by gonioscopy in primary open-angle glaucoma individuals before surgery (Description of data: A: Gonioscopic photograph showed a normal open angle, trace pigmentation in the posterior trabecular meshwork and normal insertion of the iris into a narrow ciliary body band. B: Open angle with brown pigment covering the trabecular meshwork. C: Multiple iris processes were observed in the angle. D: Gonioscopic photograph shows mild pigmentation of the trabecular meshwork)
Supplemental file 4: Representative images of the angle by gonioscopy in primary open-angle glaucoma individuals after GATT surgery (Description of data: A-C: gonioscopy showed a wide open angle and a good visualization of the posterior wall of the Schlemm’s canal. D: Blood was seen in Schlemm’s canal after trabeculectomy. E, F: Gonioscopy photograph showed localized peripheral anterior synechiae formation (arrow) in the angle after GATT surgery
Supplemental file 5: Representative images of the filtration blebs obtained by photographs (A, B, C) and slit-lamp examination (D, E, F) in individuals after TRAB surgery (Description of data: A, D: the filtration bleb was bulged and well diffused. B, E: the filtration bleb showed a multi-cavity thin-walled shape. C, F: scar-type filtration bleb showed scarring and a large number of blood vessels)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.