Abstract
Background
Abnormal blood flow patterns are known to contribute to the ascending aortic dilation in patients with bicuspid aortic valve (BAV). The present study elucidated the blood flow characteristics in the dilated ascending aorta before and after transcatheter aortic valve replacement (TAVR) using computational fluid dynamics (CFD) analysis.
Methods
We performed CFD analysis in three BAV patients with ascending aortic dilation (maximum diameter ≥ 45 mm) who underwent TAVR. The blood flow streamline was visualized to evaluate the pre- and post-operative flow velocity, severity of vortex and helix, and wall shear stress (WSS) in the ascending aorta.
Results
Before the procedure, all three patients showed abnormal blood flow patterns, with vortex and helix in the ascending aorta. Regionally elevated WSS was also observed in the lateral or posterior ascending aortic wall (16.7 Pa, 12.2 Pa, and 14.5 Pa in patient 1, 2, and 3, respectively). After the procedure, the blood flow patterns significantly improved, and the maximum WSS also decreased (4.2 Pa, 1.1 Pa, and 3.2 Pa in patient 1, 2, and 3, respectively).
Conclusion
Abnormal blood flow patterns and WSS appeared to improve after TAVR in BAV patients with ascending aortic dilation. The impact on the long-term aortic growth rate and the incidence of aortic dissection requires further studies.
Trial Registration
Changes of Ascending Aortic Diameter in Patients Undergoing Transcatheter Aortic Valve Replacement. ClinicalTrial.gov number NCT05739253. Trial registration date 20,230,212.
Keywords: Transcatheter aortic valve replacement, Computational fluid dynamics, Bicuspid aortic valve, Ascending aortic dilation
Introduction
Transcatheter aortic valve replacement (TAVR) has been approved as a therapy for severe aortic stenosis regardless of the surgical risk profile [1, 2]. However, data for patients with bicuspid aortic valve (BAV) undergoing TAVR is limited. BAV encompasses several morphologies that may pose challenges to TAVR procedure, such as elliptical annulus, bulky and asymmetric calcification, and ascending aortic dilation. Among these, ascending aortic dilation is a common feature in patients with BAV. Both genetic (aortic wall fragility) and hemodynamic (abnormal blood flow patterns) factors appear to contribute to the BAV aortopathy [3, 4]. Current guidelines recommend a combined surgical aortic valve replacement (SAVR) and aortic surgery when the diameter exceeds 45 mm to prevent aortic dissection or rupture [5, 6]. However, this aggressive strategy remains debated. For patients undergoing TAVR, it is technically difficult to simultaneously repair the dilated ascending aorta. Therefore, understanding the prognosis of untreated ascending aorta in these patients is important.
Recent developments in imaging have suggested that the blood flow dynamics in the ascending aorta may be a major contributor to the BAV aortopathy [7, 8]. Computational fluid dynamics (CFD) is a useful tool that allows for the visualization of blood flow patterns [9, 10]. It allows the calculation of flow, pressure, and wall shear stress (WSS) of the ascending aorta with exceptional spatial and temporal resolution. With the emergence of precision medicine and individualized therapy, this technique may potentially play an important role in the daily clinical practice. The aim of the present study is to compare the blood flow patterns and WSS in the dilated ascending aorta in patients with BAV before and after TAVR procedure.
Methods
Study patients
The present study was a pilot study of the clinical trial “Changes of Ascending Aortic Diameter in Patients Undergoing Transcatheter Aortic Valve Replacement” (ClinicalTrial.gov number: NCT05739253). We identified three BAV patients with ascending aortic dilation (maximum diameter ≥ 45 mm) who underwent TAVR at our center. All three patients experienced uneventful procedure and had detailed clinical data, including pre- and post-operative computed tomography angiography (CTA) and transthoracic echocardiography. We performed CFD analysis for these patients using CTA imaging. The study was approved by the institutional review board of Fuwai Hospital (date of review, 22 March 2022; approval number, 2021 − 1602), and the informed consents were obtained from all patients.
Computational fluid dynamics analysis
Aortic geometry and meshing
The aortic blood flow lumen geometries before and after the TAVR procedure were extracted from CTA scans of each patient using the commercial software Materialise Mimics 21.0 (Leuven, Belgium), and 3D aorta model were created from digital imaging and communications in medicine (DICOM) imaging data. Computational meshes were created using the commercial software ANSYS-ICEM 16.2 (ANSYS, Inc., Canonsburg, PA, USA). To verify the reliability, grid independence for the WSS was checked. We used the grid adaption command in ANSYS Fluent, and the grid refinement was halted when the results became independent of the grid size. Following mesh independence study, the final mesh contained approximately 1.5 million tetrahedral elements and 5 boundary-fitted prism layers. The geometrical model of transcatheter heart valve frame was acquired from post-operative CTA and virtually implanted in its place. We hypothesize that the prosthetic valve leaflets completely adhere to the stent during the systolic phase. This avoids the reconstruction of prosthetic leaflets geometry.
Boundary conditions and blood flow analysis
Blood was considered as an incompressible Newtonian fluid with a density of 1.06 × 103kg/m3 and a viscosity of 3.5 × 10− 3Pa·s. The inlet flow velocity profiles at the orifice of the native aortic valve (pre-operative) or the transcatheter heart valve (THV) (post-operative) were acquired by echocardiography in real time with electrocardiogram. A zero pressure condition was imposed at the outlets (innominate artery, left carotid artery, left subclavian artery, and descending aorta). The flow stimulation was carried out over 2 cardiac cycles. Time step was set to 1 × 10− 4s and the convergence criterion was set to 1 × 10− 4. The blood flow visualization and analysis were conducted using the commercial software ANSYS FLUENT 16.2 (ANSYS, Inc., Canonsburg, PA, USA).
Statistical analyses
Continuous variables were expressed as means ± standard deviations unless otherwise specified, and categorical variables were expressed as counts and proportions. Statistical analyses were performed using the Statistical Package for Social Sciences, version 23.0 (SPSS, Inc, Chicago, Ill).
Results
Table 1 showed the patients’ baseline characteristics and preoperative CTA measurements. All three patients had BAV and maximum ascending aortic diameter ≥ 45 mm. All experienced uneventful TAVR procedure and completed CTA and transthoracic echocardiography before the discharge.
Table 1.
Baseline characteristics
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age, y | 79 | 68 | 70 |
| Gender | Female | Female | Male |
| Body mass index, kg/m2 | 22.2 | 27.5 | 16.4 |
| Hypertension | Yes | Yes | No |
| Diabetes mellitus | No | No | No |
| History of coronary artery disease | No | No | No |
| Bicuspid aortic valve fusion type | Type 0 | Type 1, L/R | Type 2, L-N/R-N |
| Pre-operative echocardiography | |||
| Left ventricular ejection fraction, % | 63 | 40 | 64 |
| Peak aortic valve velocity, m/s | 4.3 | 4.5 | 5.5 |
| Mean aortic valve pressure gradient, mmHg | 60 | 47 | 69 |
| CTA-measured aortic dimensions | |||
| Annulus diameter, mm | 20.9 | 26.9 | 23.7 |
| Sinotubular junction diameter, mm | 30.5 | 33.4 | 32.4 |
| Maximal ascending aortic diameter, mm | 49.4 | 45.2 | 45.0 |
| Aortic root angulation, ° | 62 | 54 | 50 |
| Valve size implanted, mm | 23 | 26 | 23 |
| Valve type implanted | Taurus One | Venus A | KoKaValve |
| Post-operative echocardiography | |||
| Left ventricular ejection fraction, % | 68 | 42 | 72 |
| Peak aortic valve velocity, m/s | 2.7 | 2.0 | 2.4 |
| Mean aortic valve pressure gradient, mmHg | 16 | 9 | 14 |
| Paravalvular aortic insufficiency | None | Mild | Mild |
CTA: computed tomography angiography
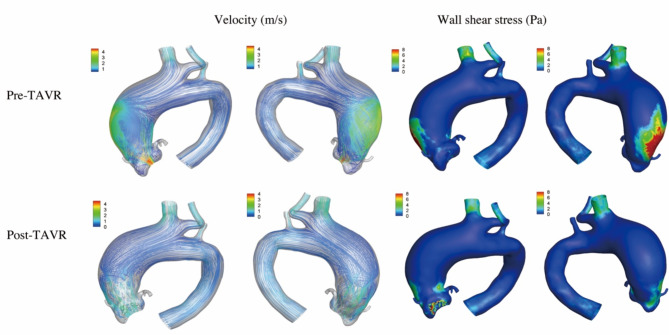
Figure 1 showed the data analysis for patient 1. The patient had a type 0 Siever’s BAV. Before the procedure, the transvalvular aortic flow was observed along the greater curvature of the ascending aorta, with the peak velocity of 4.3 m/s. The vortex was observed in the ascending aorta. The maximum WSS was 16.7 Pa on the lateral side of the ascending aorta. A 23 mm Taurus One THV was used (Peijia Medical, Suzhou, China). After the procedure, the aortic stenosis was corrected with the peak velocity of 2.7 m/s and the mean pressure gradient of 16mmHg. The blood flow patterns significantly improved, and the maximum WSS decreased to 4.2 Pa.
Fig. 1.
The computational fluid dynamics in patient 1. Before the procedure, the transvalvular aortic flow was observed along the greater curvature of the ascending aorta and the vortex was observed. The peak velocity was 4.3 m/s and the maximum wall shear stress was 16.7 Pa. After the procedure, the blood flow patterns improved. The peak velocity and the maximum wall shear stress decreased to 2.7 m/s and 4.2 Pa
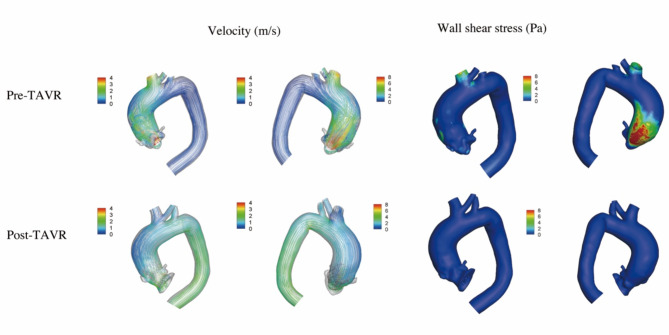
Figure 2 showed the data analysis for patient 2. The patient had a type 1 Siever’s BAV (L/R). Before the procedure, the transvalvular aortic flow was observed along the posterior side of the ascending aorta, with the peak velocity of 4.5 m/s. The blood flow was complex and tortuous, with abnormal helical flow appeared to dominate the ascending aorta. The maximum WSS was 12.2 Pa on the posterior side of the ascending aorta. A 26 mm Venus-A THV was used (Venus MedTech, Hangzhou, China). After the procedure, the aortic stenosis was corrected with the peak velocity of 2.0 m/s and the mean pressure gradient of 9mmHg. The blood flow patterns improved, and the maximum WSS decreased to 1.1 Pa.
Fig. 2.
The computational fluid dynamics in patient 2. Before the procedure, the blood flow was complex and tortuous, with abnormal helical flow appeared to dominate the ascending aorta. The peak velocity was 4.5 m/s and the maximum wall shear stress was 12.2 Pa. After the procedure, the blood flow patterns improved. The peak velocity and the maximum wall shear stress decreased to 2.0 m/s and 1.1 Pa
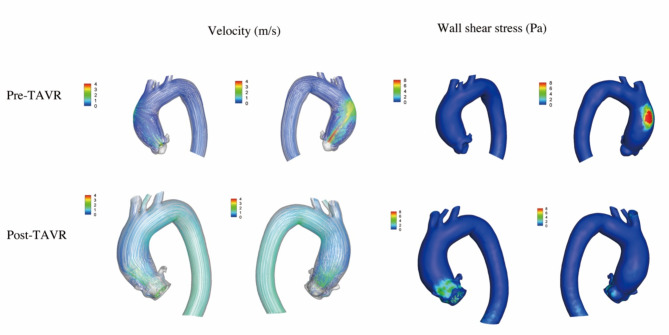
Figure 3 showed the data analysis for patient 3. The patient had a type 2 Siever’s BAV (L-N/R-N). Before the procedure, the transvalvular aortic flow was observed along the posterolateral side of the ascending aorta, with the peak velocity of 5.5 m/s. A flow jet impinged against the posterior wall of the ascending aorta, in which the WSS was the greatest (14.5 Pa). A 23 mm KoKaValve THV was used (KOKA Lifesciences, Nantong, China). After the procedure, the aortic stenosis was corrected with the peak velocity of 2.4 m/s and the mean pressure gradient of 14mmHg. The blood flow patterns and the maximum WSS decreased to 3.2 Pa.
Fig. 3.
The computational fluid dynamics in patient 3. Before the procedure, a flow jet impinged against the posterior wall of the ascending aorta. The peak velocity was 5.5 m/s and the maximum wall shear stress was 14.5 Pa. After the procedure, the blood flow patterns improved. The peak velocity and the maximum wall shear stress decreased to 2.4 m/s and 3.2 Pa
Discussion
It remains disputed whether the aortic dilation that is commonly seen in patients with BAV is related to intrinsic aortic wall fragility or altered hemodynamics. Recent advancements in dynamic blood flow imaging provide detailed flow information. Previous studies showed that patients with BAV, even with normal aortic valves, had more eccentric ascending aortic blood flow compared with those with TAV [11, 12]. When combining severe AS, the high velocity jet flow tends to exacerbate this abnormal hemodynamics [12]. In the present study, the preoperative blood flow showed marked helix or vortex in the ascending aorta, which was in accordance with previous studies. This abnormal flow pattern has been reported to be associated with regional elevation of WSS [12, 13]. WSS reflects the friction that the blood flow exerts onto the aortic wall. The prolonged exposure to altered WWS is related to the dysregulation of extracellular matrix and the degeneration of elastic fibers of the aortic wall, which may contribute to aortic dilation and increase the risk of aortic dissection [14, 15]. The present study also found regionally elevated WSS on greater curvature of the ascending aorta in all three patients before the procedure, which was in accordance with previous studies.
After the TAVR procedure, we found that the abnormal flow patterns tended to normalize, and the WSS decreased significantly in all patients. This may have important long-term clinical implications, because WSS has been shown to be regionally increased at the site of aortic dilation and aneurysm formation [16, 17]. We hypothesized that by reducing WSS and improving blood flow patterns, the risk of adverse aortic events might be decreased in the long term in patients with ascending aortic dilation who underwent TAVR. Our recent retrospective study showed that TAVR can be safely performed in patients with ascending aortic dilation (diameter ≥ 45 mm), with a similar incidence of adverse aortic events as well as survival compared to those without [18].
Previous studies by Farag et al. [19] and Trauzeddel et al. [20] both reported that compared with healthy controls, eccentric distribution of blood flow still existed after TAVR. One explanation is that the THV is implanted inside the calcified native aortic valve, inevitably resulting in a smaller effective orifice area (EOA) compared with healthy aortic valve. However, neither study compared the blood flow patterns before and after the TAVR procedure. In our study, significant improvement of blood flow and WSS was observed after the procedure. Another study by Komoriyama et al. [21] also reported that TAVR improves blood flow dynamics, especially when a larger EOA is obtained. Unfortunately, data regarding pre- and postoperative EOAs were not available in the present study. The impact of EOA on the blood flow changes and WSS after TAVR requires further studies.
There are several limitations to the present study. First, the sample size is small. Subgroup analyses regarding the differences in blood flow dynamics were not available. These factors include different BAV fusion patterns, type of THVs (self- and balloon-expandable valves), and ascending aortic geometries. The present study can be considered as a pivot study to provide feasibility and evidence for the future study. Second, similar to other CFD studies, we make a number of simplifications and assumptions. For example, the aortic wall dynamics was not simulated, and the boundary conditions for outlets were set as zero pressure. Although the aorta is characterized by a complex motion, CFD simulations under rigid-wall assumption are largely adopted. Previous studies have shown that compared with rigid wall simulation, the aortic wall displacements have minor impact on the large-scale aortic flows [22]. Previous reports also showed that when lacking patient-specific flow and pressure measurements, zero pressure for outlets can be an option [23]. Third, blood flow patterns, including vortex and helix, were only assessed qualitatively. Further studies with quantification would be more accurate and objective. Fourth, the THVs used in the present study are locally manufactured valves that have received Chinese regulatory approval. Although several studies have reported the safety and efficiency of these valves [24, 25], further studies with large sample size and long-term follow-up are necessary. Finally, the impact of post-TAVR blood flow characteristics on the ascending aorta requires long-term follow-up.
In conclusion, abnormal blood flow patterns and WSS appeared to improve after TAVR in BAV patients with ascending aortic dilation. The impact on the long-term aortic growth rate and the incidence of aortic dissection requires further studies.
Acknowledgements
None.
Abbreviations
- BAV
Bicuspid aortic valve
- CFD
Computational fluid dynamic
- CTA
Computed tomography angiography
- EOA
Effective orifice area
- SAVR
Surgical aortic valve replacement
- TAVR
Transcatheter aortic valve replacement
- THV
Transcatheter heart valve
- WSS
Wall shear stress
Author contributions
All authors have read and approved the manuscript. Methodology and manuscript writing: K.A. and W.O.Data collection and analyses: K.A., F.Z., and W.O.Conceptualization and supervision: F.Z., W.O., and X.P.
Funding
The study was supported by The Fundamental Research Funds for the Central Universities (2019PT350005), National Natural Science Foundation of China (81970444), Beijing Municipal Science and Technology Project (Z201100005420030), National High Level Talents Special Support Plan (2020-RSW02), Sanming Project of Medicine in Shenzhen (SZSM202011013), and CAMS Innovation Fund for Medical Sciences (2021-I2M-1-065).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethical approval and consent to participate
The study protocol complied with the Declaration of Helsinki and was approved by the institutional review board of Fuwai Hospital (date of review, 22 March 2022; approval number, 2021 − 1602), and the informed consents were obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmad Y, Howard JP, Arnold AD, et al. Transcatheter versus surgical aortic valve replacement in lower-risk and higher-risk patients: a meta-analysis of randomized trials. Eur Heart J. 2023;44(10):836–52. [DOI] [PubMed] [Google Scholar]
- 2.Jørgensen TH, Thyregod HGH, Ihlemann N, et al. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur Heart J. 2021;42(30):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370(20):1920–9. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez-Palomares JF, Dux-Santoy L, et al. Mechanisms of aortic dilation in patients with bicuspid aortic valve: JACC state-of-the-art review. J Am Coll Cardiol. 2023;82(5):448–64. [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. [DOI] [PubMed] [Google Scholar]
- 6.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the management of patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. 2021;143(5):e72–227. [DOI] [PubMed] [Google Scholar]
- 7.Edlin J, Nowell J, Arthurs C, et al. Assessing the methodology used to study the ascending aorta haemodynamics in bicuspid aortic valve. Eur Heart J Digit Health. 2021;2(2):271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanigk M, Burgstaller E, Latus H, et al. Aortic wall shear stress in bicuspid aortic valve disease-10-year follow-up. Cardiovasc Diagn Ther. 2023;13(1):38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarral OA, Tan MKH, Salmasi MY, et al. Phase-contrast magnetic resonance imaging and computational fluid dynamics assessment of thoracic aorta blood flow: a literature review. Eur J Cardiothorac Surg. 2020;57(3):438–46. [DOI] [PubMed] [Google Scholar]
- 10.Cilla M, Casales M, Peña E, et al. A parametric model for studying the aorta hemodynamics by means of the computational fluid dynamics. J Biomech. 2020;103:109691. [DOI] [PubMed] [Google Scholar]
- 11.Guzzardi DG, Barker AJ, van Ooij P, et al. Valve-related Hemodynamics Mediate Human Bicuspid Aortopathy: insights from Wall Shear stress mapping. J Am Coll Cardiol. 2015;66(8):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hope MD, Wrenn J, Sigovan M, et al. Imaging biomarkers of aortic disease: increased growth rates with eccentric systolic flow. J Am Coll Cardiol. 2012;60(4):356–7. [DOI] [PubMed] [Google Scholar]
- 13.Ha H, Koo HJ, Lee JG, et al. Association between flow skewness and aortic dilatation in patients with aortic stenosis. Int J Cardiovasc Imaging. 2017;33(12):1969–78. [DOI] [PubMed] [Google Scholar]
- 14.Bollache E, Guzzardi DG, Sattari S, et al. Aortic valve-mediated wall shear stress is heterogeneous and predicts regional aortic elastic fiber thinning in bicuspid aortic valve-associated aortopathy. J Thorac Cardiovasc Surg. 2018;156(6):2112–e21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guala A, Dux-Santoy L, Teixido-Tura G, et al. Wall Shear stress predicts aortic dilation in patients with bicuspid aortic valve. JACC Cardiovasc Imaging. 2022;15(1):46–56. [DOI] [PubMed] [Google Scholar]
- 16.Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255(1):53–61. [DOI] [PubMed] [Google Scholar]
- 17.Nordmeyer S, Hellmeier F, Yevtushenko P, et al. Abnormal aortic flow profiles persist after aortic valve replacement in the majority of patients with aortic valve disease: how model-based personalized therapy planning could improve results. A pilot study approach. Eur J Cardiothorac Surg. 2020;57(1):133–41. [DOI] [PubMed] [Google Scholar]
- 18.An K, Zhang F, Ouyang W, et al. Transcatheter aortic valve replacement in patients with preoperative ascending aortic diameter ≥ 45 mm. Cardiovasc Diagn Ther. 2023;13(6):939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farag ES, Vendrik J, van Ooij P, et al. Transcatheter aortic valve replacement alters ascending aortic blood flow and wall shear stress patterns: a 4D flow MRI comparison with age-matched, elderly controls. Eur Radiol. 2019;29(3):1444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trauzeddel RF, Löbe U, Barker AJ, et al. Blood flow characteristics in the ascending aorta after TAVI compared to surgical aortic valve replacement. Int J Cardiovasc Imaging. 2016;32(3):461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komoriyama H, Kamiya K, Nagai T, et al. Blood flow dynamics with four-dimensional flow cardiovascular magnetic resonance in patients with aortic stenosis before and after transcatheter aortic valve replacement. J Cardiovasc Magn Reson. 2021;23(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calò K, Capellini K, De Nisco G, et al. Impact of wall displacements on the large-scale flow coherence in ascending aorta. J Biomech. 2023;154:111620. [DOI] [PubMed] [Google Scholar]
- 23.Madhavan S, Kemmerling EMC. The effect of inlet and outlet boundary conditions in image-based CFD modeling of aortic flow. Biomed Eng Online. 2018;17(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao YB, Zhao ZG, Wei X, et al. Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: preliminary experiences in China. Catheter Cardiovasc Interv. 2017;89(S1):528–33. [DOI] [PubMed] [Google Scholar]
- 25.Zhou D, Pan W, Wang J, et al. VitaFlow™ transcatheter valve system in the treatment of severe aortic stenosis: one-year results of a multicenter study. Catheter Cardiovasc Interv. 2020;95(2):332–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.