Abstract
Background
The World Health Organization recommends that a randomised controlled trial (RCT) publishes its results in a peer-reviewed journal within 24 months of study completion. When RCTs are not published or publication is delayed, this can contribute to publication bias, which is the tendency for studies with positive or significant results to be published more frequently than studies with nonsignificant or negative results. This bias skews the available evidence, creating a distorted view of the research landscape. There is uncertainty about which activities best mitigate publication bias. This review systematically synthesises literature on activities that targeted researchers with the intention of reducing publication bias among health science researchers.
Methods
We conducted a comprehensive search in PubMed and Scopus and forward and backward citation searches. There were no restrictions on language, time or publication status. We included studies of any design that tested an activity to reduce publication bias in health research. Ideally, participants had to be investigators or researchers who had conducted, led or been involved in RCTs. The context was any research institution that conducts research. Two reviewers independently assessed titles and abstracts for eligibility, followed by duplicate full-text screening and data extraction. One reviewer collated and summarised the extracted data and arranged these using an analytical framework to describe the findings thematically. For quality assurance, a second reviewer checked the data analysis.
Results
Our database search yielded 14,185 records, with 11,754 after de-duplication. Of these, we excluded 11,728 records after title and abstract screening. We assessed 26 full texts for eligibility. One of these met the eligibility criteria. Forward and backward citation searches yielded 57 records, and 43 were eligible. We included 44 studies published between 1995 and 2022 that described activities promoting the publication of health-related research. We identified 10 broad activities that were often used in combination and concentrated on writing manuscripts.
Discussion
This review describes several strategies that have been used to assist health researchers in publishing their findings. However, our search was unable to find studies that tested activities specifically geared toward researchers conducting RCTs. Rigorous research is needed to determine effective strategies for reducing publication bias among trialists.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-024-02728-5.
Keywords: Scoping reviews, Publication bias, Randomised controlled trials, Research waste
Background
Doctors, researchers, policymakers and patients are key stakeholders who rely on the results of clinical trials to choose effective health interventions. However, many researchers report delays in publishing clinical trial results or complete non-publication [1, 2]. There is a tendency for studies with positive or significant results to be published more frequently than studies with non-significant or negative results [3, 4]. This issue is captured in the concept ‘publication bias’, which refers to non- or delayed publication of research findings [5].
Publication bias hinders research transparency and neglects researchers’ ethical contract with research participants to publish their completed trial results [6]. Making trial results publicly available is necessary to ensure that scientific knowledge informs clinical practice and policy decisions [5].
Researchers rigorously evaluate new health-related tests and interventions in studies with human participants before making the study results publicly available. Such studies often take the form of randomised controlled trials (RCTs). RCTs are the gold standard study designs to answer questions of effectiveness, i.e. whether a tested intervention works [7]. The outcomes of RCTs may allow for newer, improved interventions to become available if shown to be effective. They might inform clinical and social care guidelines, thus improving health and social care and, later, people’s health and quality of life[8].
Well-designed RCTs yield reliable, unbiased estimates for differences in outcomes between groups receiving the interventions compared in the trial. Hence, they (and the subsequent systematic reviews into which they are synthesised) are considered the best available evidence for evaluating healthcare intervention effect and causation [9, 10]. For a RCT’s results to be trusted, the study must be carefully planned, conducted, appraised, completed and reported. The scientific community entrusts trialists to transparently convey the methods and results from RCTs through peer-reviewed scientific journal publications [11].
RCTs are essential in the evidence ecosystem for health and social care. In 2022, the WHO approved resolution WHA75.8 stating that they encourage the timely open-access publication of RCTs whether they contain positive or negative interpretable results [12]. Furthermore, the WHO maintains that trials must be published in peer-reviewed journals within 24 months from completion [13]. In addition, the publication should comprehensively outline the study’s structure, methods and outcomes with clarity and transparency, irrespective of the trial’s results, and, ideally, be published in an open-access repository or publication [14, 15].
Promptly publishing RCT findings allows healthcare providers and patients to access the latest results and ensures policymakers use relevant findings. It also means that those preparing and maintaining systematic reviews can keep these as up to date as possible, further informing decision-making [16–18]. When a RCT’s results are not available, this negatively impacts those processes, thereby jeopardising evidence-based healthcare decisions and policy judgments, particularly in low-resource settings often afflicted with a high burden of diseases and weak health systems [2, 19, 20]. Evidence-based healthcare relies on access to reliable, up-to-date information from RCTs, making it essential that trial reports are of sound quality, available, comprehensive and promptly published [11, 19].
Publication bias is a major contributor to research waste. Chalmers and Glasziou [21] estimated that research waste affects up to 85% of research investments [21]. Publishing research is an essential part of efforts to avert research waste [22]. However, several studies have shown that between 25 and 50% of clinical trials are never published or are only published many years after completion [1, 23–26]. A systematic review by Dwan et al. [27] highlighted the prevalence of publication bias and outcome reporting bias, emphasising that the failure to submit manuscripts, rather than journal rejection, is a key reason RCTs remain unpublished [27]. Publication bias does a disservice to the trial participants and to societies that rely on evidence-informed decision-making to attain the best possible care [28].
The importance of this scoping review
Trialists, i.e. chief or principal investigators (PIs), are responsible for ensuring that their trial’s results are published, regardless of whether a tested intervention’s findings show no statistically significant benefit or indicate harm. Therefore, strategies to promote publishing practices are essential, and evidence is needed on interventions that might reduce publication bias [29].
One systematic review by Thaler et al. [30] identified and evaluated empirical studies of the effectiveness of interventions designed and employed to minimise publication bias, which were focused on aspects other than the preparation of the report of the RCT [30]. The review described interventions to improve prospective trial registration uptake, the use of anonymous peer review to reduce geographical bias in publishing, authors’ financial disclosure of conflict of interest and open-access publishing that may discriminate against authors from non-high-income countries. Contrary to our study, Thaler et al. [30] did not design their search to specifically identify studies that tested activities aiding trialists to publish [30].
Researchers have suggested that activities should upskill and persuade trial researchers to publish in timely ways. Persuasive activities include but are not limited to awareness raising on topics such as (a) publication bias, (b) the ethical responsibility to publish research findings and (c) the negative impact of publication bias on future research and healthcare decision-making [31–33]. Furthermore, researchers should develop the skills to prepare manuscripts for publication and adhere to reporting guidelines, such as CONSORT, which may improve their chances of journal acceptance [31, 34].
This scoping review aimed to identify, map and systematically synthesise studies that implemented and tested activities to reduce publication bias among trialists.
Methods
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist as a guide to report this study. The checklist is provided in Table S1 [35]. Although only secondary data was used in this review, we did receive approval from Stellenbosch University Human Research Ethics Committee which approved the protocol, reference number S22/06/108 (PhD).
Identifying relevant studies
Eligibility criteria
We used the Joanna Briggs Institute criteria for defining eligibility, namely the population, concept and context (PCC) framework [36]. Our initial scope intended to focus on activities targeted at researchers conducting RCTs, but our initial search did not identify any studies addressing this. Therefore, we expanded our scope and amended our search strategy to be less specific. Consequently, we included studies that described and tested any activity broadly aimed at promoting the publishing of health-related research findings among researchers in the health science field. Our rationale for modifying our study was to avoid producing an empty review, thereby averting research waste and because activities identified in this context are likely to be applicable for those involved in RCTs.
Population
Researchers or investigators (trialists) who have conducted, led or been involved in health-related research studies.
Concept
Studies that measured and compared the effects of activities that were intended to reduce publication bias of studies and described the activity.
Studies that described and assessed an implemented activity and reported an outcome measure. We considered activities to be any tasks or strategies aimed at researchers to encourage them to publish their research findings, including activities intended to help them overcome difficulties in publishing research.
Context
Any research institution or group conducting health research.
Types of study designs
We considered the following study designs for testing the activity: RCTs, experimental and quasi-experimental study designs, before and after studies, interrupted time-series studies, analytical observational studies (such as cohort and case–control and cross-sectional studies) and descriptive observational study designs. We did not include case reports, publications simply describing activities and opinion papers where the authors present their ideas or opinions without describing or commenting on previously or currently implemented or tested activities.
Search strategy
Our information specialist developed a comprehensive search strategy for PubMed and Scopus without restrictions on language, date or publication status. We also considered the relevant terms that reviews similar to ours have used in their search strategies when developing our search strategy [30, 37]. Our search strategy consisted of keywords and index terms adapted for each database and information source (Table S2). To confirm our search strategy’s accuracy, we checked that our results included all previously known studies similar to our topic. Lastly, to identify additional reports not found by our database search, we conducted backwards and forward citation searches on reviewed full texts from the screening phase [38].
Selecting studies for inclusion
Screening and selecting records
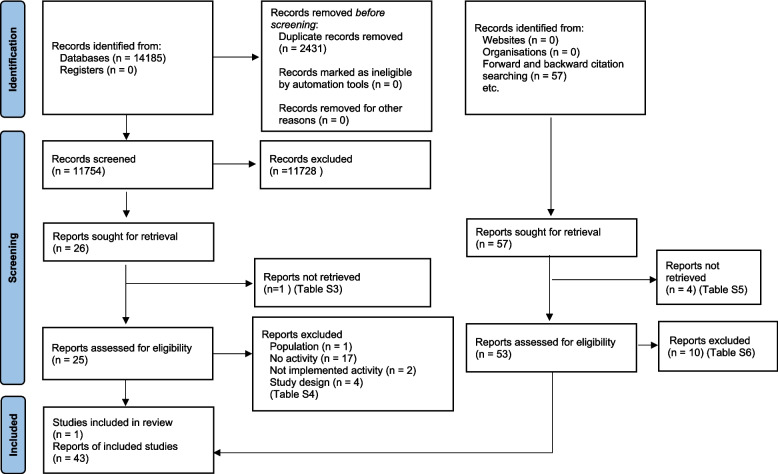
We exported all retrieved studies from the bibliographic databases into Rayyan — a free software for managing records for systematic reviews [39]. One author (A. H.) removed the duplicates. A. H. and a second reviewer independently and in duplicate screened all titles and abstracts for potentially eligible studies. We resolved disagreements through discussion, and if unresolved, we included the record for the full-text assessment phase. Both reviewers used a pre-designed eligibility checklist to independently inspect the full articles to determine whether they met the inclusion criteria. A. H. contacted study authors for electronic copies of unobtainable articles. The reviewers resolved disagreements through discussion with a third reviewer where necessary. We tabulated the ineligible studies. A. H. received a full text from a corresponding author [40] that was identified through forward citation [40]. We used the PRISMA flow diagram to depict our scoping review study selection process (Fig. 1) [41].
Fig. 1.
PRISMA 2020 flow diagram for new systematic reviews
Data extraction
Two reviewers independently extracted data using a pre-specified, pre-piloted data extraction form. A third reviewer assessed the data extraction forms for inconsistencies. The data extracted included article characteristics (e.g. author names, year of publication, country) and contextual factors (e.g. study aims/objectives, sample size, the reason for the activity, participant’s work requirements, participant’s faculty, activity provider, activity facilitator, activity type/format, activity period).
Outcomes
We extracted and reported the following outcomes:
Number of publications
Number of manuscripts submitted for publication
Number of publications in progress
Number of pre-prints
These were the primary outcomes of interest. No other outcomes were included.
Synthesis of results
Data analysis and presentation
A. H. conducted data analysis, collated and summarised the extracted data and arranged these using an analytical framework [42]. We grouped the studies by activity formats, summarised by activity types (e.g. live in-person or virtual lectures, workshops, tutorials, alert systems or others). We compared the contextual factors and outcomes mentioned above.
A. H. manually coded and synthesised the data methods for the extracted data from the included studies. A second reviewer checked the data analysis as a quality control measure.
A. H. conducted a descriptive analysis of the extracted characteristics. These are presented as an overview of the types of activities.
Results
The database search retrieved 14,185 records. We screened 11,754 records after de-duplication, and 11,728 records did not meet eligibility based on title and abstract screening. We reviewed 26 studies for full-text eligibility assessment. Of the 26 studies, we were unable to retrieve one study (Table S3) [43]. We excluded 24 ineligible studies and provided reasons (Table S4). As a result of finding one eligible study, we conducted forward and backward citation searches on the 26 studies we sought from the screening process.
We identified 57 records through forward and backward citation searching. Of the 57 reports, we could not retrieve four reports (Table S5), and 10 did not meet our eligibility criteria (Table S6). We included 43 additional studies. Table 1 provides the characteristics of the 44 included studies. The table summarises their descriptions, study designs, participant demographics, types of activities and activity effectiveness. The PRISMA flow diagram depicts the process (Fig. 1) [41].
Table 1.
Characteristics of included studies
|
Author (year) Country |
Study design | Participant demographics | Activity | Publication outcomes measured |
|---|---|---|---|---|
|
Al-Imari L. (2016) Canada [44] |
Descriptive single group |
Work requirements: Clinicians Faculty: Medicine Publication experience: NR |
Provider: Academic Facilitator: Writing expert Sample size: 8 Period: 6 months |
Pre-prints: NR Publications reported as an outcome: NR Number of publications: NR Manuscript submissions: NR Manuscripts in progress: NR |
|
Arrazola J. (2020) USA [45] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Epidemiology Publication experience: No |
Provider: Academic Facilitator: Experience faculty member Sample size: 39 Period: 2 days in person session, 6 months formal mentorship |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 17 Manuscript submissions: 24 Manuscripts in progress:15 |
|
Bellicoso D. (2022) Canada [46] |
Descriptive single group |
Work requirements: Clinicians Faculty: General Publication experience: Some |
Provider: Academic Facilitator: Experience faculty member Sample size: 31 Period: 3 h |
Pre-prints: NR Publications reported as an outcome: NR Number of publications: NR Manuscript submissions: NR Manuscripts in progress: NR |
|
Bourgault A. M. (2022) USA [47] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Nursing Publication experience: NR |
Provider: Academic Facilitator: Faculty member Sample size: 25 Period: 18 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 15 Manuscript submissions:19 Manuscripts in progress: 39 |
|
Brandon C. (2015) USA [48] |
Descriptive single group |
Work requirements: Clinicians Faculty: Medicine Publication experience: Yes |
Provider: Academic Facilitator: Experience faculty member Sample size: 6 Period: 12 months |
Pre-prints: Yes Publications reported as an outcome: Yes Number of publications: 4 Manuscript submissions:5 Manuscripts in progress: NR |
|
Buffington A. (2021) USA [49] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Medicine Publication experience: No |
Provider: Academic Facilitator: Experience faculty member Sample size: 23 Period: 4–9 h |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 50 Manuscript submissions: NR Manuscripts in progress: NR |
|
Cable C. T. (2013) USA [50] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Medicine Publication experience: No |
Provider: Academic Facilitator: Experience faculty member Sample size: 7 per retreat Period: 1-month total contribution and 1 week of actual retreat |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 8 Manuscript submissions: 7 Manuscripts in progress: NR |
|
Cope V. C. (2016) Australia [51] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Nursing Publication experience: No |
Provider: Academic Facilitator: Experience faculty member Sample size: 7 Period: 5 days |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 4 Manuscript submissions: 0 Manuscripts in progress: NR |
|
Dankoski M. (2012) USA [52] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: General Publication experience: Yes |
Provider: Academic Facilitator: Experience faculty member Sample size: 200 Period: 2 days |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 23 Manuscript submissions: NR Manuscripts in progress: NR |
|
Duncanson K. (2018) Australia [53] |
Descriptive two group |
Work requirements: Clinicians Faculty: General Publication experience: NR |
Provider: Nonacademic affiliation Facilitator: Experience faculty member Sample size: 52 Period: 40 h over the 6 weeks |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 21 Manuscript submissions: 26 Manuscripts in progress: NR |
|
Files J. A. (2008) USA [54] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Medicine Publication experience: Yes |
Provider: Academic Facilitator: Experience faculty member Sample size: 4 Period: 10 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 3 Manuscript submissions: 3 Manuscripts in progress: NR |
|
Fischer-Cartlidge E. (2020) USA [55] |
Descriptive single group |
Work requirements: Clinicians Faculty: Nursing Publication experience: No |
Provider: Academic Facilitator: Experience faculty member Sample size: 89 Period: 12 weeks |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 22 Manuscript submissions: 29 Manuscripts in progress: NR |
|
Fleming L. W. (2017) USA [56] |
Descriptive two group |
Work requirements: Academics/lecturers Faculty: Pharmacy Publication experience: Yes |
Provider: Academic Facilitator: Experience faculty member Sample size: 18 Period: NR |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 29 Manuscript submissions: NR Manuscripts in progress: NR |
|
Franks A. M. (2018) USA [57] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Pharmacy Publication experience: NR |
Provider: Academic Facilitator: Experience faculty member Sample size: 49 Period: 6 monthly sessions over 6 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 10 Manuscript submissions: 12 Manuscripts in progress:49 |
|
Harris S. (2003) USA [58] |
Descriptive single group |
Work requirements: Clinicians Faculty: Medicine Publication experience: Yes |
Provider: Academic Facilitator: Experience faculty member Sample size: 10 Period: 26 meetings in 36 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 28 Manuscript submissions: NR Manuscripts in progress:36 |
|
Harvey D. (2020) Australia [59] |
Descriptive single group |
Work requirements: Clinicians Faculty: Allied health Publication experience: No |
Provider: Academic Facilitator: Experience faculty member Sample size: 9 Period: 8 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 2 Manuscript submissions: 3 Manuscripts in progress: NR |
|
Hekelman F. P. (1995) USA [60] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: General Publication experience: NR |
Provider: Academic Facilitator: Experience faculty member Sample size: 40 Period: 12 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 16 Manuscript submissions: NR Manuscripts in progress: NR |
|
Jackson D. (2009) Australia [61] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Nursing Publication experience: Yes |
Provider: NR Facilitator: Writing expert Sample size: 39 Period: 3 days |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 17 Manuscript submissions: 37 Manuscripts in progress: NR |
|
Kooker (2015) USA [62] |
Descriptive single group |
Work requirements: NR Faculty: Nursing Publication experience: NR |
Provider: Academic Facilitator: Experience faculty member Sample size: NR Period: Once monthly for 12 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 12 Manuscript submissions: 13 Manuscripts in progress: Yes, no details |
|
Kulage K. M. (2016) USA [63] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Nursing Publication experience: NR |
Provider: Academic Facilitator: Experience faculty member Sample size: 17 Period: NR |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 15 Manuscript submissions: NR Manuscripts in progress: NR |
|
Kwan P. P. (2021) USA [64] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: General Publication experience: NR |
Provider: Academic Facilitator: Faculty member Sample size: 33 Period: 6 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 22 Manuscript submissions: 28 Manuscripts in progress: NR |
|
Murray R. (2008) UK [65] |
Descriptive single group |
Work requirements: Clinicians Faculty: Allied health Publication experience: NR |
Provider: NR Facilitator: Writing expert Sample size: 14 Period: 6 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 6 Manuscript submissions: 7 Manuscripts in progress: NR |
|
Ness V. (2014) UK [66] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Nursing Publication experience: Yes |
Provider: Academic Facilitator: Faculty member Sample size: 5 Period: NR |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 4 Manuscript submissions: 5 Manuscripts in progress:1 |
|
Noone J. (2019) USA [67] |
Descriptive single group |
Work requirements: Faculty: General Publication experience: No |
Provider: Academic Facilitator: Experience faculty member Sample size: 19 Period: 5 days |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 23 Manuscript submissions: NR Manuscripts in progress: NR |
|
Oakley M. (2012) USA [68] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Dental Publication experience: NR |
Provider: NR Facilitator: Writing expert Sample size: 8 Period: 4-year period |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 6 Manuscript submissions: 1 Manuscripts in progress: NR |
|
Oshiro J. (2020) USA [69] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Medicine Publication experience: Yes |
Provider: Academic Facilitator: Experience faculty member Sample size: 25 Period: 4 h |
Pre-prints: NR Publications reported as an outcome: NR Number of publications: NR Manuscript submissions: NR Manuscripts in progress: NR |
|
Pololi L. (2005) USA [70] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Medicine Publication experience: Yes |
Provider: Academic Facilitator: Writing expert Sample size: NR Period: Once monthly for 2 years |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: Unclear Manuscript submissions: 27 Manuscripts in progress: NR |
|
Pololi L. (2015) USA [71] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Medicine Publication experience: NR |
Provider: Academic Facilitator: Experience faculty member Sample size: 12 Period: 12 months |
Pre-prints: NR Publications reported as an outcome: NR Number of publications: NR Manuscript submissions: NR Manuscripts in progress:66 |
|
Reader S. (2015) USA [72] |
Descriptive single group |
Work requirements: Clinical educators Faculty: Medicine Publication experience: NR |
Provider: Academic Facilitator: Experience faculty member Sample size: 10 Period: 14 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 3 Manuscript submissions: NR Manuscripts in progress: NR |
|
Remein C. D. (2022) USA [73] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: General Publication experience: NR |
Provider: Academic Facilitator: Experience faculty member Sample size: 18 Period: 1 day |
Pre-prints: NR Publications reported as an outcome: NR Number of publications: NR Manuscript submissions: NR Manuscripts in progress: NR |
|
Rickard C. M. (2009) Australia [74] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: General Publication experience: Yes |
Provider: Academic Facilitator: Writing expert Sample size: 8 Period: 5 days |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 33 Manuscript submissions: NR Manuscripts in progress: NR |
|
Ross R. G. (2016) USA [75] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: General Publication experience: Yes |
Provider: Academic Facilitator: Experience faculty member Sample size: 134 Period: 2 years |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 337 Manuscript submissions: NR Manuscripts in progress: NR |
|
Sabouni A. (2017) Egypt [76] |
Descriptive single group |
Work requirements: Clinicians Faculty: General Publication experience: NR |
Provider: NR Facilitator: Faculty member Sample size: 159 Period: 2 months (12 lectures) |
Pre-prints: NR Publications reported as an outcome: NR Number of publications: NR Manuscript submissions: NR Manuscripts in progress: NR |
|
Salas-Lopez D. (2012) USA [77] |
Descriptive single group |
Work requirements: Clinicians Faculty: General Publication experience: Yes |
Provider: Nonacademic affiliation Facilitator: Writing expert Sample size: NR Period: 24 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 5 Manuscript submissions: 6 Manuscripts in progress: NR |
|
Santucci A. K. (2008) USA [78] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Mental health Publication experience: NR |
Provider: Academic Facilitator: Faculty member Sample size: 5 Period: 24 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: Unclear Manuscript submissions: NR Manuscripts in progress: NR |
|
Shah J. (2010) NR [79] |
Descriptive single group |
Work requirements: NR Faculty: NR Publication experience: No |
Provider: Writesim TCExam Programme Facilitator: Mentor Sample size: 15 Period: NR |
Pre-prints: NR Publications reported as an outcome: NR Number of publications: NR Manuscript submissions: NR Manuscripts in progress: NR |
|
Sommers P. S. (1996) USA [80] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: General Publication experience: Yes |
Provider: Academic, journal editor Facilitator: Writing expert Sample size: 35 Period: 2 days |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 19 Manuscript submissions: NR Manuscripts in progress: NR |
|
Sonnad S. S. (2011) USA [43] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Medicine Publication experience: No |
Provider: Academic Facilitator: Faculty member Sample size: 51 Period: NR |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 4 Manuscript submissions: NR Manuscripts in progress: NR |
|
Stanley I. H. (2017) USA [81] |
Descriptive single group |
Work requirements: Clinicians Faculty: Mental health Publication experience: No |
Provider: Academic Facilitator: Experience faculty member Sample size: 23 Period: NR |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 4 Manuscript submissions: NR Manuscripts in progress: NR |
|
Steinert Y. (2008) Canada [82] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: General Publication experience: NR |
Provider: Academic Facilitator: Experience faculty member Sample size: 24 Period: 6 months |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 85 Manuscript submissions: 14 Manuscripts in progress: NR |
|
Vogt M. (2021) USA [83] |
Descriptive two group |
Work requirements: Clinicians Faculty: Nursing Publication experience: NR |
Provider: Academic Facilitator: NR Sample size: 16 Period: 7 weeks |
Pre-prints: NR Publications reported as an outcome: NR Number of publications: NR Manuscript submissions: NR Manuscripts in progress: NR |
|
von Isenburg M. (2017) USA [82] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: General Publication experience: NR |
Provider: Academic Facilitator: Faculty member Sample size: 31 Period: 16 weeks |
Pre-prints: NR Publications reported as an outcome: NR Number of publications: NR Manuscript submissions: 11 Manuscripts in progress: NR |
|
Weiss (2022) USA [40] |
Descriptive single group |
Work requirements: Clinicians Faculty: Medicine Publication experience: No |
Provider: Academic Facilitator: Experience faculty member Sample size: 6 Period: 6 years (monthly meeting of 60–90 min) |
Pre-prints: NR Publications reported as an outcome: Yes Number of publications: 31 Manuscript submissions: NR Manuscripts in progress: NR |
|
Wortman-Wunder E. (2020) USA [83] |
Descriptive single group |
Work requirements: Academics/lecturers Faculty: Allied health Publication experience: No |
Provider: Academic Facilitator: Experience faculty member Sample size: 126 Period: 15 h (weekly 2.5-h sessions) |
Pre-prints: NR Publications reported as an outcome: NR Number of publications: NR Manuscript submissions: NR Manuscripts in progress: NR |
Abbreviation: NR Not reported
There was heterogeneity in the study designs, methods and type of activity. Therefore, we summarised and presented the results using a narrative, thematic approach. Based on these findings, we broadly present the characteristics of the types of activities.
Description of the studies
The included studies were published from 1995 until 2022. Forty studies used descriptive single-group study designs. The remaining three were descriptive two-group study designs. They described and implemented activities that promote the publishing of health-related research. Most of the studies were conducted in high-income countries, with the majority from the USA (n = 31).
Types of activities
We identified 10 broad activities with various combinations (Table S7) where we mapped the activities to their studies and categorises the activities into broad delivery formats, i.e. facilitator-led (n = 36 studies), structured programmes (n = 25 studies), writing retreats (n = 10 studies) and software programmes (n = 1 studies).
The studies were multicomponent, combining, on average, two activities. The most commonly used combinations were structured programmes which were facilitator-led (n = 25), followed by facilitator-led with one or more additional facilitator-led activities (n = 11).
The studies that implemented facilitator-led approaches as part of their activity package incorporated the following formats: writing mentors (n = 16), writing groups (n = 6), individual or group peer-support groups (n = 27) and medical writers (n = 7). The studies that used structured programmes included writing programmes (n = 20), workshops (n = 12) and writing courses (n = 1).
Using the descriptions from the included studies, we defined the structured programmes in the following way. Programmes ran over a week, generally consisting of interactive training and lectures. All programmes in our study included a facilitator-led activity. The majority of programmes covered topics on writing style and editing (n = 14), structuring a manuscript (n = 13), specific requirements for different sections in a manuscript (n = 11) and how to choose a journal (n = 9). Furthermore, studies reported using a structured curriculum (n = 4) and covered good practice techniques such as transparency in reporting (n = 2) or reporting guidelines (n = 1).
We considered a workshop similar to a programme but shorter (i.e. less than 5 days). Five studies used a workshop and included facilitator-led components. Workshops covered fewer topics than the writing programme. Topics included were constructing manuscript-specific sections (n = 7), writing style and editing (n = 5) and choosing journals (n = 3).
Retreats were broadly described as uninterrupted writing time away from work at conducive venues within a reasonable distance from home and work. The venues provided catering to optimise writing time. The retreats ranged from 1 to 4 nights away. The participants would set out writing targets, which usually incorporated facilitator-led activities such as group or individual peer support (n = 7) and mentoring (n = 6). Rarely, retreats formed part of a structured programme such as workshops (n = 2) and a programme (n = 1). When part of a structured programme, the retreats would more likely include topics on writing style and editing (n = 1), structuring a manuscript (n = 1), specific requirements for different sections in a manuscript (n = 3) and how to choose a journal (n = 2).
One study developed and tested software which helped researchers with their writing style, editing, structure and specific requirements for different sections in a manuscript. It included a facilitator-led component, allowing the writer to ask questions.
Activity effectiveness
The study designs used and the information reported do not allow estimates of the effectiveness of the activities reported in the studies in this scoping review. There were 34 studies that reported publications as an outcome; of these, two studies did not provide the exact number of publications (see Table 1). Twenty studies reported the number of manuscripts submitted at the end of the study, and 7 studies indicated that there were manuscripts in progress. Lastly, one study reported on pre-prints as an outcome.
Discussion
Our review describes several strategies used to assist researchers in publishing their research findings. Despite the call from numerous organisations for timely publication of clinical trial results, we did not identify studies that implemented an activity explicitly for clinical trialists. We, therefore, report on studies implementing activities supporting a broader range of health researchers to publish their study findings. By mapping the activity components of eligible studies, we found that studies commonly reported multipronged approaches consisting of structured programmes with a facilitator-led component.
We have described these multipronged activities and their components to provide guidance and options for research groups and institutions to consider these approaches for supporting the timely publication of studies, including trials.
We found that none of the included studies implemented sound experimental methodological designs to assess the effectiveness of their approach. Therefore, there is still a gap in knowledge about which activities are effective at minimising publication bias among trialists [37].
Through discussions with colleagues, we know of noteworthy yet unpublished formal and informal activities in practice. These may take on different forms than those described in our study, including but not limited to informal mentoring, learning through shadowing in the job environment, “trial and error” and using books or online publications that guide researchers on writing for publication [37]. In addition, administrators, policymakers and research methodologists have emphasised the need to upskill and persuade researchers to promote the publishing of their trials. Different approaches that we have not mentioned above could also be taken to upskill and persuade researchers to promote the publishing of their trials, such as informing researchers about publication bias, the ethical responsibility of publishing research findings and the negative impact of publication bias on practice and research, primarily evidence-based healthcare decision-making.
Researchers have access to several online resources, such as uploaded instructional video recordings, webinars, writing templates, writing tutors and the CONSORT guideline to aid the writing process [84]. Other options include formal prerequisite courses for trialists when registering a trial. The course content could address frequent problems of publishing trials and of using tools such as CONSORT to guide the reporting process, the importance of publishing, the dangers of publication bias and its effects on the evidence ecosystem, including content on selective reporting, plagiarism and publishing in open-access journals [3, 85, 86]. In addition to writing focused activities, alternative approaches exist to encourage the publication of clinical trials. For example, the International Standard Randomised Controlled Trial Number (ISRCTN) Registry recently launched the transparency tracker [87]. This prompts trialists to update their trial records in the ISRCTN Registry. One year after the planned overall end date for a trial, the transparency tracker prompts the trialists to update the end date if the trial has not ended or to report where to find the publication or a basic summary of results. Following this, 6 months after the planned publication date, trialists are prompted to update the end date if the trial has not ended or to report where to find a publication or a basic summary of results. Furthermore, trialists are asked to explain if the study record has not been updated for 2 years, and there is no publication or summary of results.
Trial registries or ethics committees may also consider an extreme yet appropriate approach whereby they hold research groups accountable for publication bias. For example, similar to the ISRCTN’s trial tracker, if the trial results are not published, trialists or their institutions might be flagged and logged onto an online repository for violating transparency standards.
Strengths and limitations
Searching for studies in this field is challenging. Our scoping review provides a general overview of the published literature related to publication bias. An information specialist helped design our search strategies, yet our initial search strategies did not find any eligible records that met the aims of our review. Consequently, in an attempt to find eligible studies, our information specialist modified the search strategy to broaden our yield. However, this proved futile even though our final search yielded more than 10,000 records, and we found only one eligible study. Noting this, we conducted forward and backward citation searches on the 22 articles that were checked in full text and identified an additional 57 records. None of our search iterations had retrieved these records.
Of note, we rarely found terminologies related to publication bias and trialists in the title/abstract screening or the full-text eligibility assessments. Possible reasons for this could be that studies (a) addressed publication bias or trialists in their full texts, but the authors did not explicitly mention these terms in the title or abstract, (b) the main focus of the article was not solely on publication bias or trialists, and (c) databases have not indexed articles using terms such as “publication bias” and “trialist”.
It is also possible that researchers who have done studies that would be eligible for this review have, themselves, contributed to publication bias by not publishing their research. This might be especially likely if studies tested activities that were intended to help trialists to publish their clinical trial results but found these activities to be ineffective.
Implications for practice and future research
Although trial registers have become an avenue to disseminate trial results, journal research articles remain the main way to communicate trial findings with a diverse and extensive audience. We identified broad activities that could be adapted to trialists to minimise publication bias, but trialists commonly report that the lack of time is the main reason they either do not submit manuscripts or publish [88–90]. Therefore, in seeking to design and implement an appropriate activity targeted at trialists, future research should investigate the challenging factors in the publication process that may make the publication process time-consuming. They should also seek advice from regional trialists to address these reasons, to make the submission and publication process more efficient.
Conclusion
We found that most activities were multipronged, commonly designed as structured programmes and led by facilitators to advise on manuscript writing, providing writing time and offering feedback from peers and mentors. Despite the prevalence of publication bias and its significant impact on research waste, we found no research explicitly targeting activities to encourage trialists to publish their findings. Therefore, rigorous research is needed to determine effective strategies for reducing publication bias among those conducting RCTs.
Supplementary Information
Additional file 1: Table S1. Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist. Table S2. An example of the search strategy of PubMed. Table S3. Reports not retrieved. Table S4. Reports excluded. Table S5. Reports not retrieved. Table S6. Reports excluded. Table S7. Characteristics of included studies activities.
Acknowledgements
We would like to thanks Oliver for her input into the search strategy. The following individuals helped with screening: Emily Potgieter (E. P.), Laylah Ryklief (L. R.) and Keisha De Gouveia (K. D. G.).
Authors’ contributions
Conceptualisation, AH, TK and MC. Methods and data collection tools development, AH, TK and MC. Searching, AH. Screening, AH, EP, LR, and KDG. Data extraction, AH, EP and LR. Data analysis, AH. AH wrote the first draft of the manuscript, and AH revised this and subsequent drafts with input from all authors. All authors approved the final version of the manuscript.
Funding
The authors declare that they have not received funding to conduct this study.
Data availability
The database provided in this article is available to use.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM. Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ. 2012;344:d7292. [DOI] [PMC free article] [PubMed]
- 2.Chen R, Desai NR, Ross JS, Zhang W, Chau KH, Wayda B, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352: i637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings. Health Technol Assess. 2010;14(8):iii–ix−xi, 1–193. [DOI] [PubMed] [Google Scholar]
- 4.Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta‐analysis. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication bias in meta‐analysis: Prevention, assessment and adjustments. 2005. p. 1–7. 10.1002/0470870168.ch1.
- 5.Minogue V, Cooke M, Donskoy A-L, Vicary P, Wells B. Patient and public involvement in reducing health and care research waste. Res Involv Engagem. 2018;4(5):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan VMB, Farquhar CM. Is there any hope that we can reduce research wastage and prevent publication bias? J Hosp Man Health Pol. 2019;3(1):1–3. [Google Scholar]
- 7.Feinstein AR, Horwitz RI. Double standards, scientific methods, and epidemiologic research. N Engl J Med. 1982;307(26):1611–7. [DOI] [PubMed] [Google Scholar]
- 8.Australian Clinical Trials. What is a clinical trial? Canberra: National Health and Medical Research Council; 2023 [7 November 2023]. Available from: https://www.australianclinicaltrials.gov.au/about/how-they-work.
- 9.Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. The Lancet. 2002;359(9305):515–9. [DOI] [PubMed] [Google Scholar]
- 10.Siegfried N, Clarke M, Volmink J. Randomised controlled trials in Africa of HIV and AIDS: descriptive study and spatial distribution. BMJ. 2005;331(7519):742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieseler B. Beyond journal publications–a new format for the publication of clinical trials. Z Evid Fortbild Qual Gesundhwes. 2017;120:3–8. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Strengthening clinical trials to provide high- quality evidence on health interventions and to improve research quality and coordination. In: Seventy- fifth World Health Assembly. Geneva; 2022. Available: https://apps.who.int/gb/ebwha/pdf_files/WHA75/A75_ACONF9-en.pdf.
- 13.World Health Organization. Joint statement on public disclosure of results from clinical trials. Geneva: World Health Organization; 2017. Available from: https://www.who.int/news/item/18-05-2017-joint-statement-on-registration.
- 14.Schulz KF AD, Moher D for the CONSORT Group. CONSORT 2010 statement: update guidelines for reporting parallel group randomized trials, which was published simultaneously in nine journals, see the EQUATOR Network link [website] [Available from: https://www.equator-network.org/reporting-guidelines/consort/.
- 15.World Health Organization. WHO policy on open access Geneva: World Health Organization [website] [Available from: https://www.who.int/about/policies/publishing/open-access.
- 16.Ross JS, Lehman R, Gross CP. The importance of clinical trial data sharing: toward more open science. Circ Cardiovasc Qual Outcomes. 2012;5(2):238–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumholz HM. Open science and data sharing in clinical research: basing informed decisions on the totality of the evidence. Am Heart Assoc; 2012:141–2. [DOI] [PubMed]
- 18.Whitty CJM. What makes an academic paper useful for health policy?. BMC Med. 2015;13:301. 10.1186/s12916-015-0544-8. [DOI] [PMC free article] [PubMed]
- 19.Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM. Publication of NIH funded trials registered in ClinicalTrials. gov: cross sectional analysis. BMJ. 2012;344:d7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzoli L, Flacco ME, D’Addario M, Capasso L, De Vito C, Marzuillo C, et al. Non-publication and delayed publication of randomized trials on vaccines: survey. BMJ. 2014;348: g3058. [DOI] [PubMed] [Google Scholar]
- 21.Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;374(9683):86–9. [DOI] [PubMed] [Google Scholar]
- 22.Glasziou P, Chalmers I. Paul Glasziou and Iain Chalmers: Is 85% of health research really “wasted”? The BMJ. 2016.
- 23.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337(8746):867–72. [DOI] [PubMed] [Google Scholar]
- 24.Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. 1998;279(4):281–6. [DOI] [PubMed] [Google Scholar]
- 25.Ross JS, Mocanu M, Lampropulos JF, Tse T, Krumholz HM. Time to publication among completed clinical trials. JAMA Intern Med. 2013;173(9):825–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials. Gov: a cross-sectional analysis. PLoS Med. 2009;6(9):e1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwan K, Gamble C, Williamson PR, Kirkham JJ, Group RB. Systematic review of the empirical evidence of study publication bias and outcome reporting bias—an updated review. PLoS ONE. 2013;8(7): e66844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan AW, Song F, Vickers A, Jefferson T, Dickersin K, Gotzsche PC, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet. 2014;383(9913):257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke M, Hopewell S, Chalmers L. Reports of clinical trials should begin and end with up-to-date systematic reviews of other relevant evidence: a status report. J R Soc Med. 2007;100(4):187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaler K, Kien C, Nussbaumer B, Van Noord MG, Griebler U, Klerings I, et al. Inadequate use and regulation of interventions against publication bias decreases their effectiveness: a systematic review. J Clin Epidemiol. 2015;68(7):792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14(8):1–220. [DOI] [PubMed] [Google Scholar]
- 32.Malički M, Marušić A. Is there a solution to publication bias? Researchers call for changes in dissemination of clinical research results. J Clin Epidemiol. 2014;67(10):1103–10. [DOI] [PubMed] [Google Scholar]
- 33.Kien C, Nußbaumer B, Thaler KJ, Griebler U, Van Noord MG, Wagner P, et al. Barriers to and facilitators of interventions to counter publication bias: thematic analysis of scholarly articles and stakeholder interviews. BMC Health Serv Res. 2014;14(1):551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carroll HA, Toumpakari Z, Johnson L, Betts JA. The perceived feasibility of methods to reduce publication bias. PLoS ONE. 2017;12(10): e0186472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 36.Peters MDJ GC, McInerney P, Munn Z, Tricco AC, Khalil H. Scoping reviews. In: Aromataris E MZ, editor. Joanna Briggs Institute Reviewer's Manual. JBI; 2020. p. 407–52.
- 37.Galipeau J, Moher D, Campbell C, Hendry P, Cameron DW, Palepu A, et al. A systematic review highlights a knowledge gap regarding the effectiveness of health-related training programs in journalology. J Clin Epidemiol. 2015;68(3):257–65. [DOI] [PubMed] [Google Scholar]
- 38.Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Searching for and selecting studies. Cochrane Handbook for systematic reviews of interventions. 2019:67–107.
- 39.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss BD, Stillwater BJ, Aldulaimi S, Cunningham JK, Gachupin FC, Koleski J, et al. Writing support group for medical school faculty—a simple way to do it. Teaching and Learning in Medicine. 2022:1–8. [DOI] [PubMed]
- 41.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 43.Sonnad SS, Goldsack J, McGowan KL. A writing group for female assistant professors. J Natl Med Assoc. 2011;103(9–10):811–5. [DOI] [PubMed] [Google Scholar]
- 44.Bellicoso D, Valenzano TJ, Topolovec-Vranic J. Effectiveness of a manuscript writing workshop on writing confidence amongst nursing and health disciplines clinicians. J Med Imaging Radiat Sci. 2022. [DOI] [PubMed]
- 45.Bourgault AM, Galura SJ, Kinchen EV, Peach BC. Faculty writing accountability groups: a protocol for traditional and virtual settings. J Prof Nurs. 2022;38:97–103. [DOI] [PubMed] [Google Scholar]
- 46.Brandon C, Jamadar D, Girish G, Dong Q, Morag Y, Mullan P. Peer support of a faculty “writers’ circle” increases confidence and productivity in generating scholarship. Acad Radiol. 2015;22(4):534–8. [DOI] [PubMed] [Google Scholar]
- 47.Buffington A, Lange C, Bakker C, Nanney M, Roberts W, Berge J, et al. The Collaborative Scholarship Intensive: a research-intensive course to improve faculty scholarship. Fam Med. 2021;53(5):355–8. [DOI] [PubMed] [Google Scholar]
- 48.Cable CT, Boyer D, Colbert CY, Boyer EW. The writing retreat: a high-yield clinical faculty development opportunity in academic writing. J Grad Med Educ. 2013;5(2):299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cope VC, Sundin D, Smyth A, Wang C, Baum G, Ewens B, et al. The hidden benefits of writing retreats: academic development and social interaction for nurses. J Nurs Educ Pract. 2016;6(11):73–9. [Google Scholar]
- 50.Dankoski M, Palmer M, Banks J, Brutkiewicz R, Walvoord E, Hoffmann-Longtin K, et al. Academic writing: supporting faculty in a critical competency for success. The Journal of Faculty Development. 2012;26(2):47–54. [Google Scholar]
- 51.Duncanson K, Webster EL, Schmidt DD. Impact of a remotely delivered, writing for publication program on publication outcomes of novice researchers. Rural Remote Health. 2018;18(2):4468. [DOI] [PubMed] [Google Scholar]
- 52.Files JA, Blair JE, Mayer AP, Ko MG. Facilitated peer mentorship: a pilot program for academic advancement of female medical faculty. J Womens Health. 2008;17(6):1009–15. [DOI] [PubMed] [Google Scholar]
- 53.Fischer-Cartlidge E. An evidence-based approach to increasing nurses’ publication rates. AJN The American Journal of Nursing. 2020;120(8):50–5. [DOI] [PubMed] [Google Scholar]
- 54.Fleming LW, Malinowski SS, Fleming JW, Brown MA, Davis CS, Hogan S. The impact of participation in a research/writing group on scholarly pursuits by non-tenure track clinical faculty. Curr Pharm Teach Learn. 2017;9(3):486–90. [DOI] [PubMed] [Google Scholar]
- 55.Franks AM. Design and evaluation of a longitudinal faculty development program to advance scholarly writing among pharmacy practice faculty. Am J Pharm Educ. 2018;82(6). [DOI] [PMC free article] [PubMed]
- 56.Harris S, Wiebe C, Knell E, Berger S. A physician peer support writing group. Fam Med. 2003;35(3):195–201. [PubMed] [Google Scholar]
- 57.Harvey D, Barker R, Tynan E. Writing a manuscript for publication: an action research study with allied health practitioners. Focus on Health Professional Education: A Multi-disciplinary Journal. 2020;21(2):1–16. [Google Scholar]
- 58.Hekelman F, Gilchrist V, Zyzanski S, Glover P, Olness K. An educational intervention to increase faculty publication productivity. Fam Med. 1995;27(4):255–9. [PubMed] [Google Scholar]
- 59.Jackson D. Mentored residential writing retreats: a leadership strategy to develop skills and generate outcomes in writing for publication. Nurse Educ Today. 2009;29(1):9–15. [DOI] [PubMed] [Google Scholar]
- 60.Kooker BM, Latimer R, Mark DD. Successfully coaching nursing staff to publish outcomes. J Nurs Adm. 2015;45(12):636–41. [DOI] [PubMed] [Google Scholar]
- 61.Kulage KM, Larson EL. Implementation and outcomes of a faculty-based, peer review manuscript writing workshop. J Prof Nurs. 2016;32(4):262–70. [DOI] [PubMed] [Google Scholar]
- 62.Kwan PP, Sharp S, Mason S, Saetermoe CL. Faculty writing groups: the impact of protected writing time and group support. Int J Educ Res Open. 2021;2:100100. [DOI] [PMC free article] [PubMed]
- 63.Murray R, Newton M. Facilitating writing for publication. Physiotherapy. 2008;94(1):29–34. [Google Scholar]
- 64.Ness V, Duffy K, McCallum J, Price L. Getting published: reflections of a collaborative writing group. Nurse Educ Today. 2014;34(1):1–5. [DOI] [PubMed] [Google Scholar]
- 65.Noone J, Young HM. Creating a community of writers: participant perception of the impact of a writing retreat on scholarly productivity. J Prof Nurs. 2019;35(1):65–9. [DOI] [PubMed] [Google Scholar]
- 66.Oakley M, Vieira AR. The endangered clinical teacher-scholar: a promising update from one dental school. J Dent Educ. 2012;76(4):454–60. [PubMed] [Google Scholar]
- 67.Oshiro J, Caubet SL, Viola KE, Huber JM. Going beyond “not enough time”: barriers to preparing manuscripts for academic medical journals. Teach Learn Med. 2020;32(1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pololi L, Knight S, Dunn K. Facilitating scholarly writing in academic medicine. J Gen Intern Med. 2004;19(1):64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pololi LH, Evans AT. Group peer mentoring: an answer to the faculty mentoring problem? A successful program at a large academic department of medicine. J Contin Educ Heal Prof. 2015;35(3):192–200. [DOI] [PubMed] [Google Scholar]
- 70.Reader S, Fornari A, Simon S, Townsend J. Promoting faculty scholarship–an evaluation of a program for busy clinician-educators. Canadian Medical Education Journal. 2015;6(1): e43. [PMC free article] [PubMed] [Google Scholar]
- 71.Remein CD, Childs E, Beard J, Demers LB, Benjamin EJ, Wingerter SL. “Getting Started”: a pilot introductory narrative writing session for interprofessional faculty in academic health sciences. Adv Med Educ Pract. 2022;13:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rickard CM, McGrail MR, Jones R, O’Meara P, Robinson A, Burley M, et al. Supporting academic publication: evaluation of a writing course combined with writers’ support group. Nurse Educ Today. 2009;29(5):516–21. [DOI] [PubMed] [Google Scholar]
- 73.Ross RG, Greco-Sanders L, Laudenslager M. An institutional postdoctoral research training program: increasing productivity of postdoctoral trainees. Acad Psychiatry. 2016;40(2):207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sabouni A, Chaar A, Bdaiwi Y, Masrani A, Abolaban H, Alahdab F, et al. An online academic writing and publishing skills course: help Syrians find their voice. Avicenna journal of medicine. 2017;7(03):103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salas-Lopez D, Deitrick L, Mahady ET, Moser K, Gertner EJ, Sabino JN. Getting published in an academic-community hospital: the success of writing groups. J Gen Intern Med. 2012;27(1):113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santucci AK, Lingler JH, Schmidt KL, Nolan BA, Thatcher D, Polk DE. Peer-mentored research development meeting: a model for successful peer mentoring among junior level researchers. Acad Psychiatry. 2008;32(6):493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shah J, Rajgor D, Vaghasia M, Phadtare A, Pradhan S, Carvalho E, et al. WriteSim TCExam-an open source text simulation environment for training novice researchers in scientific writing. BMC Med Educ. 2010;10(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sommers P, Muller J, Bailiff P, Stephens G. Writing for publication: a workshop to prepare faculty as medical writers. Fam Med. 1996;28(9):650–4. [PubMed] [Google Scholar]
- 79.Stanley IH, Hom MA, Chu C, Joiner TE. Increasing research productivity and professional development in psychology with a writing retreat. Scholarsh Teach Learn Psychol. 2017;3(3):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steinert Y, McLeod PJ, Liben S, Snell L, Steinert Y, McLeod PJ, et al. Writing for publication in medical education: the benefits of a faculty development workshop and peer writing group. Med Teach. 2008;30(8):e280–5. [DOI] [PubMed] [Google Scholar]
- 81.Vogt M, Leslie Coonfare D, NPD-BC CS. Graduate nursing students’ scholarly writing: a program evaluation. Int J Nurs & Healt Car Scie. 2021;1(08).
- 82.von Isenburg M, Lee LS, Oermann MH. Writing together to get AHEAD: an interprofessional boot camp to support scholarly writing in the health professions. Journal of the Medical Library Association: JMLA. 2017;105(2):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wortman-Wunder E, Wefes I. Scientific writing workshop improves confidence in critical writing skills among trainees in the biomedical sciences. Journal of Microbiology & Biology Education. 2020;21(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGauran N, Wieseler B, Kreis J, Schüler Y-B, Kölsch H, Kaiser T. Reporting bias in medical research-a narrative review. Trials. 2010;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Das N, Panjabi M. Plagiarism: why is it such a big issue for medical writers? Perspect Clin Res. 2011;2(2):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.BioMed Central (BMC). Transparency Tracker: Springer nature; 2023 [Available from: https://www.isrctn.com/search/tracker.
- 88.Smyth R, Jacoby A, Altman DG, Gamble C, Williamson PR. The natural history of conducting and reporting clinical trials: interviews with trialists. Trials. 2015;16(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al-Imari L, Yang J, Pimlott N. Peer-support writing group in a community family medicine teaching unit: Facilitating professional development. Can Fam Physician. 2016;62(12):e724–30. [PMC free article] [PubMed] [Google Scholar]
- 90.Arrazola J, Polster M, Etkind P, Moran JS, Vogt RL. Lessons learned from an intensive writing training course for applied epidemiologists. Public Health Rep. 2020;135(4):428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist. Table S2. An example of the search strategy of PubMed. Table S3. Reports not retrieved. Table S4. Reports excluded. Table S5. Reports not retrieved. Table S6. Reports excluded. Table S7. Characteristics of included studies activities.
Data Availability Statement
The database provided in this article is available to use.