Abstract
Objective
The establishment of clinical registries is essential for the comprehensive evaluation of surgical outcomes. In 2006, the Schulthess Shoulder Arthroplasty Registry (SAR) was launched to systematically assess safety, implant longevity, functional outcomes, pain levels, quality of life, and patient satisfaction in individuals undergoing shoulder arthroplasty. This paper aims to outline the registry data and demonstrate how it is leveraged to improve clinical outcomes. Additionally, we provide guidance for organizations currently collecting or planning to collect similar data.
Participants
Our SAR systematically records adult patients’ data undergoing either anatomic or reverse shoulder joint replacement at the Schulthess Clinic. Both primary and revision surgeries are comprehensively documented within the registry.
Current outcomes
From March 2006 to December 2023, the SAR included 98% of eligible operations. A total of 2301 patients were recruited, accounting for 3576 operations and 14,487 person-years of follow-up. At baseline, the mean age was 71 (range: 20–95), with 65% being female patients. The most prevalent indication was cuff tear arthropathy (46%), and the mean preoperative Constant Score was (31 ± 15). Notably, functional recovery peaked at 12 months postoperatively, displaying no clinically significant deterioration during the initial ten follow-up years in the overall cohort (including both primary arthroplasty and revisions). The registry has been instrumental in addressing various clinical and methodological inquiries, focusing particularly on comparing different implant configurations and surgical techniques to optimize functional recovery. Additionally, SAR data played a pivotal role in substantiating the clinical significance and reliability of radiological monitoring for cortical bone resorption, scapular notching, and glenoid component loosening.
Keywords: Shoulder arthroplasty, Shoulder joint replacement, Clinical registry, Schulthess Shoulder Arthroplasty Registry (SAR), Functional outcomes, Implant longevity, Patient-reported outcomes
Introduction
Registries play a pivotal role in advancing the field of Shoulder Arthroplasty (SA) by serving as comprehensive repositories of patient information. The significance of registries lies in their ability to systematically collect and disseminate important patient information related to SA outcomes. By documenting a wide array of patient outcomes, including surgical methods, postoperative complications, and long-term functional results, registries contribute to a deeper understanding of safety and effectiveness of clinical practices [1].
Shoulder arthroplasty is recommended in the following conditions: osteoarthritis, rheumatoid arthritis, rotator cuff arthropathy, avascular necrosis and posttraumatic arthritis as well as certain conditions after failed previous surgeries.
Within the realm of SA, the Schulthess Local Shoulder Arthroplasty Registry (SAR), situated in Zurich, Switzerland, offers a comprehensive cohort profile for both anatomic and reverse shoulder arthroplasty. This resource provides valuable insights into the multifaceted factors impacting patient outcomes. Registries like SAR facilitate evidence-based decision-making by offering a comprehensive view of real-world scenarios, helping clinicians refine their approaches and optimize patient care protocols. In addition, the systematic collection of patient-reported outcomes within such registries is pivotal, as it reflects the subjective experiences and perspectives of individuals undergoing SA as well as systematic documentation of various implant designs and configurations.
In addition, our local registry plays a fundamental role in monitoring the performance of orthopedic implants over time. This includes tracking the longevity of implants, detecting potential failures or problems, and informing decisions about the selection of implants in our clinical practice. The SAR contributes to post-market surveillance of orthopedic devices and implants. This continuous monitoring helps regulatory authorities and manufacturers identify any safety concerns and take appropriate actions if needed.
Collectively, our SAR has been used for benchmarking and quality assurance purposes, comparing their performance and outcomes with local or international standards. This allowed our institution to identify areas for improvement and adopt our clinical practices. In essence, the SAR emerge as a crucial tool in enhancing both the clinical knowledge base and the overall quality of patient care.
The aim of this paper is to outline our registry data and provide details on how we leverage our registry. Additionally, we aim to offer guidance for other organizations currently collecting or planning to collect similar data.
Population description
The registry comprises a retrospective cohort of adult patients who underwent anatomic or reverse shoulder joint replacement or implant revision at Schulthess Clinic in Zurich, Switzerland. Data collection for the registry is ongoing, incorporating both retrospective and prospective data. The registry’s completeness is determined by comparing registered cases to all eligible cases in the hospital billing system (congruency rate). The overall data acquisition from March 2006 to December 2023 was 98%. After excluding the initiation phase of the first 6 months, the congruency rate increased to 99%.
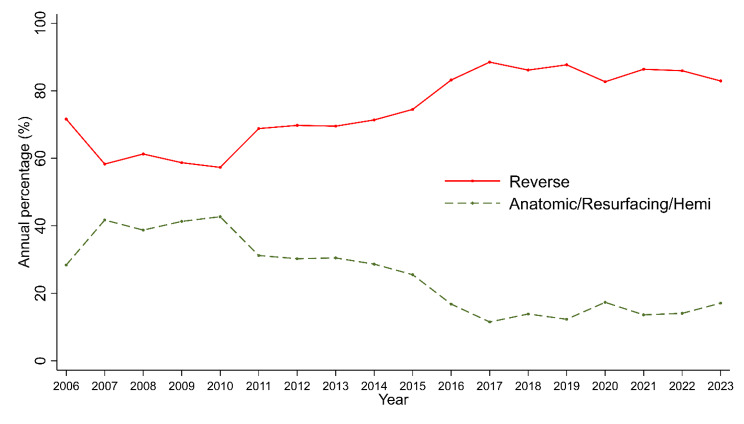
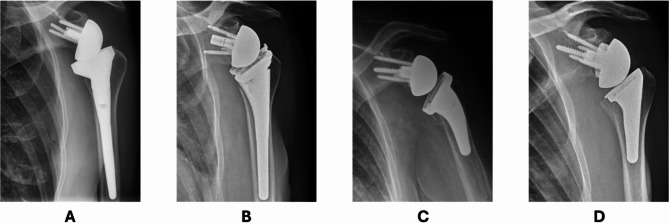
Mean Constant Score (CS), Shoulder Pain and Disability Index (SPADI) and Subjective Shoulder Value (SSV) at baseline were 31.7, 36.6 and 40.5, respectively. Mean preoperative pain was 5.9 (± 2.6, scale (0 = no pain, 10 = maximum pain)) and mean QuickDASH score was 52.4 (± 18.4). The most common indication was cuff tear arthropathy (46%), followed by primary osteoarthritis (26%); see Table 1. Reverse shoulder arthroplasty (RSA) became increasingly common, rising from 71% in 2006–2019 to 85% in 2019–2023 (Fig. 1). Within the patient population treated with a RSA, three distinct subgroups regarding implant design can be identified. First, the classic Grammont design with a medialized and distalized center of rotation (Fig. 2A), second, the Lateralized design, with a lateralized center of rotation (Fig. 2B). The last subgroup of RSA implant design has been developed more recently, using a lateralized-distalized center of rotation (Fig. 2C and D). Between 2006 and 2023, 48% of all RSAs implanted had the Grammont design, 41% of shoulders had the lateralized design and 11% had the lateralized-distalized design. The distribution among these subgroups underwent significant shift between 2019 and 2023 with lateralized-distalized becoming the most prominent design.
Table 1.
Descriptive characteristics
| Variable | Parameter | N (%) | Mean (SD) |
|---|---|---|---|
| Age | 3576 | 71.3 (10.1) | |
| Age category | |||
| <=40 | 30 (1) | ||
| > 40–50 | 112 (3) | ||
| > 50–60 | 325 (9) | ||
| > 60–70 | 909 (25) | ||
| > 70–80 | 1556 (44) | ||
| > 80–90 | 626(18) | ||
| > 90 | 18 (1) | ||
| Sex | |||
| Female | 2309(65) | ||
| Male | 1267(35) | ||
| BMI | 1989 | 26.9 (5.1) | |
| ASA | |||
| ASA 1: Healthy Patient | 156 (4) | ||
| ASA 2: Mild Systemic Disease | 1770(50) | ||
| ASA 3: Severe Systemic Disease | 1602 (45) | ||
| ASA 4: Severe Systemic Disease that is constant threat to life | 364(1) | ||
| Admission type | |||
| Illness | 2510(75) | ||
| Accident | 839 (25) | ||
| Primary Indication | |||
| Cuff Tear Arthropathy | 1530(46) | ||
| Rheumatoid Arthritis | 93 (3) | ||
| Prosthesis Revision | 411 (12) | ||
| Acute Fracture | 62(2) | ||
| Posttraumatic | 387 (8) | ||
| Primary Osteoarthritis | 878 (26) | ||
| Humeral Head Necrosis | 67 (2) | ||
| Other | 10 (0) | ||
| Preoperative Pain (0–10) | 3293 | 5.9 (2.6) | |
| Constant Score (0-100) | 3064 | 31.7 (15.2) | |
| SPADI (0-100) | 3204 | 36.6 (20.3) | |
| QuickDASH (0-100) | 3189 | 52.4 (18.4) | |
| Subjective Shoulder Value | 1773 | 40.5 (20.8) | |
| Abduction (0-120°) | 3453 | 69.9 (32.7) | |
| Flexion (0-120°) | 3454 | 81.2 (37.2) | |
| Abduction strength (Affected arm; kg) | 3418 | 0.8 (1.9) | |
| Abduction strength (Unaffected arm; kg) | 3135 | 4.9 (3.5) | |
| EQ-VAS (0-100) | 1835 | 67.5 (18.3) | |
Note. BMI, body mass index; EQ-VAS, EuroQol Visual Analogue Scale; QuickDASH, Quick Disabilities of Arm, Shoulder, and Hand; SPADI, Shoulder Pain and Disability Index
Fig. 1.
Annual number of RSA (%)
Fig. 2.
A Distalized medialized design; B Lateralized design; C and D Distalized-Lateralized design
This consistently high data acquisition can be attributed to three factors: (i) the implementation of a technological solution using FileMaker Pro Advanced (V.18, Claris International, California, USA), which flags each prosthesis implantation for the cohort staff when the operation is scheduled in the electronic clinical record; (ii) sufficient funding for study assistance and data management, allowing close monitoring of the operation plan; (iii) the high motivation and commitment of the surgeons to quality control and scientific activities.
Patient reported outcomes (PROMs)
Objective shoulder function
At each clinical examination the following active and passive range of motion (ROM) parameters are evaluated; flexion, abduction, internal and external rotation at 90° and external rotation at 0°. In addition, several functional clinical tests are performed, such as functional external rotation tests as parts of the CS, drop-arm sign, lag sign, Hornblower sign, lift-off test and the Belly-Press test are performed. Abduction strength of both arms is measured using a spring balance (Pesola AG, Schindellegi, Switzerland).
Self-report shoulder assessments
The SPADI was developed to measure the pain and disability associated with shoulder pathology. The SPADI is a self-administered index consisting of 13 items divided into two subscales: pain and disability. Thirty-seven male patients with shoulder pain were used in a study to examine the measurement characteristics of the SPADI [2].
The CS [3, 4] is one of the most commonly used assessment tools for various shoulder pathologies. Although it is not a gold standard, it has good psychometric properties and has excellent ability to detect clinically significant changes. It assesses four parameters: pain, activities of daily living, strength, and mobility. It is considered user-friendly, although the measurement of strength requires some extra effort. A higher score corresponds to better shoulder function. The CS is a relatively unique tool because it combines patient-reported outcomes (pain and activities of daily living: 35 points), performance measurement, and clinician-reported outcomes (strength and mobility: 65 points).
The SSV [5] is an easily administered, reliable, responsive, and valid measure of shoulder function in athletes that is highly correlated with other PROMs. numeric rating scale 0-100) is a self-report administered, reliable, responsive, and valid measure of shoulder function in patients undergoing SA that is highly correlated with other PROMs. All self-report data will be assessed using REDCap, which is a free, secure, web-based application designed to support data capture for research studies [7].
Quality of life
The EQ-5D is a measure of self-reported health outcomes that is applicable to a wide range of health conditions and treatments. It consists of two parts: a descriptive system (Part I) and a visual analogue scale (VAS) (Part II). Part I of the scale consists of 5 single-item dimensions including: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has a 3-point response scale designed to indicate the level of the problem. Part II uses a vertical graduated VAS (thermometer) to measure health status, ranging from worst imaginable health state to best imaginable health state. The extended version of the EQ-5D includes a valuation task which is used only for valuation studies. There is also an optional set of demographic questions [8].
Patient satisfaction
Patients are asked postoperatively whether they would agree to undergo the same operation again and to what extent their situation changed as compared to pre-operation in terms of shoulder function and quality-of-life (5-levels ranging from ‘much better’ to ‘much worse’).
Expectations
Patients are asked postoperatively to what extent their overall expectations of the operation were met (0 = not at all; 10 = fully).
Radiographic outcomes
Pathological radiological outcomes are assessed based on the internationally recognized radiological protocols specifically developed for monitoring SA.
Our group has developed a radiographic monitoring tool based on international consensus for the SA Monitoring including Core Set of Radiographic Parameters [9]. Starting from January 2020, ultrasound is performed for both anatomic and reverse implants.
Adverse event documentation
Adverse Events (AEs) and complications in SA are evaluated based on the internationally published core set of AEs. The assessment includes considerations such as the timing of occurrence (intraoperative or postoperative), the location (local or systemic), whether the event qualifies as a Serious Adverse Event, the affected tissues or physiological systems, potential association with the implant. Detailed documentation of revision surgeries covers specific reasons for revision, such as infection, glenoid periprosthetic fracture, humeral periprosthetic fracture, dislocation or instability, glenoid component loosening, humeral component loosening, rotator-cuff problems, prosthesis failure, as well as other factors. Death incidents are obtained passively from relatives and the family physician. Additionally, during scheduled follow-up time points, attempts are made to contact the patient multiple times over the phone. If information is not available, insurance companies may be contacted to obtain this information.
Assessments timeline
Clinical and functional assessments, AE assessments, and radiological evaluations are conducted pre-operatively and subsequently at 6 months, 1 year (optional), 2 years, 5 years, 10 years, and 15 years (see Fig. 3). Following the 5-year mark, follow-up visits are scheduled at 5-year intervals until either prosthesis revision or patient withdrawal from the registry due to various reasons, such as declining overall health or decease. Before each clinical visit, patients are provided with questionnaires assessing patient-reported outcomes. Electronic questionnaires distributed via REDCap are used or paper copies depending on patients’ preference.
Fig. 3.
Data acquisition and completeness
Data integrity and monitoring
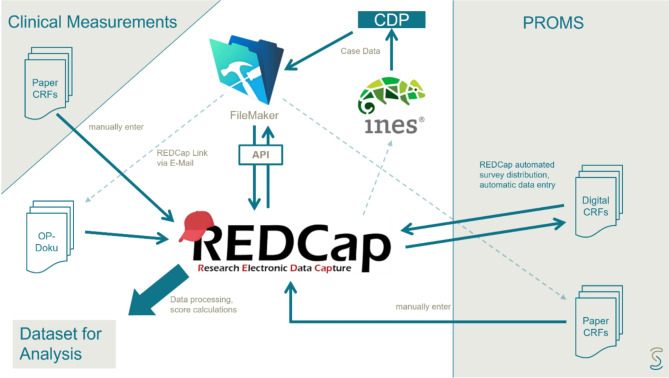
All data were captured using REDCap, which is a free, secure, web-based application designed to support data capture for research studies hosted under Schulthess clinic.
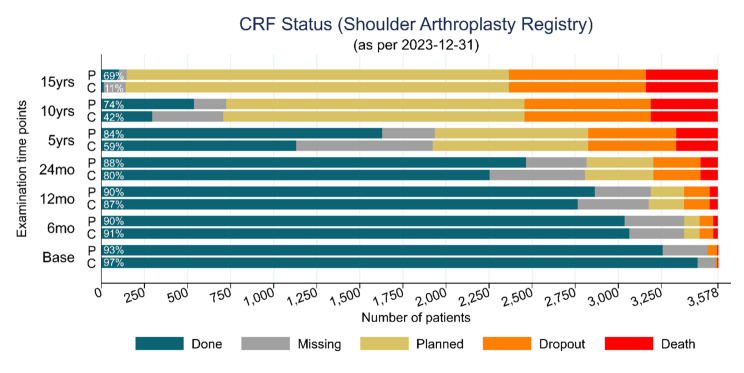
Data collection was conducted through an adjunct REDCap to FileMaker Pro method utilizing the SQL Server. (see Fig. 4 for an overview of the registry workflow) FileMaker Pro is a powerful software platform that enables users to create custom applications for managing data. It allows us to design, develop, and deploy database solutions tailored to our registry’s specific needs. Participants’ information and study-related data were securely recorded and organized within REDCap’s structured framework. Leveraging SQL Server and FileMaker Pro ensures robust data storage, retrieval, and management [3]. Overall, data completeness at 6 months was 90%, 2 years 86%, 5 years, 82% and 10 years 69% and 15 years 57% (Fig. 5).
Fig. 4.
Overview of registry workflow
Fig. 5.
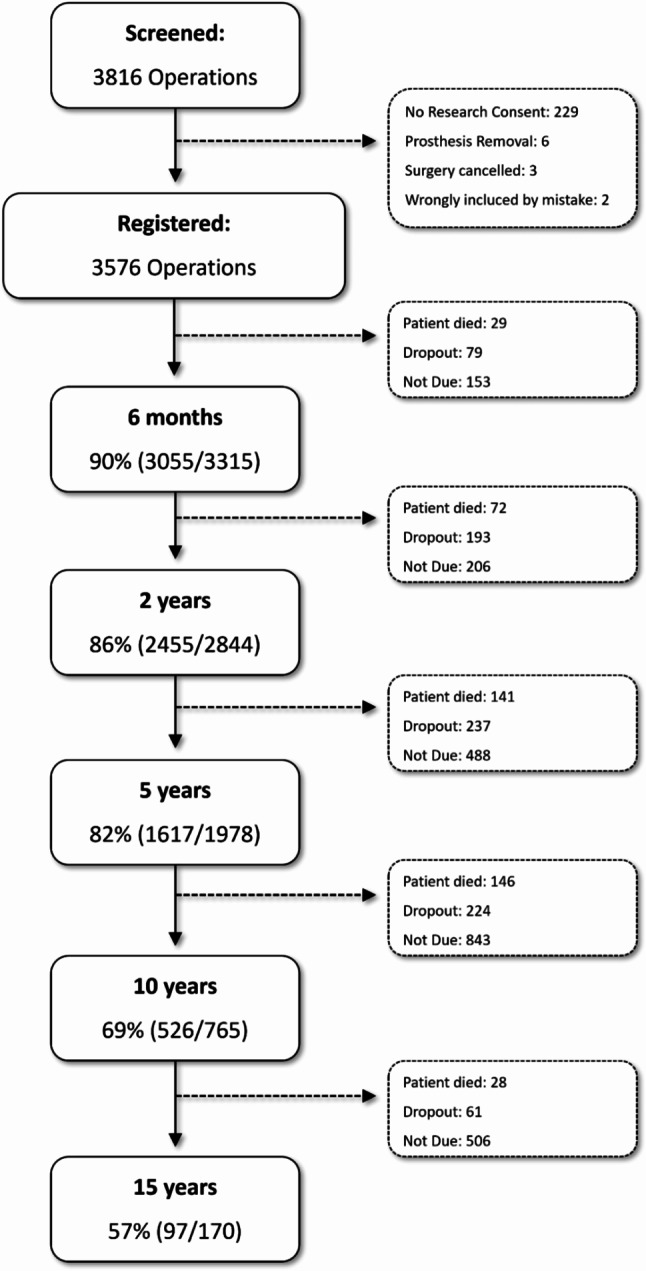
Patient Registry Flow Chart
Main findings
Since the first description of this cohort in 2020, a significant number of findings have been published. A study conducted by Grob and colleagues demonstrated that radiographic parameters displaying medialization and cranialization after anatomic total shoulder arthroplasty (aTSA). Anatomic total shoulder arthroplasty with a cemented pegged glenoid seems to be a useful predictor of impaired shoulder function in case of glenoid loosening [10].
Early loosening of the glenoid component was identified in aTSA, specifically when employing a stemless humeral component paired with a pegged polyethylene glenoid component. As a result, we discontinued the usage of this specific device and promptly notified Swissmedic, Switzerland’s national regulatory body for medical products, and the respective company in order to take all necessary actions [11].
Similarly, a retrospective study was conducted involving 65 patients with primary aTSA for glenohumeral osteoarthritis [12]. The study aimed to compare two convertible metal-back glenoid systems, both stemmed and stemless designs, to assess their respective performance regarding complications and the necessity for subsequent revision surgeries. Results demonstrated that after 5 years, both stemmed and stemless aTSA with metal-back glenoid components demonstrated similar clinical outcomes. Revisions rates were also found to be comparable between the two designs.
Other interesting work published by Endell and colleagues demonstrated that five years post-RSA, patients who resumed predominantly upper extremity sports exhibited comparable shoulder functional outcomes to individuals who did not engage in any sports and those participating in lower extremity sports [13].
Similarly, several articles have been published that show the influence of lateralization on clinical outcomes and AEs. In a recent study, where Freislederer and colleagues investigated patients with cuff tear arthropathy, results demonstrated a better preservation of external rotation and a reduced rate of scapular notching for a lateralized RSA (lateralized glenosphere and 135° neck-shaft angle (NSA) in contrast to the classic Grammont design without glenoidal lateralization and with a 155° NSA [14]. In another study Friesenbichler and colleagues demonstrated that achieving good to excellent shoulder abduction required enhancing the glenohumeral contribution, which correlated with the postoperative strength of abduction [15]. In a recent investigation, we examined three designs of RSA: the Grammont design, the lateralized and lateralized and distalized design, specifically in patients with Cuff Tear Arthropathy (CTA). We controlled for anatomical and pathomorphologic confounding factors such as scapular anatomy, implant position, center of rotation, humeral offset, implant characteristics, rotator cuff status and tear pattern among others. The groups were defined as follows: group 1 (inlay, 155° humeral inclination, 36 + 2 mm eccentric glenosphere (n = 50)), group 2 (inlay, 135° humeral inclination, 36 + 4 mm lateralized glenosphere (n = 141)) and group 3 (onlay, 145° humeral inclination, + 3 mm lateralized base plate, 36 + 2 mm eccentric glenosphere (n = 35)). Our results revealed comparable functional outcome scores using the Constant Score. However, patients with a lateralized and distalized RSA configuration demonstrated improved flexion and abduction capabilities, along with fewer occurrences of scapular notching. Moreover, enhanced rotation was noted in patients with either of the lateralized RSA setups compared to the traditional Grammont design [16]. In a comparative study [17], outcomes were assessed in RSA patients with advanced CTA, stratified into “lateralized” (LAT) and “non-lateralized” (NONLAT) groups based on differing prosthetic designs: the LAT group, characterized by a 135° humeral inclination and a 36 ± 4 mm lateralized glenosphere, and the NONLAT group, characterized by a 155° humeral inclination and a 36 ± 2 mm eccentric glenosphere. LAT patients demonstrated improved external rotation and greater capability to reach lumbar vertebra 3 (L3). Conversely, NONLAT patients exhibited higher rates of inferior glenosphere overhang, center of rotation medialization, and scapular notching. Notably, LAT patients had higher humeral offset and lateralization shoulder angle. Overall, the lateralized prosthesis yielded superior rotation and less scapular notching compared to the non-lateralized design in CTA patients. RSA safety related data from our registry demonstrated that despite some of our patients being over 85 years old with multiple comorbidities, our elderly patients demonstrated significant clinical improvement in daily activities, with high satisfaction rates. Radiographic examination at 24 months post-surgery confirmed sufficient implant stability [18]. Assessing revisions rates, prior findings from our registry is that the incidence of revisions following primary RSA in our patient registry is minimal. However, a considerable number of supplementary interventions were observed, which, although not involving component revision, could potentially affect the ultimate outcome post-RSA [19]. In a recent retrospective investigation (N = 641) we aimed to analyze the effect of scapulothoracic orientation and posture types on clinical outcome after RSA using 2 years follow data [20]. Patients were classified into posture types A, B, and C using a recognized method that assesses scapular internal rotation on preoperative cross-sectional imaging. Results demonstrated that type C posture influences the 2-year clinical outcome of RSA patients in terms of worse flexion, abduction, SPADI and pain.
Patient reported outcomes over time
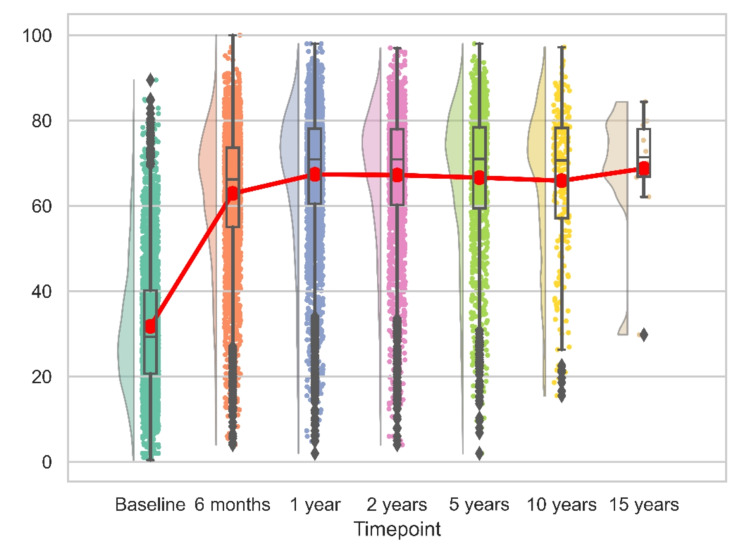
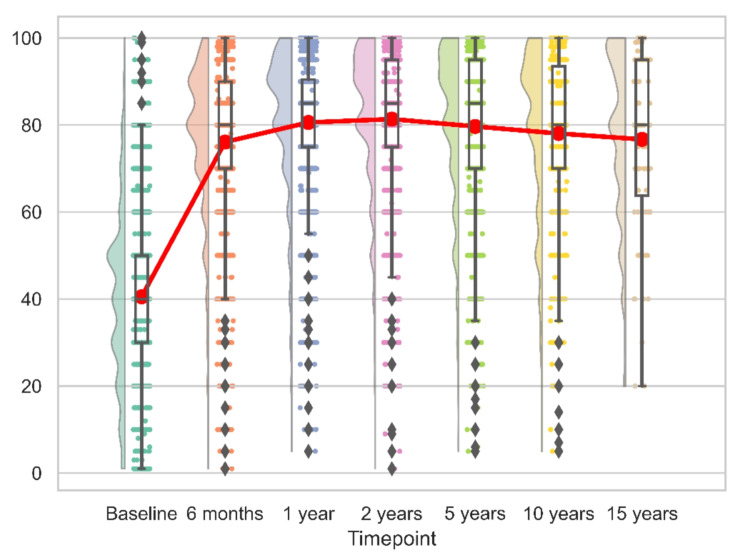
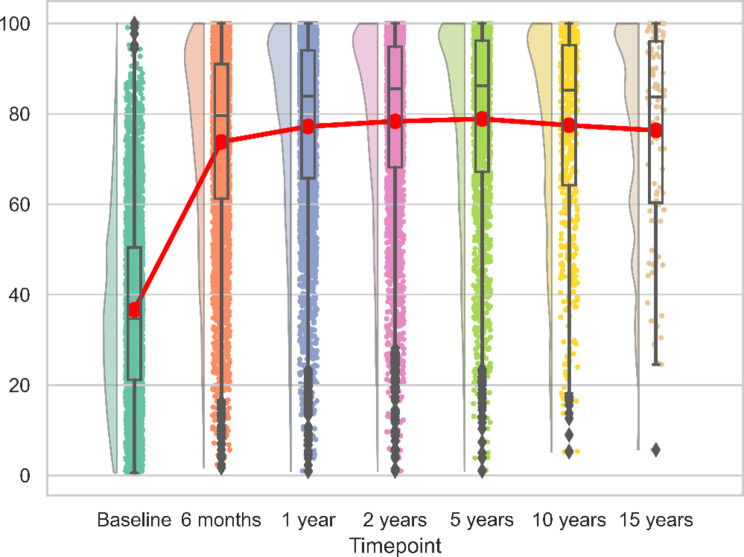
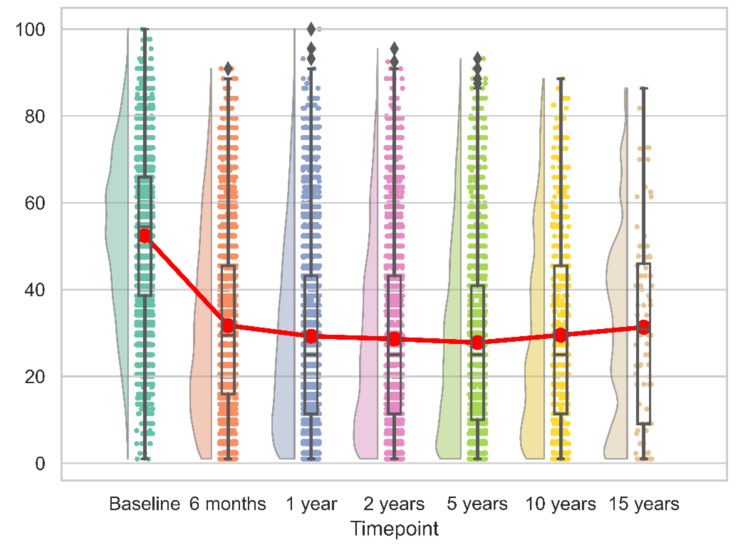
For both reverse and anatomic implants, the peak recovery, as measured by CS, SSV, SPADI and QuickDASH was observed within the first 12 months post-operation. This pattern remained consistent over the initial 15 years, with no significant changes observed (Figs. 6, 7, 8 and 9). We calculated the percentages of patients failing to achieve minimal clinically important differences (MCID) at each time point. For the CS, this proportion declined from 11.7% at 6 months to 8.8% at 24 months post-operation, with an MCID of 9.4 points. However, at later time points, the percentage increased to 13%.
Fig. 6.
Longitudinal CS
Fig. 7.
Longitudinal SSV
Fig. 8.
Longitudinal SPADI
Fig. 9.
Longitudinal QuickDASH
A similar trend was noted with the SPADI, where 26.6% of patients did not achieve an improvement beyond the MCID of 20.6 points at 6 months, compared to 20% at 24 months post-surgery. By the long-term follow-up, this percentage rose to 25.4%.
The QuickDASH, which has an MCID of 8 points, exhibited a comparable trend. The proportion of patients not reaching the MCID decreased from 25.2% at 6 months to 22% at 24 months, before increasing again to 29.6% at the 15-year follow-up.
In contrast, the SSV presented a different pattern. Although the percentage of treatments failing to achieve the MCID of 26.6 points declined from 33.3% at 6 months to 26.7% at 24 months, there was no subsequent increase throughout the long-term recovery period. However, it is important to note that the MCID for the SSV was derived from a cohort of patients with proximal humerus fractures and may not be directly applicable to those undergoing SA [21].
Adverse events and revisions rates
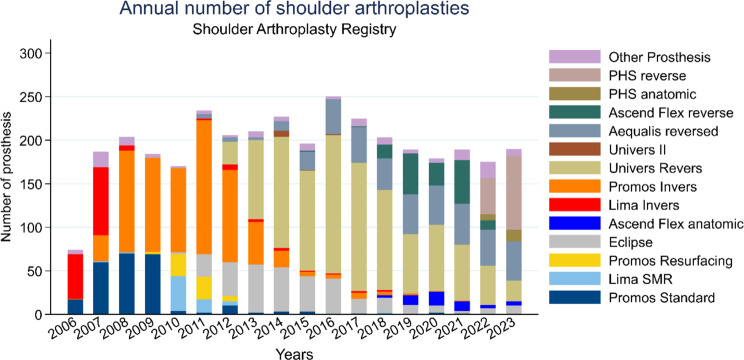
Our registry data played a crucial role in monitoring AEs and assessing implant survival rates. By systematically collecting and analyzing information on patient outcomes, complications, and device performance over time, our registry provided valuable insights into the safety and effectiveness of implants. The documentation of AEs in our registry included the nature of the event, its severity, timing, possible contributing factors, and patient response. Accurate and thorough documentation enables healthcare teams to analyze trends, identify potential patterns or systemic issues, and implement preventive measures to mitigate future occurrences. Average annual implant surgeries are approximately 200 cases and frequency of implant type can be seen in Fig. 10 with Perform Humeral Reverse (Stryker Inc, Kalamazoo, MI, USA) being the most prominent currently. The overall pooled revision rate was 0.02 per implant, corresponding to 11% revisions 10 years post implantation and 5% revisions 5 years post-implantation in agreement with data from other registries. Overall, our dropout rate was 0.06 per implant per year corresponding to a dropout percentage of 40% at 10 years and 20% at 5 years.
Fig. 10.
Annual Number of surgeries by implant type
Discussion
The aim of this article was to describe our registry data. For internal quality control purposes, the primary emphasis is placed on identifying and addressing implants exhibiting unexpectedly high early failure rates. This allows us to prioritize the selection of the most suitable implants for our patient population.
Our patient registry serves as a valuable tool for both patients and clinicians alike. By systematically collecting and organizing patient data, registries facilitate comprehensive clinical and radiological outcomes, and AEs. For patients, our SAR offers the opportunity to contribute to medical research and improve healthcare outcomes by providing access to cutting-edge treatments and clinical trials. Moreover, our registry contributes to patient care by facilitating clinicians in monitoring disease progression, tracking the evolution of implant designs, tailoring treatment plans, and identifying potential risk factors. For our surgeons, this registry provides valuable insights into treatment effectiveness, safety profiles, and long-term outcomes, thereby supporting evidence-based decision-making and quality improvement initiatives and identify alarmingly high rates of implant failures. Overall, our patient registry plays a crucial role in advancing medical knowledge, improving patient care, and fostering collaboration between patients, clinicians, and researchers.
Future directions and the use of artificial intelligence and machine learning
The integration of artificial intelligence (AI) and machine learning (ML) in the analysis of SA registry data represents a pioneering approach with profound implications for the field of orthopedics. Leveraging the wealth of information within the registry, AI and ML technologies have the potential to revolutionize our understanding of surgical outcomes. These advanced analytics can facilitate predictive modeling, enabling the identification of intricate patterns and associations within vast datasets. For instance, in preoperative planning, ML modeling can analyze imaging data to precisely segment and identify relevant anatomical structures. Recently developed computerized planning programs utilizing computerized tomography with 3D modeling aim to enhance the accuracy of parameter assessment and offer automated values for glenoid measurement indices. This technological advancement aids surgeons in virtually trialing implants preoperatively and optimizing their size and fit. At Schulthess clinic we have been utilizing these technologies, namely Blueprint (Stryker Inc, Kalamazoo, MI, USA) and Virtual Implant Positioning (Arthrex Inc, Naples, FL, USA) [22, 23] successfully for the past couple of years. This has allowed us to choose the appropriate sizes of different components of TSA which can help minimize the risk of intraoperative fractures, postoperative instability, overstuffing of the joint, and impingement on the rotator cuff. While occasional discrepancies may arise between surgical decisions made during planning and the implants actually utilized, their significance remains pivotal [24].
Following surgery, ML holds considerable potential in forecasting longitudinal outcomes, encompassing functional abilities, pain management, and patient satisfaction. This prognostication is rooted in the comprehensive longitudinal data gathered within our registries, taking into account patient demographics and surgical specifics. The incorporation of ML into SA is currently in progress. For instance, Ramkumar and colleagues [25] investigated an iPhone application utilizing a ML software development kit for assessing a patient’s shoulder ROM in four arcs. This application demonstrated clinical efficacy in accurately capturing and analyzing the intricate spatial movements of the shoulder joint, as evidenced by comparable ROM angle measurements between the software development kit and manual goniometer [25]. Likewise, Predict+ (Exactech, Gainesville, FL, USA), a newly introduced clinical decision support tool, employs AI algorithms to anticipate individualized patient outcomes post SA [26].
In the context of our SA registry, AI and ML applications may enhance the accuracy of prognostic assessments, enable personalized treatment planning, and contribute to more precise risk classification. Additionally, these technologies offer the prospect of real-time clinical decision support, assisting healthcare professionals in optimizing patient care strategies based on the latest data trends. The utilization of AI and ML in conjunction with SA registry data heralds a new era in evidence-based orthopedic research, fostering innovation and ultimately enhancing the quality of patient outcomes.
Registry future trajectories
With the first patients approaching the 20-year follow-up landmark within the next two years, the SAR is poised to continue providing crucial data on long-term functional and pain outcomes, implant survivorship, revision rates, patient satisfaction, pain, and quality of life. In addition to research and quality control, the integration of pre-operative 3D planning, AI and ML methodologies into future directions holds significant promise. The application of AI and ML on registry data can enhance predictive modeling, personalized treatment planning, and real-time clinical decision support, marking a transformative advancement in the utilization of cutting-edge technologies to optimize patient care.
Acknowledgements
We would like to specifically express our gratitude to Schwyzer HK, former Chair of the Shoulder and Elbow Department, for his initiation of the registry and invaluable leadership, and to Laurent Audigé, Head of Research in Shoulder, Elbow, Hand at Schulthess, for his dedicated efforts in advancing research in this field. Without their visionary work, much of the progress reflected in our data and publications would not have been possible. The authors would also like to thank Beatrice Weber for her contribution to data collection.
Author contributions
AL and MS conceived of the presented idea and research methodology and prepared the first draft of the manuscript. TS was involved in planning, data collection and analysis. PM and FF participated in data interpretation and editing the manuscript. All authors reviewed the final version of the manuscript.
Funding
No specific financial source of funding. Support was provided by the Schulthess Clinic.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study received approval by the Ethics Committee of Zurich (Kantonale Ethikkommission [KEK], Stampfenbachstrasse 121, CH-8090 Zurich, Switzerland; KEK-ZH-Nr. 2014–0483) and is performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients provided written informed consent prior to patient enrolment / data collection and use of their data for research purposes.
Consent for publication
Not applicable.
Competing interests
We declare the following financial interests/personal relationships, which may be considered as potential competing interests: M.S. and F.F. report personal fees from Stryker, outside the submitted work. P.M. reports personal fees from Arthrex, outside the submitted work. A.L. and T.S., their immediate family, or any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Delaunay C. Registries in orthopaedics. Orthop Traumatol Surg Res. 2015;101(1 Suppl):S69–75. [DOI] [PubMed] [Google Scholar]
- 2.Breckenridge JD, McAuley JH. Shoulder Pain and Disability Index (SPADI). J Physiother. 2011;57(3):197. [DOI] [PubMed] [Google Scholar]
- 3.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987(214):160–4. [PubMed]
- 4.Vrotsou K, Ávila M, Machón M, Mateo-Abad M, Pardo Y, Garin O, et al. Constant-Murley score: systematic review and standardized evaluation in different shoulder pathologies. Qual Life Res. 2018;27(9):2217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the constant score. J Shoulder Elb Surg. 2007;16(6):717–21. [DOI] [PubMed] [Google Scholar]
- 6.Dorado K, Schreiber KL, Koulouris A, Edwards RR, Napadow V, Lazaridou A. Interactive effects of pain catastrophizing and mindfulness on pain intensity in women with fibromyalgia. Health Psychol Open. 2018;5(2):2055102918807406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group TE. EuroQol -- a new facility for the measurement of health- related quality of life. 1990;16(3):199–208. [DOI] [PubMed]
- 9.I. G. Premium and protection of several procedures for dealing with outliers when sample sizes are moderate to large. Technometrics. 1973;14(2):385–404.
- 10.Grob A, Freislederer F, Marzel A, Audige L, Schwyzer HK, Scheibel M. Glenoid Component Loosening in Anatomic Total Shoulder Arthroplasty: Association between Radiological predictors and clinical Parameters-An observational study. J Clin Med. 2021;10(2). [DOI] [PMC free article] [PubMed]
- 11.Kraus M, Illner J, Warnhoff M, Audige L, Moro F, Brunner M, et al. editors. Unacceptably high loosening and revision rates after anatomical shoulder total arthroplasty with cemented pegged polyethylene glenoid component. Berlin, Germany: DKOU; 2023. [Google Scholar]
- 12.Kraus M, Illner J, Warnhoff M, Brunner M, Schneller T, Lazaridou A et al. Complications and revisions in metal-backed anatomic total shoulder arthroplasty: a comparative study of Revision Rates between Stemless and stemmed Humeral Components. J Shoulder Elb Surg. 2024. [DOI] [PubMed]
- 13.Endell D, Audige L, Grob A, Schwyzer HK, Glanzmann M, Marzel A et al. Impact of sports activity on medium-term clinical and radiological outcome after reverse shoulder arthroplasty in Cuff Deficient Arthropathy; an Institutional Register-based analysis. J Clin Med. 2021;10(4). [DOI] [PMC free article] [PubMed]
- 14.Freislederer F, Toft F, Audige L, Marzel A, Endell D, Scheibel M. Lateralized vs. classic Grammont-style reverse shoulder arthroplasty for cuff deficiency Hamada stage 1–3: does the design make a difference? J Shoulder Elb Surg. 2022;31(2):341–51. [DOI] [PubMed] [Google Scholar]
- 15.Friesenbichler B, Grassi A, Grobet C, Audige L, Wirth B. Is limited shoulder abduction associated with poor scapulothoracic mobility after reverse shoulder arthroplasty? Arch Orthop Trauma Surg. 2021;141(4):587–91. [DOI] [PubMed] [Google Scholar]
- 16.Freislederer FMP, Audige L, Schneller T, Ameziane Y, Trefzer R, Imiolczyk JP, Scheibel M. Analysis of three different reverse shoulder arthroplasty designs for cuff tear arthropathy – the combination of lateralization and distalization provides best mobility. BMC Musculoskelet Disord. 2024;in press. [DOI] [PMC free article] [PubMed]
- 17.Freislederer F, TF, Imiolczyk JP, Scheibel M, Audige L. Advanced cuff tear arthropathy: classic indication for a Grammont-style reverse shoulder arthroplasty implant or does bipolar lateralization perform better? Seminars Arthroplasty: JSES. 2024:17–26.
- 18.Endell D, Audige L, Imiolczyk JP, Scheibel M, Freislederer F. Is it worth the risk? Clinical and radiographic outcomes 24 months after reverse shoulder arthroplasty in an advanced geriatric population. JSES Int. 2022;6(5):795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glanzmann MC, Audige L, Schwyzer HK, Kolling C. Re-intervention and revision rates following primary reverse total shoulder arthroplasty - review of a local shoulder arthroplasty registry. Int Orthop. 2020;44(11):2365–70. [DOI] [PubMed] [Google Scholar]
- 20.Moroder P, Siegert P, Coifman I, Ruttershoff K, Spagna G, Scaini A et al. Scapulothoracic orientation has a significant influence on the clinical outcome after reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2024. [DOI] [PubMed]
- 21.Dabija DI, Jain NB. Minimal clinically important difference of shoulder outcome measures and diagnoses: a systematic review. Am J Phys Med Rehabil. 2019;98(8):671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iannotti J, Baker J, Rodriguez E, Brems J, Ricchetti E, Mesiha M, et al. Three-dimensional preoperative planning software and a novel information transfer technology improve glenoid component positioning. J Bone Joint Surg Am. 2014;96(9):e71. [DOI] [PubMed] [Google Scholar]
- 23.Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elb Surg. 2015;24(2):302–9. [DOI] [PubMed] [Google Scholar]
- 24.Bedeir YH, Tabeayo E, Chou TA, Gruson KI. Accuracy and reliability of computerized Surgical Planning Software in Anatomic Total Shoulder Arthroplasty. Cureus. 2023;15(4):e37400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramkumar PN, Haeberle HS, Navarro SM, Sultan AA, Mont MA, Ricchetti ET, et al. Mobile technology and telemedicine for shoulder range of motion: validation of a motion-based machine-learning software development kit. J Shoulder Elb Surg. 2018;27(7):1198–204. [DOI] [PubMed] [Google Scholar]
- 26.Exactech. Patient-Specific Outcome Predictor. https://www.exac.com 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.