Abstract
Background
Lower respiratory tract infections (LRTIs) are one of the leading causes of hospital admissions among children. In this study, we aimed to describe the epidemiological characteristics of viral pathogens associated with LRTIs in hospitalized children in Yan’an; this has yet to be reported in the literature and may guide public health interventions and resource allocation in this region.
Methods
Between June 2021 and May 2023, we conducted a retrospective analysis of the results of viral detection using oral pharyngeal swabs from 4565 children with LRTIs in the Inpatient Department of Yan’an University Affiliated Hospital. Eleven respiratory viruses, including influenza A virus (Flu A), influenza A H1N1 virus (H1N1), seasonal influenza A H3N2 virus (H3N2), influenza B virus (Flu B), parainfluenza virus (HPIV), adenovirus (HADV), bocavirus (HBoV), rhinovirus (HRV), metapneumovirus (HNPV), coronavirus (HCoV), and respiratory syncytial virus (HRSV), were confirmed by applying a multiplex real-time polymerase chain reaction (PCR) kit for respiratory viruses. We evaluated the epidemiological features of infections caused by respiratory pathogens, including aging, gender and the seasonal variations of different pathogens, and explored the high-risk factors associated with virus-caused pneumonia.
Results
At least one virus was detected in all 4565 cases; the positivity rate was 27.95%. We also detected a total of 1,276 cases with mixed infections (with two or more viruses). Of the positive cases, 59.3% were male and 40.7% were female (x2 = 0.41, P = 0.68). The highest positivity rates for respiratory pathogens were observed for HRSV, HRV, and HADV, at 5.98%, 5.67%, and 4.38%, respectively. We also observed variations in the number and positivity rates of respiratory pathogen infections by season, age and gender. HPIV (x2 = 12.05, P < 0.05) and HADV (x2 = 11.73, P < 0.05) were more common in children under three years-of-age. Notably, with the exception of the 1 to < 3 years age group, males consistently demonstrated elevated infection rates across other age groups.
Conclusions
Our analysis revealed that respiratory pathogen infections varied by gender, season, and age in the enrolled population of children.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-024-05300-1.
Keywords: Children, Respiratory tract infections, Respiratory virus, Epidemiology
Background
Respiratory viruses are the main cause of lower respiratory tract illnesses (LRTIs) in the pediatric population [1]. The Global Burden of Disease (2019) study reported that LRTIs are the second highest cause of health burden in children [2] while severe pneumonia was reported as the major cause of morbidity and mortality in children, especially those under five years-of-age [3]. These LRTIs are also known to impose significant financial strain on families [4]. According to the World Health Organization (WHO), LRTIs and pneumonia account for more than 4 million deaths annually [5].
Over the last few decades, the diagnostic work up of clinical infections has changed significantly, especially with the rapid development of molecular diagnostic methods [6, 7]. In particular, the multiplex polymerase chain reaction (PCR) assay has been confirmed as a significantly more advanced tool for the clinical detection of potential pathogens. This method is used to detect a wide range of respiratory pathogens, including, but not limited to, influenza viruses (A and B), human rhinovirus (HRV), respiratory syncytial virus (HRSV), human adenovirus (HADV), human parainfluenza viruses (HPIV), and human metapneumovirus (HMPV), providing a comprehensive approach to identifying potential infectious agents in respiratory illnesses [8, 9].
In Yan’an, previous studies have not employed a multiplex PCR assay platform to assess local epidemic pathogens and their detection rates in this specific population. This study aimed to describe the epidemiological characteristics of eleven respiratory viruses detected in pediatric patients, focusing on factors such as age, gender, and seasonal variations of different pathogens. In addition, we explored the high-risk factors associated with pneumonia caused by these viruses.
Participants and methods
This retrospective study was approved by the Ethics Committee of the Yan’an University Affiliated Hospital, China (Reference: S-S20230003).
Study site
This study was performed at the Pediatric Inpatient Department of Yan’an University Affiliated Hospital in Yan’an, Shanxi, China. Yan’an is located in the northern part of Shaanxi Province, Yan’an has a plateau continental monsoon climate; the northern part of this region has a semi-arid climate while the southern part has a semi-humid climate.
Participants
Our study included all hospitalized children between the 1st of June 2021 and the 31st of May 2023 who had been diagnosed with LRTIs in the Inpatient Department of Yan’an University Affiliated Hospital. Patients were included if they met the diagnostic criteria for acute upper respiratory tract infection or acute lower respiratory tract infection according to the national diagnostic criteria for pediatric respiratory tract infection. Patients were excluded if there was no pathogenic testing data available. These children had all been subjected to 11 respiratory pathogen tests. For each patient, we collated a range of data from an electronic medical records system, including hospitalization number, sex, age, diagnosis, and the results of diagnostic tests. A total of 4,565 patients (2,664 males and 1,901 females) were included in our analysis (ages ranged from 0 to 14 years). The patients were divided into four groups by age: <1 year (group I), 1–< 3 years (group II), 3–< 6 years (group III), and ≥ 6 years (group IV). Based on the climatic conditions of China, the four seasons were categorized as follows: March, April, and May were considered to be spring; June, July, and August were considered to be summer; September, October, and November were considered to be autumn; and December, January, and February of the next year were considered to be winter. The temperature and precipitation data in Yan’an were gathered from the China Meteorological Administration Government Website (https://www.cma.gov.cn).
Specimen collection
Throat swabs for the nucleic acid testing of respiratory pathogens were collected within 24 h of a child’s admission by trained pediatric nurses. The collection process was as follows: (1) cleaning the child’s mouth and teeth to ensure that the area was free from any debris or contaminants, and (2) the application of a flocking swab to reach the child’s pharyngeal isthmus. The flocking swab was inserted gently into the child’s mouth and directed towards the pharyngeal isthmus, the narrow passage between the back of the mouth and the throat. The swab was then used to wipe the posterior pharyngeal wall and bilateral tonsils; rotational movements were used to maximize the contact surface. After sampling, the swab was swiftly extracted from the child’s pharyngeal isthmus and immediately placed in a sample tube containing 3 ml of the sample solution provided with the kit(ResP® 13 Respiratory Pathogen Multiplex Detection Kit, NINGBO HEALTH GENE TECHNOLOGIES Co., LTD). The sample tube was then sent for examination within 30 min to ensure the integrity of the collected sample.
PCR capillary electrophoresis fragment analysis for eleven respiratory pathogens
Assays were developed for 11 respiratory pathogens, including influenza A virus (Flu A), influenza A H1N1 virus (H1N1), seasonal influenza A H3N2 virus (H3N2), influenza B virus (Flu B), parainfluenza virus (HPIV), respiratory syncytial virus (HRSV), bocavirus (HBoV), rhinovirus (HRV), metapneumovirus (HNPV), coronavirus (HCoV), and adenovirus (HADV). This test comprehensively covers the clinical high positive rate and severe high-risk viruses, and can achieve the joint detection of multiple pathogens, thus providing a comprehensive, accurate and rapid detection method for the clinical diagnosis of acute respiratory infection and the differential diagnosis of COVID-19. For each patient, throat swabs were collected and multiple fluorescence quantitative PCR tests were carried out in accordance with the instructions provided in a Respiratory Pathogen Detection Kit (Ningbo Haishi Gene Technology Co., Ltd., Ningbo, China). Eleven sets of specific primers were employed, and one-step reverse transcription polymerase chain reaction (RT-PCR) was performed in a single tube to amplify target fragments. The nucleic acid samples were amplified through a series of RT-PCR steps: first, pretreatment at 25℃ (5 min) for one cycle, reverse transcription at 50℃ (15 min) for one cycle, and pre-denaturation at 95℃ (2 min) for one cycle; secondly, denaturation at 94℃ (30 s), annealing at 65℃ (30 s), and extension at 72℃ (60 s) for six cycles, a step that was repeated until the annealing temperature reached 60℃; there was a 1℃ touchdown every six cycles. third, denaturation at 94℃ (30 s), annealing at 60℃ (30 s), and extension at 72℃ (60 s) for 29 cycles; finally, the products were extended at 72℃ (10 min) for one cycle and were kept at 4℃ for one cycle. To separate amplification products of different lengths, we performed capillary electrophoresis and the GenomeLab GeXP Genetic Analysis System (Beckman Coulter). The samples were analyzed by trained professionals who then generated a report based on their findings.
Statistical analysis
Data are presented as number [n(%)] and were tested by Chi-squared tests with the Bonferroni correction method in SPSS Statistics for Windows, version 29.0 (IBMCorp., Armonk, N.Y., USA). Generalized linear regression was used to assess the relationships between independent and dependent variables while controlling for potential confounders. Then, we calculated odds ratios (ORs) with 95% confidence intervals (CIs). The models were adjusted for age, gender and season. Data were analyzed by R software (The R Foundation; http://www.r-project.org; version 4.2.1) and EmpowerStats software (www.empowerstats.net, X&Y solutions, Inc. Boston, Massachusetts). GraphPad Prism 9 software was used for mapping. A P-value < 0.05 was considered to be statistically different.
Results
General characteristics of the enrolled patients
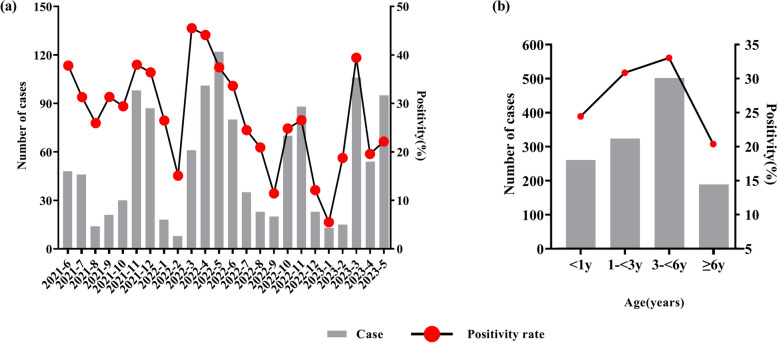
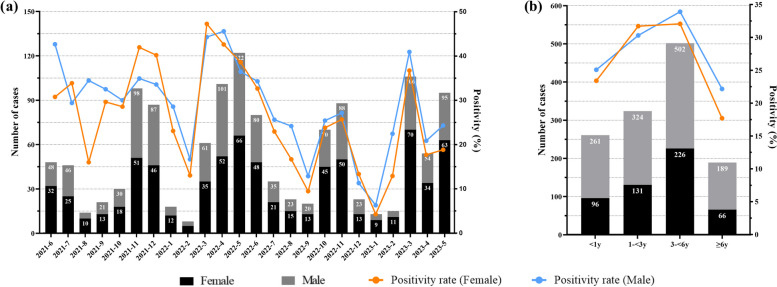
Of the 4565 cases included in our analysis, 1276 were positive for at least one virus, with a total positivity rate of 27.95%. There were 2664 males and 1901 females included in our analysis, with a mean age of 3.3 ± 2.9 years; 1068 were under 1 year-of-age, 1050 were 1–3 years-of-age, 1519 were 3–6 years-of-age, and 928 were over 6 years-of-age. Positive cases involved 757 (59.3%) males and 519 (40.7%) females. The positive rates of the 11 respiratory pathogen assays were 5.98% (HRSV), 5.67% (HRV), 4.38% (HADV), 3.68% (HNPV), 2.83% (HPIV), 2.74% (Flu A), 1.97% (H3N2), 1.82% (H1N1), 1.80% (Flu B), 1.10% (HBoV), and 0.35% (HCoV), respectively. There was no significant difference in gender between the groups in terms of positive pathogen detection = 0.41, P = 0.68), although the age groups showed a statistically significant difference ( = 57.05, P < 0.001, Table 1). As shown in Fig. 1, the number and positive rates of respiratory pathogen detection varied by season and age. The number and positive rate of respiratory virus testing exhibited a trough during winter, especially in January and February. However, the total positive rate of the 11 viral infections peaked in March on an annual basis. The positive rates of respiratory pathogen detection were highest in the 3–< 6 years-of-age group. There are significant differences in respiratory virus infection rates from June 2021 to May 2024 based on gender and age (P < 0.001), as illustrated in Fig. 2. The infection proportions for both males and females, along with various age groups, exhibit significant variation across the months (Supplemental Table E1 and E2). Specifically, females showed higher infection rates in July 2021, November 2021, December 2021, March 2022, May 2022, and December 2022. In contrast, males had higher infection rates in the remaining months. And except for the 1-<3 years age group, male infection rates were generally higher.
Table 1.
General characteristics of children infected with respiratory pathogens
| Flu A | H1N1 | H3N2 | Flu B | HPIV | HADV | HBoV | HRV | HNPV | HCoV | HRSV | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | n = 125 | n = 83 | n = 90 | n = 82 | n = 129 | n = 200 | n = 50 | n = 259 | n = 168 | n = 16 | n = 273 | |
| Sex (Male) | 80 (64) | 56 (67.47) | 55 (61.11) | 41 (50) | 76 (58.91) | 122 (61) | 29 (58) | 151 (58.3) | 95 (56.55) | 8 (50) | 167 (61.17) | 0.609 |
| Age (years) | ||||||||||||
| < 1 | 17 (13.6) | 10 (12.05) | 11 (12.22) | 6 (7.32) | 29 (22.48) | 26 (13) | 5 (10) | 65 (25.1) | 20 (11.9) | 4 (25) | 101 (37) | < 0.001 |
| 1-< 3 | 18 (14.4) | 16 (19.28) | 11 (12.22) | 10 (12.2) | 42 (32.56) | 55 (27.5) | 23 (46) | 72 (27.8) | 36 (21.43) | 3 (18.75) | 75 (27.47) | |
| 3-< 6 | 54 (43.2) | 27 (32.53) | 44 (48.89) | 39 (47.56) | 51 (39.53) | 78 (39) | 22 (44) | 81 (31.27) | 102 (60.71) | 6 (37.5) | 83 (30.4) | |
| ≥ 6 | 36 (28.8) | 30 (36.14) | 24 (26.67) | 27 (32.93) | 7 (5.43) | 41 (20.5) | 0 (0) | 41 (15.83) | 10 (5.95) | 3 (18.75) | 14 (5.13) |
The information provided is displayed as a percentage (n). The chi-squared examination for discrete variables. InfA is an abbreviation for influenza A, H1N1 refers to influenza A H1N1 virus, H3N2 represents seasonal influenza A H3N2 virus, Flu B stands for influenza B virus, HPIV denotes parainfluenza virus, HADV signifies adenovirus, HBoV indicates bocavirus, HRV represents rhinovirus, HNPV refers to metapneumovirus, HCoV stands for coronavirus, and HRSV represents respiratory syncytial virus
Fig. 1.
The detection of pathogens in children with acute respiratory infections. a Monthly distributions of the pathogens detected, and b the distribution of pathogens by age
Fig. 2.
The detection of pathogens in children with acute respiratory infections by gender. a Monthly distributions of the pathogens detected, and b the distribution of pathogens by age
Seasonal, age and gender distribution of various respiratory pathogens
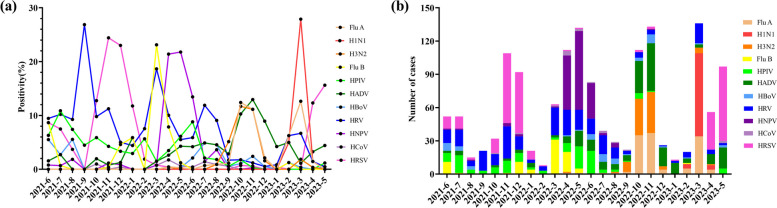
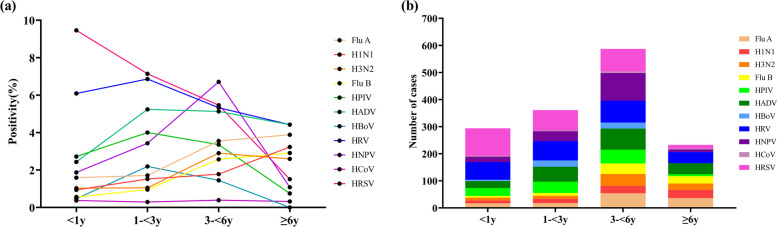
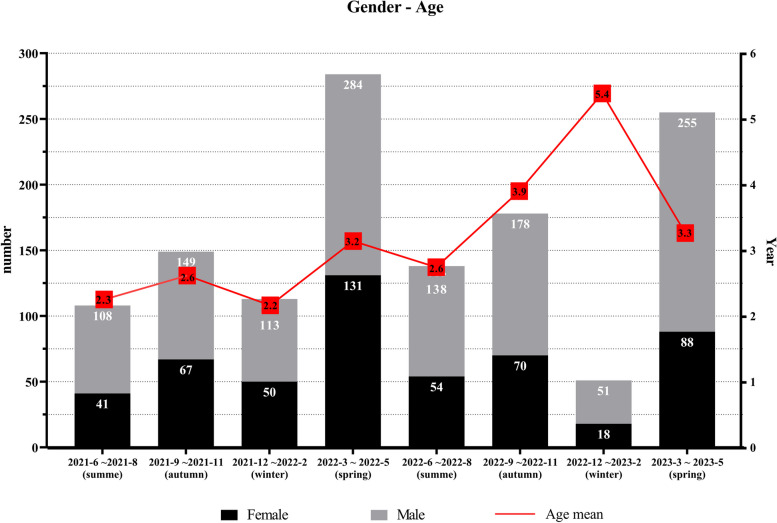
As shown in Fig. 3, the positivity rate and number of HADV detections exhibited multiple peaks in various months, and were highest during winter (October and November) in 2022, especially in terms of FluA and H3N2. The HRSV peak occurred in November and December 2021 but fell rapidly in January. Thereafter, the prevalence of HRSV remained low until April 2023. In 2021, the first wave of HRV peaked in September, followed by a second and third wave in March and July 2022, but exhibited a clear trough in December and January every year. The positivity of FluB peaked in March 2022 whereas H1N1 peaked in the same month in 2023. Subsequently, peak HNPV positivity was detected in spring and summer (April to June) in 2022. HRSV and HRV predominated in the < 3 years-of-age group (Fig. 4). HNPV were common in the 3–6 years-of-age group, whereas HBoV and HRV were predominant in the ≥ 6 years-of-age group. Figure 5 illustrates the trends in respiratory virus infections, highlighting the gender distribution and mean age of children with viral respiratory tract infections across different seasons. The mean age indicates a general upwards trend, suggesting that the mean age of infected individuals tended to increase over time, reaching a peak of 5.3 years between Dec 2022 to Feb 2023, coinciding with peak infection rates.
Fig. 3.
Monthly distributions showing the detection of 11 respiratory pathogens: a the positive rate of pathogen detection, and b the number of pathogens
Fig. 4.
The detection of eleven respiratory pathogens in children stratified by age: a the positivity rate for pathogenic detection, and b the number of pathogens detected
Fig. 5.
Gender Distribution and Mean Age of Children with Viral Respiratory Tract Infections by each Season (June 2021 to May 2024)
Single/co-infections and association between age/gender and type of virus infection
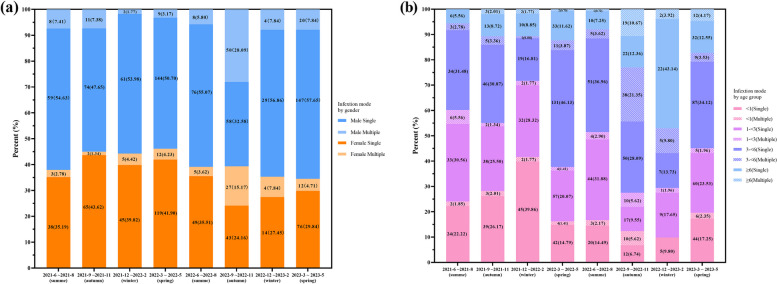
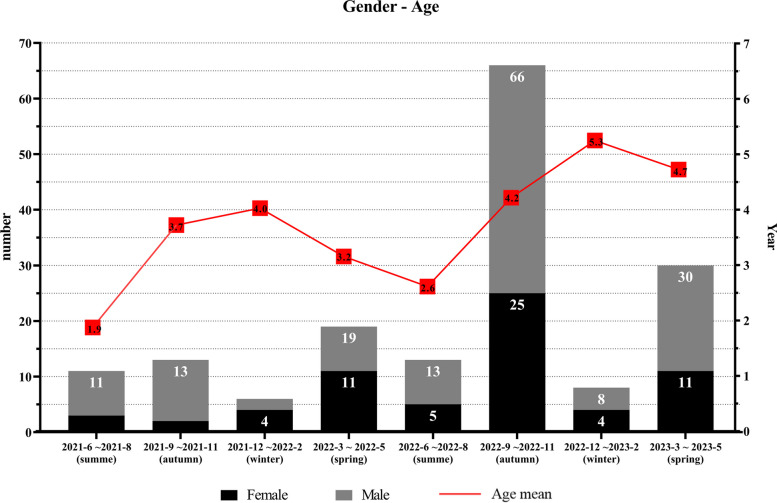
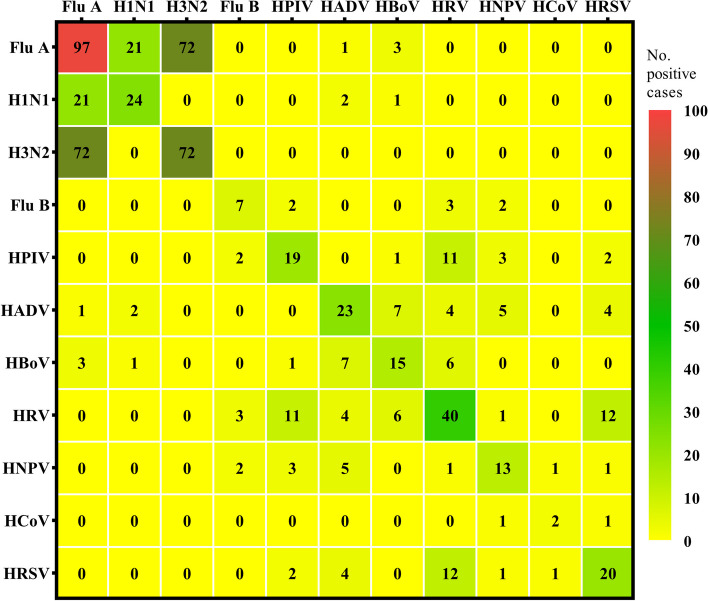
The proportion of viral infection among the 1276 virus-positive children, and the number of samples tested for 11 types of viruses, was categorized quarterly, along with their proportion of total samples, as shown in Supplemental Table E3. Overall, from June 2021 to May 2024, single virus infections consistently dominated across all seasons. We also observed that the proportion of single virus infections in boys was significantly higher than that in girls We also found that multiple infections significantly increased between September and November 2022, particularly among males and children over 3 years-of-age (Fig. 6). In other periods, the proportion of dual infections was relatively low, with the lowest occurrence observed from November 2021 to February 2022 (Supplemental Table E4 and Fig. 7). The most common co-infection of two respiratory viruses was Flu A and H3N2, followed closely by Flu A and H1N1 (Fig. 8). After stratifying by gender and age, a comparison of the number and proportion of dual infections revealed that dual infections were significantly more common in children under 6 years of age, with boys being more likely to be infected than girls (Supplemental Table E5). In addition, the number of triple virus infections rose significantly between September and November 2022, with minimal occurrences in other time frames. A summary of the patterns of triple and quadruple infections can be found in Supplemental Table E6. Notably, during the same period, a 5-year-old girl tested positive for four viruses simultaneously for the first time: Flu A, H3N2, HPIV, and HADV.
Fig. 6.
Percentage of single and multiple virus detections by season, gender, and age group (June 2021 to May 2024). a The number and percentage of single virus and multiple virus tests for boys and girls across each season. b The number and percentage of single virus and multiple virus tests categorized by four age groups for each season
Fig. 7.
Gender Distribution and Mean Age of Children with dual Viral Respiratory Tract Infections by each Season (June 2021 to May 2024)
Fig. 8.
Heat map shows co-infection of two respiratory viruses
The associations between age/season/gender and various respiratory pathogens
As shown in Tables 2 and 3, multivariate regression analysis revealed that FluA infections occurred more often among children aged ≥ 6 years-age and in autumn (P < 0.01); H1N1 was also commonly found in children aged ≥ 6 years-of-age and occurred more commonly in spring (P < 0.01). H3N2 predominated in children aged 3–6 years and majorly in the autumn season (P < 0.01); FluB was commonly detected in children aged ≥ 6 years-of-age and was mostly detected during spring (P < 0.01). HPIV was mostly detected in children aged < 3 years-of-age and mainly during summer (P < 0.01). HADV was mostly detected in children aged < 3 years-of-age and tended to peak in the autumn (P < 0.01). HBoV was commonly detected in children aged 1–3 years-of-age and mainly in summer (P < 0.01). HRV was mostly detected in children aged < 3 years-of-age and peaked in the summer (P < 0.01). HNPV was most commonly detected in children aged 3–6 years-of-age and in spring (P < 0.01). HCoV was mostly detected in children aged < 1 year-of-age and ≥ 6 years-of-age (P < 0.01) but peaked in spring. HRSV was most commonly detected in children aged < 1 year-of-age and during the autumn and winter (P < 0.01). Table 4 shows a significant relationship between gender and the risk of respiratory virus infections. For all viruses except Flu B, HRV, and HCoV, females exhibited lower odds ratios (ORs), thus suggesting a reduced risk of infection compared to males, although this difference was not statistically significant.
Table 2.
Multivariable-adjusted associations between age and respiratory pathogen infections
| Flu A | H1N1 | H3N2 | Flu B | HPIV | HADV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aOR | P | aOR | P | aOR | P | aOR | P | aOR | P | aOR | |
| Age(years) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | |||||
| <1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| (reference) | (reference) | (reference) | (reference) | (reference) | (reference) | ||||||
| 1-<3 | 1.142 | 0.699 | 1.483 | 0.336 | 1.145 | 0.756 | 1.621 | 0.353 | 1.33 | 0.251 | 2.357 |
| 0.582~2.239 | 0.665~3.309 | 0.489~2.681 | 0.585~4.491 | 0.818~2.163 | 1.462~3.800 | ||||||
| 3-<6 | 1.991 | 0.015 | 1.531 | 0.257 | 2.513 | 0.008 | 4.572 | 0.001 | 1.22 | 0.405 | 2.214 |
| 1.141~3.437 | 0.733~3.201 | 1.277~4.945 | 1.917~10.904 | 0.764~1.950 | 1.404~3.493 | ||||||
| ≥6 | 2.013 | 0.02 | 4.937 | <0.001 | 1.713 | 0.148 | 6.659 | <0.001 | 0.289 | 0.004 | 1.661 |
| 1.115~3.633 | 2.37~10.285 | 0.826~3.555 | 2.728~16.257 | 0.125~0.665 | 1.005~2.745 | ||||||
| p | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| HBoV | HRV | HNPV | HCoV | HRSV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | aOR | P | aOR | P | aOR | P | aOR | P | aOR | P | |
| Age(years) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | ||||||
| <1 | 1 | 1 | 1 | 1 | 1 | ||||||
| (reference) | (reference) | (reference) | (reference) | (reference) | |||||||
| 1-<3 | <0.001 | 4.015 | 0.005 | 1.045 | 0.804 | 1.558 | 0.12 | 0.667 | 0.597 | 0.793 | 0.15 |
| 1.510~10.674 | 0.737~1.483 | 0.891~2.724 | 0.148~2.999 | 0.579~1.087 | |||||||
| 3-<6 | 0.001 | 3.072 | 0.025 | 0.812 | 0.23 | 3.22 | <0.001 | 0.928 | 0.909 | 0.567 | <0.001 |
| 1.152~8.191 | 0.578~1.141 | 1.97~5.264 | 0.259~3.32 | 0.417~0.770 | |||||||
| ≥6 | 0.048 | 0 | 0.99 | 0.726 | 0.122 | 0.749 | 0.462 | 1.014 | 0.986 | 0.138 | <0.001 |
| 0~. | 0.485~1.089 | 0.347~1.618 | 0.224~4.581 | 0.078~0.243 | |||||||
| p | < 0.001 | < 0.001 | < 0.001 | 0.467 | < 0.001 |
The age and season were taken into account when adjusting the model. Abbreviations used include InfA for influenza A, H1N1 for influenza A H1N1 virus, H3N2 for seasonal influenza A H3N2 virus, Flu B for influenza B virus, HPIV for parainfluenza virus, HADV for adenovirus, HBoV for bocavirus, HRV for rhinovirus, HNPV for metapneumovirus, HCoV for coronavirus, and HRSV for respiratory syncytial virus
Table 3.
Multivariable-adjusted associations between season and respiratory pathogen infections
| Flu A | H1N1 | H3N2 | Flu B | HPIV | HADV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | P | OR | P | OR | P | OR | P | OR | P | OR | |
| Season | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | |||||
| Spring | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| (reference) | (reference) | (reference) | (reference) | (reference) | (reference) | ||||||
| Summer | 0.104 | < 0.01 | 0 | 0.99 | 0.31 | 0.27 | 0.487 | 0.03 | 3.268 | < 0.01 | 1.077 |
| 0.025~0.433 | 0~. | 0.038~2.529 | 0.258~0.92 | 2.112~5.056 | 0.675~1.719 | ||||||
| Autumn | 2.515 | < 0.01 | 0 | 0.99 | 16.487 | < 0.01 | 0.021 | < 0.01 | 1.211 | 0.46 | 2.254 |
| 1.689~3.744 | 0~. | 7.556~35.974 | 0.003~0.153 | 0.727~2.017 | 1.575~3.226 | ||||||
| Winter | 0.444 | 0.03 | 0.075 | <0.01 | 0.938 | 0.93 | 0.569 | 0.06 | 0.827 | 0.57 | 1.568 |
| 0.213~0.927 | 0.027~0.208 | 0.241~3.656 | 0.317~1.021 | 0.432~1.583 | 1.018~2.417 | ||||||
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| HBoV | HRV | HNPV | HCoV | HRSV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | OR | P | OR | P | OR | P | OR | P | OR | P | |
| Season | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | ||||||
| Spring | 1 | 1 | 1 | 1 | 1 | ||||||
| (reference) | (reference) | (reference) | (reference) | (reference) | |||||||
| Summer | 0.76 | 12.877 | < 0.01 | 1.673 | < 0.01 | 0.719 | 0.08 | 0.919 | 0.89 | 0.434 | < 0.01 |
| 4.97~33.365 | 1.212~2.309 | 0.497~1.039 | 0.281~3.008 | 0.275~0.683 | |||||||
| Autumn | < 0.01 | 4.106 | < 0.01 | 1.089 | 0.61 | 0.046 | < 0.01 | 0.293 | 0.12 | 1.219 | 0.203 |
| 1.44~11.706 | 0.786~1.509 | 0.017~0.124 | 0.062~1.376 | 0.899~1.653 | |||||||
| Winter | 0.04 | 1.061 | 0.94 | 0.562 | 0.01 | 0.039 | < 0.01 | 0.206 | 0.137 | 1.27 | 0.156 |
| 0.204~5.511 | 0.361~0.875 | 0.01~0.158 | 0.026~1.654 | 0.913~1.765 | |||||||
| P | < 0.001 | < 0.001 | < 0.001 | 0.535 | < 0.001 |
The age and season were taken into account when adjusting the model. Abbreviations used include InfA for influenza A, H1N1 for influenza A H1N1 virus, H3N2 for seasonal influenza A H3N2 virus, Flu B for influenza B virus, HPIV for parainfluenza virus, HADV for adenovirus, HBoV for bocavirus, HRV for rhinovirus, HNPV for metapneumovirus, HCoV for coronavirus, and HRSV for respiratory syncytial virus
Table 4.
Multivariable-adjusted associations between season and respiratory pathogen infections
| Flu A | H1N1 | H3N2 | Flu B | HPIV | HADV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | P | OR | P | OR | P | OR | P | OR | P | OR | |
| Gender | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | |||||
| Male | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| (reference) | (reference) | (reference) | (reference) | (reference) | (reference) | ||||||
| Female | 0.749 | 0.13 | 0.679 | 0.109 | 0.84 | 0.436 | 1.371 | 0.163 | 0.962 | 0.831 | 0.871 |
| 0.515~1.089 | 0.422~1.091 | 0.542~1.302 | 0.880~2.134 | 0.671~1.377 | 0.651~1.167 | ||||||
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| HBoV | HRV | HNPV | HCoV | HRSV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | OR | P | OR | P | OR | P | OR | P | OR | P | |
| Gender | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | ||||||
| Male | 1 | 1 | 1 | 1 | 1 | ||||||
| (reference) | (reference) | (reference) | (reference) | (reference) | |||||||
| Female | 0.356 | 0.991 | 0.974 | 1.008 | 0.951 | 0.991 | 0.956 | 1.387 | 0.515 | 0.901 | 0.420 |
| 0.559~1.757 | 0.781~1.302 | 0.720~1.364 | 0.518~3.714 | 0.699~1.161 | |||||||
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
The age and season were taken into account when adjusting the model. Abbreviations used include InfA for influenza A, H1N1 for influenza A H1N1 virus, H3N2 for seasonal influenza A H3N2 virus, Flu B for influenza B virus, HPIV for parainfluenza virus, HADV for adenovirus, HBoV for bocavirus, HRV for rhinovirus, HNPV for metapneumovirus, HCoV for coronavirus, and HRSV for respiratory syncytial virus
Discussion
Between early 2020 and December 2022, a wide range of stringent non-pharmaceutical interventions (NPIs) were used to combat the COVID-19 pandemic, including mask-wearing, school closures, and social distancing. This strategy not only reduced the transmission of the Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) but also influenced the prevalence patterns of other common respiratory viruses [10]. For instance, social distancing, mask-wearing, and enhanced hygiene practices contributed to a marked decline in the circulation of influenza and other respiratory viruses, which typically follow seasonal patterns. Health data from various regions showed a significant drop in flu cases during periods when COVID-19 restrictions were in place, thus suggesting that the interventions for controlling the pandemic inadvertently suppressed the transmission of these other viruses. This phenomenon has provided a unique opportunity to study the effects of public health strategies on a range of respiratory illnesses, potentially offering insights into more effective approaches to manage viral diseases in the future. Due to the strict implementation of these NPIs, the incidence of SARS-CoV-2 was significantly reduced, and individuals infected with the virus were promptly transferred to specialized infectious disease hospitals for appropriate medical care. Consequently, the scope of this study on hospitalized children did not involve individuals diagnosed with COVID-19.
The rigorous enforcement of NPIs effectively curbed the transmission of SARS-CoV-2 [11]. As a result, the infection rate of other respiratory pathogens typically linked to acute respiratory infections was reduced further, dropping to 27.9% compared to its previous value prior to the COVID-19 pandemic. In this study, we found that the highest positivity rates for respiratory pathogens were observed for HRSV, HRV, and HADV, at 5.98%, 5.67%, and 4.38%, respectively. This study reveals that even amidst the implementation of NPIs in our region, HRSV infections continued to demonstrate significant recurrence, accompanied by pronounced epidemic peaks. It is noteworthy that with the gradual implementation of NPIs, HRSV infections experienced a prolonged period of low incidence; however, they remain prone to potential seasonal outbreaks or epidemics. During this period, however, rhinoviruses experienced three epidemic peaks. Although the positive rate of HRV infections has been declining, the potential for a resurgence of HRV outbreaks remains uncertain following the relaxation of these measures. Additionally, it is important to note that HAD exhibited a gradually increasing positive rate during the later stages of the NPIs, reaching a peak in the winter of 2022 (October and November). Previous research conducted in South Korea indicates that under clinical surveillance systems, the scale of localized transmission of HRSV and six other viruses, including HRV, is approximately half of what it was prior to the NPIs [12]. Additionally, reports from Taiwan highlight instances of HRSV infections occurring post-relaxation of NPIs, despite strict limitations on international entry, with no concurrent influenza outbreaks observed [13]. Interestingly, the impact of NPIs was not uniform across all common respiratory viruses: rhinoviruses and adenoviruses continued to circulate in Australia during the pandemic, even after the initial disruption of normal transmission [14]. However, during the implementation of COVID-19-related restrictions, studies found that the genetic diversity of RSV in Australia significantly decreased [15]. Conversely, epidemiological modeling studies conducted in the United States by Baker et al. [16]. suggested that prolonged NPI periods may lead to an accumulation of infants susceptible to HRSV, thereby increasing the likelihood of localized transmission post-NPIs. This underscores the necessity for ongoing and vigilant monitoring of HRSV recurrence in real-world scenarios, particularly in the context of lifting NPIs. Simultaneously, we observed that in the later stages of the NPIs, peaks of Influenza A virus and H3N2 infections also emerged. Notably, these two viruses were the most prevalent in the dual infection cases identified in this study, indicating a clear co-infection phenomenon. This suggests that as NPIs were relaxed, the interactions between different respiratory viruses intensified, leading to an increased incidence of co-infections. The concurrent peaks of Influenza A and H3N2 highlight the potential for complex epidemiological dynamics in the post-NPIs environment, raising concerns about the implications for public health and the management of respiratory viral infections. Understanding these interactions is crucial for developing effective strategies to monitor and control viral infections in populations, especially during the respiratory virus season.
These findings align with a previous study conducted in Shanghai, which reported a similar reduction in the infection rate of viral respiratory pathogens to 27.5% [17]. In the present study, our analyses revealed that HRSV, HRV, and HADV were the most prevalent pathogens detected in children suffering from LRTIs, rather than HIPV and HMPV [18]. This finding suggests that the implementation of NPIs may have influenced the prevalence and pathogenicity of common respiratory pathogens, such as influenza, RSV, rhinovirus, and adenovirus. The observed reduction in infection rates and the overall impact on the transmission dynamics of these viruses highlight the potential broader effects of NPIs on respiratory health beyond their intended target of SARS-CoV-2. This has significant implications for public health strategies and warrants further investigation into the long-term consequences of pandemic control measures on the epidemiology of respiratory infections.
In addition, our analyses confirmed the prevalence of common respiratory pathogens during the winter and spring seasons; this information was consistent with previous research findings [19]. Based on the significant differences in respiratory virus infection rates found in this study regarding gender and age, it was first noted that males had higher infection rates in most months, which may be related to biological response differences in virus infections between genders. For example, the immune system of males may be more easily suppressed than that of females in certain situations. Additionally, male children may participate in outdoor activities more frequently, increasing their chances of exposure to viruses, which may further lead to an increase in infection rates. In the analysis of age groups, it was found that, except for the 1-<3 years age group, males generally had higher infection rates in other age groups. Furthermore, we observed that these occurrences were more prominent in children aged 3–6 years-of-age. This could be attributed to the fact that children in this age group had recently started attending kindergarten and engaging in collective activities, thus making it challenging for them to consistently adhere to NPIs during outdoor activities [20].
The rate of respiratory viral infections is usually seasonal [21], with peaks occurring during specific times of the year, often in the fall and winter months. The epidemiology of these infections can vary across different countries and populations [22, 23], and is influenced by factors such as climate, population density, social behaviors, and healthcare infrastructure. For example, in temperate regions, influenza and RSV infections typically exhibit distinct seasonal patterns, while in tropical regions, the patterns may differ due to less pronounced seasonal variations. In addition, variations in vaccination coverage, age distribution, and immune status within populations can further impact the epidemiological patterns of respiratory viral infections. Understanding these patterns in more detail is crucial for developing targeted public health interventions and optimizing healthcare resources to effectively mitigate the impact of respiratory pathogens. Moreover, the pattern of viral spread usually occurs between October, November, and March, with peak incidence in January and February [24]. In the present study, localized outbreaks of influenza viruses were observed in October, November, and March, with different circulating strains each year. However, unlike previous epidemics, we revealed the underestimation of cases in both January and February. This underestimation was identified by the comprehensive analysis of surveillance data, including clinical testing, hospital admissions, and community-based monitoring. The general findings of this study align with previous research [25–27], as HRSV and HRV were identified as the most detectable respiratory pathogens during this period. The underestimation of cases in January and February may be attributed to factors such as limited testing capacity, asymptomatic or mild cases going unreported, and challenges in differentiating COVID-19 from other respiratory infections based on clinical symptoms alone. This highlights the importance of robust surveillance systems and accurate diagnostic tools to capture the true burden of respiratory infections, especially during public health emergencies. Furthermore, it is worth noting that the peak prevalence of HRSV occurred between October and December 2021; this was followed by a sharp decline which did not continue into 2022. This observation raises the possibility of interference between respiratory viruses, where the presence of one virus affects the transmission or pathogenicity of another [28]. Interference in this context may be caused by a phenomenon known as viral interference, where the immune response triggered by one virus can impede the replication or spread of a different virus [24]. Furthermore, competition for susceptible hosts and resources within the host’s respiratory tract may also contribute to the observed patterns of viral prevalence. Understanding the mechanisms of interference and competition between respiratory viruses is essential for elucidating the complex dynamics of co-circulating pathogens and their implications for public health interventions and vaccine development. In addition, it is possible that the peak of SARS-CoV-2 infection during the summer months could have potentially influenced the prevalence of H1N1 [29]. Furthermore, previous research has shown that HRSV tends to circulate more among infants and exhibits a decreasing trend as age increases [28]. This suggests that infants and young children are more susceptible to HRSV infections, while older individuals may have acquired immunity or reduced susceptibility due to previous exposure. Understanding the age-related patterns of HRSV circulation is crucial for targeting vaccination efforts and implementing preventive measures to protect the most vulnerable populations, particularly infants and young children, from severe respiratory illness caused by HRSV. This suggests that NPIs may not have exerted a significant influence on the seasonality and population characteristics of HRSV. However, this does not rule out the possibility that the stricter enforcement of NPIs could be associated with increasing age. It is important to consider the potential impact of NPIs on different age groups and population subgroups when evaluating their effectiveness in mitigating the transmission of respiratory viruses. In addition, the interplay between NPIs, age-related immunity, and viral circulation dynamics warrants further investigation to better understand the complex interactions shaping the epidemiology of HRSV and inform public health strategies aimed at controlling respiratory infections.
In contrast, HRV can be detected in almost every season; this is because HRV is a non-enveloped virus, and is relatively resistant to ethanol-containing disinfectants [30]; furthermore, this virus can survive on environmental surfaces over a prolonged period of time [31]. In this study, we found that HRV was prevalent throughout the year, except for the winter, and was common in all age groups. Prior to the COVID-19 epidemic, higher hospitalization rates were observed in years where the predominant circulating virus was influenza in southeast China [32]. However, with the implementation of NPIs, the dominant strains of influenza virus have not changed [33], although there has been a significant decline in the detection rate over time, which is now reported to be 6.4%. Similar trends have been observed in other areas such as Shanghai [17], Hong Kong [34], and New Zealand [35], where the implementation of NPIs has resulted in a reduction in the detection rate of influenza virus.
There are some limitations to our study that should be considered. Firstly, we did not take into account bacterial infections and infections caused by atypical pathogens. Atypical pathogens are agents that cause respiratory infections but are not detected by standard bacterial cultures or methods, often requiring specific serological or molecular tests for diagnosis. Examples include Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila. These organisms can lead to clinical presentations that are similar to those caused by typical respiratory pathogens, and their exclusion from our analysis could have potentially contributed to an underestimation of the total burden of respiratory infections recorded in our data. Secondly, the observation period was not extensive enough to provide a comprehensive understanding of the trends and dynamics of respiratory infections over time. Additionally, patients with comorbidities, such as high body mass index (BMI), asthma, and hay fever were not excluded from the analysis. Our primary focus was the trends of pathogen prevalence; the collection of comprehensive data regarding comorbidities was limited, particularly among hospitalized patients. This limitation may affect the interpretation of our results, as the presence of these conditions could influence the severity of respiratory infections and the associated pathogen profiles.
In conclusion, this study highlights the need for age, gender and season-specific surveillance and prevention strategies, which could lead to more effective control of respiratory infections in pediatric populations. To build on the knowledge from this study, future research should aim to explore the underlying mechanisms that contribute to the observed variations in pathogen prevalence, such as differences in immunity, social behavior, and environmental factors. Additionally, longitudinal studies could provide a better understanding of the long-term health impacts of these infections and the effectiveness of interventions over time. The development of more targeted vaccines and treatment protocols, considering these demographic factors, could also be an essential next step in reducing the disease burden among children.
Conclusion
Our analysis found that the prevalence of respiratory pathogen infections varied by age, gender and season in the enrolled population of children. The highest positivity rates were observed for HRSV, HRV, and HADV. Influenza A and H3N2 were more common in the autumn season, while H1N1 and influenza B were more common in the spring. HPIV and HADV were more prevalent in children under 3 years-of-age, while HBoV and HRV predominated in children aged 1–3 years-of-age. The findings highlight the importance of considering gender, season, and age when studying respiratory pathogen infections in children.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- ADV
Adenovirus
- BMI
Body mass index
- H1N1
Influenza A H1N1 virus
- CIs
Confidence intervals
- H3N2
Seasonal influenza A H3N2 virus
- HADV
Adenovirus
- HBoV
Bocavirus
- HCoV
Human coronavirus
- HMPV
Human metapneumovirus
- HNPV
Metapneumovirus
- HPIV
Human parainfluenza virus
- HRV
Rhinovirus
- HRSV
Human respiratory syncytial virus
- InfA
Influenza A
- InfB
Influenza B
- LRTIs
Lower respiratory tract illnesses
- NPIs
Non-pharmaceutical interventions
- ORs
Odds ratios
- PCR
Polymerase chain reaction
- RT-PCR
Reverse transcription polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
Authors’ contributions
Acquisition of data: S.L., Y.F, X.Z., Q.Y. and Z.X; Analysis and interpretation of data: S.L. and N.F.; Drafting the article: S.L and Z.X.; Critical revision of the manuscript for important intellectual content: S.L, Z.X. and Y.L. All authors approved the final version of the manuscript submitted.
Funding
This study was supported by the Yan’an University Graduate Education Innovation Program (YCX2023121) and Bejing Health Alliance Charitable Foundation (B21181FN). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
The data of the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Yan’an University Affiliated Hospital (Ethics Approval No. S-S20230003). The requirement for informed consent was waived owing to the retrospective observational nature of the study. The decision not to require informed consent was upheld by the Ethics Committee of the Yan’an University Affiliated Hospital of Medicine. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lucion MF, Juárez MDV, Pejito MN, Orqueda AS, Bollón LR, Mistchenko AS, Gentile Á. Impact of COVID-19 on the circulation of respiratory viruses in a children’s hospital: an expected absence. Arch Argent Pediatr. 2022;120(2):99–105. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim Aet al: Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019:a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–1222. [DOI] [PMC free article] [PubMed]
- 3.Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyu HH, Vongpradith A, Sirota SB, Novotney A, Troeger CE, Doxey MC, Bender RG, Ledesma JR, Biehl MH, Albertson SB, et al. Age-sex differences in the global burden of lower respiratory infections and risk factors, 1990–2019: results from the global burden of Disease Study 2019. Lancet Infect Dis. 2022;22(11):1626–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marciniuk D, Schraufnagel D, Ferkol T, Fong K, Joos G, Varela V. Forum of International Respiratory Societies. The Global Impact of Respiratory Disease–Second Edition. In. Sheffield: European Respiratory Society; 2017.
- 6.Lin W-H, Chiu H-C, Chen K-F, Tsao K-C, Chen Y-Y, Li T-H, Huang Y-C, Hsieh Y-C. Molecular detection of respiratory pathogens in community-acquired pneumonia involving adults. J Microbiol Immunol Infect. 2022;55(5):829–37. [DOI] [PubMed] [Google Scholar]
- 7.Rappo U, Schuetz Audrey N, Jenkins Stephen G, Calfee David P, Walsh Thomas J, Wells Martin T, Hollenberg James P, Glesby Marshall J. Impact of early detection of respiratory viruses by Multiplex PCR assay on clinical outcomes in adult patients. J Clin Microbiol. 2016;54(8):2096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sim JY, Chen Y-C, Hsu W-Y, Chen W-Y, Chou Y, Chow JC, Lai Y-C, Tang H-J, Chen C-C, Ho C-H, et al. Circulating pediatric respiratory pathogens in Taiwan during 2020: dynamic change under low COVID-19 incidence. J Microbiol Immunol Infect. 2022;55(6):1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato M, Takashita E, Katayose M, Nemoto K, Sakai N, Fujisaki S, Hashimoto K, Hosoya M. Clinical and Virologic impacts of respiratory viral co-infections in Children with Influenza. Pediatr Infect Dis J. 2023;42(8):e268–73. [DOI] [PubMed] [Google Scholar]
- 10.Lynch JP III, Kajon AE. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and advances in treatment and Prevention. Semin Respir Crit Care Med. 2016;37(04):586–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi HJ, Kim NY, Eom SA, Kim-Jeon MD, Oh SS, Moon BS, Kwon MJ, Eom JS. Effects of non-pharmacological interventions on respiratory Viruses Other Than SARS-CoV-2: Analysis of Laboratory Surveillance and Literature Review from 2018 to 2021. jkms. 2022;37(21):e172–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Kim HY, Lee M, Ahn JG, Baek JY, Kim MY, Huh K, Jung J, Kang JM. Respiratory Syncytial Virus Outbreak without Influenza in the Second Year of the Coronavirus Disease 2019 pandemic: a National Sentinel Surveillance in Korea, 2021–2022 season. J Korean Med Sci. 2022;37(34):e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Roh YH, Ahn JG, Kim MY, Huh K, Jung J, Kang J. M. respiratory syncytial virus and influenza epidemics disappearance in Korea during the 2020–2021 season of COVID-19. Int J Infect Dis. 2021;110:29–35. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan SG, Carlson S, Cheng AC, Chilver MB, Dwyer DE, Irwin M, Kok J, Macartney K, MacLachlan J, Minney-Smith C, Smith D, Stocks N, Taylor J. Barr, I. G. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. 2020;25:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eden JS, Sikazwe C, Xie R, Deng YM, Sullivan SG, Michie A, Levy A, Cutmore E, Blyth CC, Britton PN, Crawford N, Dong X, Dwyer DE, Edwards KM, Horsburgh BA, Foley D, Kennedy K, Minney-Smith C, Speers D, Tulloch RL. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat Commun. 2022;13(1):2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci U S A. 2020;117(48):30547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu M, Liu P, Su L, Cao L, Zhong H, Lu L, Jia R, Xu J. Comparison of respiratory pathogens in Children with Lower Respiratory Tract infections before and during the COVID-19 pandemic in Shanghai, China. Front Pead. 2022;10:881224. [DOI] [PMC free article] [PubMed]
- 18.Xin W, You L, Xin M, Erin B, Harry C, Harish N. Global hospital admissions and in-hospital mortality associated with all-cause and virus-specific acute lower respiratory infections in children and adolescents aged 5–19 years between 1995 and 2019: a systematic review and modelling study. BMJ Global Health. 2021;6(7):e006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Reeves RM, Wang X, Bassat Q, Brooks WA, Cohen C, Moore DP, Nunes M, Rath B, Campbell H, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Global Health. 2019;7(8):e1031–45. [DOI] [PubMed] [Google Scholar]
- 20.Li ZJ, Yu LJ, Zhang HY, Shan CX, Lu QB, Zhang XA, Ren X, Zhang CH, Wang YF, Lin SH, et al. Broad impacts of Coronavirus Disease 2019 (COVID-19) pandemic on Acute Respiratory infections in China: an observational study. Clin Infect Dis. 2022;75(1):e1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scafetta N. Distribution of the SARS-CoV-2 pandemic and its monthly Forecast based on seasonal climate patterns. In: Int J Environ Res Public Health. 2020;17(10):3493. [DOI] [PMC free article] [PubMed]
- 22.Wu Z, Zhang R, Liu D, Liu X, Zhang J, Zhang Z, Chen S, He W, Li Y, Xu Y, et al. Acute respiratory distress syndrome caused by human adenovirus in adults: a prospective observational study in Guangdong, China. Front Med. 2022;8:791163. [DOI] [PMC free article] [PubMed]
- 23.Vashisht R, Mirzai S, Koval C, Duggal A. Adenovirus-associated acute respiratory distress syndrome: need for a protocol-based approach. Indian J Crit Care Med. 2020;24(5):367–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurskaya OG, Prokopyeva EA, Sobolev IA, Solomatina MV, Saroyan TA, Dubovitskiy NA, Derko AA, Nokhova AR, Anoshina AV, Leonova NV, et al. Changes in the etiology of Acute Respiratory infections among children in Novosibirsk, Russia, between 2019 and 2022: the impact of the SARS-CoV-2 Virus. Viruses. 2023;15(4):934. [DOI] [PMC free article] [PubMed]
- 25.Ding Q, Xu L, Zhu Y, Xu B, Chen X, Duan Y, Xie Z, Shen K. Comparison of clinical features of acute lower respiratory tract infections in infants with RSV/HRV infection, and incidences of subsequent wheezing or asthma in childhood. BMC Infect Dis. 2020;20(1):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sapra M, Kirubanandhan S, Kanta P, Ghosh A, Goyal K, Singh MP, Ratho RK. Respiratory viral infections other than SARS CoV-2 among the north Indian patients presenting with acute respiratory illness during the first COVID-19 wave. VirusDisease. 2022;33(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read JF, Bosco A. Decoding susceptibility to respiratory viral infections and Asthma Inception in Children. Int J Mol Sci. 2020;21(17):6372. [DOI] [PMC free article] [PubMed]
- 28.Cooksey GLS, Morales C, Linde L, Schildhauer S, Guevara H, Chan E, Gibb K, Wong J, Lin W, Bonin BJ. Severe acute respiratory syndrome coronavirus 2 and respiratory virus sentinel surveillance, California, USA, May 10, 2020–June 12, 2021. Emerg Infect Dis. 2022;28(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azzari C, Baraldi E, Bonanni P, Bozzola E, Coscia A, Lanari M, Manzoni P, Mazzone T, Sandri F, Checcucci Lisi G, et al. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital J Pediatr. 2021;47(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savolainen-Kopra C, Korpela T, Simonen-Tikka M-L, Amiryousefi A, Ziegler T, Roivainen M, Hovi T. Single treatment with ethanol hand rub is ineffective against human rhinovirus—hand washing with soap and water removes the virus efficiently. J Med Virol. 2012;84(3):543–7. [DOI] [PubMed] [Google Scholar]
- 31.Winther B, McCue K, Ashe K, Rubino J, Hendley JO. Rhinovirus contamination of surfaces in homes of adults with natural colds: transfer of virus to fingertips during normal daily activities. J Med Virol. 2011;83(5):906–9. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Zhang X, Shan W, Gao J, Hua J, Tian J, Ding Y, Zhang J, Chen L, Song Y. Influenza-associated hospitalization in children younger than 5 years of age in Suzhou, China, 2011–2016. Pediatr Infect Dis J. 2019;38(5):445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Chen L, Ding Y, Zhang J, Hua J, Geng Q, Ya X, Zeng S, Wu J, Jiang Y, et al. Viral etiology of medically attended influenza-like illnesses in children less than five years old in Suzhou, China, 2011–2014. J Med Virol. 2016;88(8):1334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowling BJ, Ali ST, Ng TWY, Tsang TK, Li JCM, Fong MW, Liao Q, Kwan MYW, Lee SL, Chiu SS, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5(5):e279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang QS, Wood T, Jelley L, Jennings T, Jefferies S, Daniells K, Nesdale A, Dowell T, Turner N, Campbell-Stokes P, et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun. 2021;12(1):1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of the current study are available from the corresponding author on reasonable request.