Abstract
Background
Bypass graft surgery is a key surgical intervention for ischemic heart disease (coronary bypass graft surgery) and critical limb ischemia (peripheral bypass graft surgery). Graft occlusion remains a significant clinical problem for both types. Further research into the pathobiological mechanisms of graft occlusion are needed in order to design targeted therapeutic strategies.
Methods
Three Large White female pigs (mean weight 52.3 +/- 4.4 kg) received general anaesthesia prior to surgery. The external jugular vein was harvested bilaterally, and a bilateral femoral peripheral arterial bypass was performed, with the superficial femoral artery permanently ligated. The grafts were interrogated immediately post operatively on-table using Medistim MiraQ transit time flowmetry system (Medistim, Oslo, Norway) to assess graft performance. On postoperative day three, the pigs were returned to the operating room, and the grafts were interrogated once again using transit time flowmetry.
Results
Six out of six (100%) successful bilateral EJV to femoral artery bypass grafts were performed. All pigs were successfully recovered, and returned to the operating room at postoperative day 3. The wounds were re-opened and the grafts were inspected. Postoperative graft assessment was performed with transit time flowmetry using the Medistim MiraQTM system (Medistim, Oslo, Norway), demonstrating all grafts were patent (100%).
Conclusion
This model may serve as a platform to gain further mechanistic insight into graft failure pathobiology. By combining a bilateral graft model with gold-standard transit time flowmetry, longitudinal experimentation of targeted therapeutic interventions to combat graft failure may be further studied with improved objectivity.
Keywords: Cardiovascular, Bypass, Thrombosis, Graft failure, Atherosclerosis
Background
Bypass graft surgery continues to be an important durable intervention for both peripheral arterial occlusive disease (infrainguinal or peripheral bypass grafting) and ischemic heart disease (coronary artery bypass grafting, or CABG). For peripheral bypass, autologous vein is the gold standard conduit. For CABG, revascularization using arterial conduits (internal mammary or radial arteries) offers superior long-term patency, although autologous vein is still frequently used, particularly for multivessel disease. Saphenous vein grafts have high failure rates, with 3–12% occluding before hospital discharge, 8–25% failing at 1 year, and only 50–60% remaining patent after a decade [1]. One- and 12-month failure rates for coronary bypass vein grafts approach 12% and 20%, and one-month failure rates for infrainguinal bypass grafts have been reported at 4.9%. These are predominantly due to occlusive thrombosis [2]. The use of antiplatelet agents, such as aspirin, have been shown to be beneficial in maintaining early graft patency [3].
Animal models are critical tools for the study of pathobiological mechanisms which cause graft failure, including endothelial injury, neointimal hyperplasia and atherosclerosis. Previous limitations of small animal models of bypass surgery include that they can be technically difficult to perform and reliably reproduce due to their small vascular anatomy, the relatively low volume of tissue available for analysis, and limited extent of intimal hyperplasia in certain species which can make interpreting the efficacy of any trialled therapeutic strategies difficult [4]. Furthermore, small animal models lack similarity to human physiology with regard to hemodynamics and are therefore primarily of mechanistic value [5].
This is in contrast with large animal models, including canine, porcine, and ovine models, which are limited in availability and costly to maintain, but are generally better able to mimic human physiology. Furthermore, porcine models of coronary and peripheral atherosclerosis offer further advantages over rodent models, such as genetic expression and development of high-risk atherosclerotic lesions which are similar to humans [6]. No animal model is perfectly suited to address all requirements, however, testing grafts in large animals is essential for evaluation of long-term patency, graft remodeling, and ultimately, clinical translation [7].
The aim of this study was to design a contemporary, large animal model of peripheral bypass surgery, performed bilaterally, in order to create a within-subjects platform for further mechanistic investigation of graft pathobiology. Given the widespread uptake of intraoperative transit time flowmetry (TTFM) in cardiovascular surgery as a means of quality control [8], contemporary models should also integrate this important validation tool. The bilateral graft design allows within-subject comparison of both explanted grafts at an early postoperative timepoint, and allows greater statistical power to be achieved for any given animal number compared to a between-subjects design. The use of external jugular vein as conduit and femoral artery as the target vessel allows minimal size mismatch. TTFM has been integrated in this model at both intraoperative and postoperative timepoints in order to match contemporary validation practices.
Methods
Full animal ethics review and approval was obtained (University of Queensland Animal Ethics Committee, AE000145). Three Large White female grower pigs were sourced and housed according to local ethical best practice guidelines for humane care of animals, including appropriate living space, access to enrichment food, bedding, and ad libitum access to water. Baseline representative characteristics of the pigs used for the study are shown in Table 1.
Table 1.
Representative baseline characteristics of study animals
| Parameter | Value | Reference range | Units |
|---|---|---|---|
| Number of animals (N) | 3 | ||
| Species | Large White pig background | ||
| Age | 13–14 | Weeks | |
| Gender | Female | ||
| Weight | 52.3 +/- 4.4 | Kg | |
| Hb | 96.7 +/- 14 | 110–160 | g/L |
| Plt | 341.0 +/- 102 | x10^9/L | |
| WCC | 14.4 +/- 3 | 11–22 | x10^9/L |
| Na | 144.0 +/- 3 | 140–150 | mmol/L |
| K | 6.0 +/- 1 | 4.7–7.1 | mmol/L |
| Urea | 3.5 | 3.0-8.5 | mmol/L |
| Cr | 0.1 | 0.09–0.24 | mmol/L |
| Protein | 49.7 +/- 7 | 79–89 | g/L |
| Albumin | 28.7 +/- 2 | 19–39 | g/L |
| Bilirubin | 3.3 | 0–17 | umol/L |
| ALP | 230.7 +/- 45 | 120–400 | IU/L |
| AST | 20.3 +/- 6 | 32–84 | IU/L |
| ALT | 40.0 +/- 1 | IU/L | |
| GGT | 37.0 +/- 9 | 10 to 60 | IU/L |
| PT | 13.4 | 11–12 | Seconds |
| APTT | 14.5 +/-1 | 34–39 | Seconds |
Where applicable, values are stated as mean +/- SD unless otherwise stated. Due to laboratory technical issues, some blood tests were repeated on separate animals of the same breed to complete the laboratory panel
Animal acquisition and housing
Pigs were sourced, transported, housed and allowed to acclimatize for a minimum of 5–7 days prior to surgery. Regular monitoring of animal health and welfare was performed by qualified personnel daily, with any concerns over animal health being escalated to a qualified Veterinarian. Animals deemed unfit to undergo surgery did not proceed to premedication or induction of anesthesia.
Anesthesia
The nominated pig was examined for fitness for anesthesia/surgery. Signs such as coughing, diarrhea, emaciation, weakness / lethargy, paleness, or severe skin lesions were discussed with a Consultant Veterinarian. Small wounds or transport diarrhoea were treated where necessary prior to study initiation. The animal was fasted 12 h prior to the scheduled procedure, and brought to the holding room inside a mobile cage prior to the injection of premedication. The weight of the pig was recorded in order to calculate drug dosages.
Premedication, induction and intubation
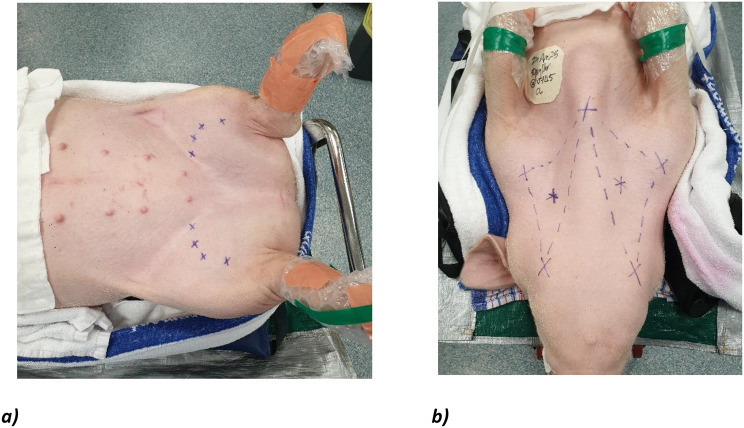
Pigs were administered midazolam (45 mg PO) 30 min prior to injectable premedication. An intramuscular injection of methadone (0.25 mg/kg), ketamine (25 mg/kg) and dexmedetomidine (0.01 mg/kg) were administered in the thigh using a 23G needle fitted to a syringe, and pigs were allowed to sedate for 10–15 min. Pigs were then mask ventilated with isoflurane. The lateral ear vein was cannulated with a 24G cannula and alfaxalone (1 mg/kg IV) was administered to allow for intubation. Monitoring including pulse oximetry, capnography, and gas monitoring was commenced. Pigs were intubated with a size 7.5–8.0 endotracheal tube. Pigs were scrubbed with soapy chlorhexidine to remove particulate material from the skin and prepared with chlorhexidine in 5% ethanol. Pigs were transferred to the operating room and positioned on the operating table in a dorsal recumbent position, and its limbs were immobilised using tape or gauze bandaging (Fig. 1).
Fig. 1.
Positioning of the pig in dorsal recumbent position. a) Ultrasound-guided marking of bilateral femoral arteries) and b) landmarks for dissection of bilateral external jugular veins, at the intersection of a triangle made by the sternal notch, ramus of the mandible, and shoulder joint
Maintenance of anesthesia and ventilation
The pig was connected to the anesthesia circuit and isoflurane and oxygen flow rate were adjusted accordingly. A rebreathing circuit was employed. The dose of isoflurane was adjusted to 1–3% depending on depth of anesthesia. Fentanyl patches (50-100ug/hr transdermal) were applied prior to incision for analgesia. Additional bolus doses of propofol (0.1 mg/kg) were administered PRN for tremors, and supplemental analgesia was provided throughout surgery with additional doses of methadone (0.1 mg/kg IV) and ketamine (10 mg/kg IV). Intravenous fluids were administered at a rate of 3 to 6 mls/kg/hr via the lateral ear vein throughout the operation. Eye ointment was applied for ocular lubrication. IV cephazolin 22 mg/kg q6hr was given immediately prior to incision for surgical prophylaxis.
Surgical approach
The lower abdomen and inguinal region was clipped. The course of the femoral artery was mapped under Doppler ultrasound guidance (Fig. 1a)). A sterile preparation of a surgical field was created by applying aqueous povidone-iodine or chlorhexidine solution from the lower abdomen and bilateral inguinal regions to the level of the distal femurs. The sternum, ramus of mandible and tip of the shoulder were marked bilaterally as landmarks for jugular vein dissection. A point halfway between the mandible and shoulder was then used as a guide for the location of the external jugular vein, using landmarks previously reported (Fig. 1b) [9]. The neck was prepared from mandible to sternum. Sterile surgical drapes were placed over the animal around the prepared field in square fashion.
Arterial exposure
The common femoral artery (CFA), profunda femoris artery (PFA), superficial femoral artery (SFA), saphenous artery (SA) and saphenous vein (SV) were exposed by sharp dissection of the overlying fascia, and retraction of overlying gracilis and adductor muscles. Proximal and distal control of the above vessels was gained using silastic loops. The CFA, PFA, and SFA were identified, and dissected free from their surrounding adventitia.
Conduit harvest and preparation
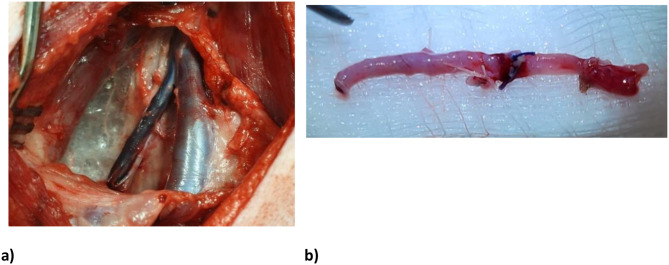
A longitudinal incision was made at the centre of the previously marked triangle in the neck. Dissection to the sternocleidomastoid muscle was performed, and it was retracted laterally. Further dissection using low-power cautery was performed to the internal jugular vein (IJV), with subsequent identification of the carotid artery lying deeper and medially, and the external jugular vein (EJV) lying laterally. The EJV was dissected free from surrounding adventitial tissue using a minimal touch technique. Small tributaries were ligated using Vicryl ties or surgical clips. The calibre of EJV was assessed and carefully checked to match the calibre of the femoral artery.
Fig. 2.
a) Exposure of the left external and internal jugular veins, b) harvested EJV conduit. Great care was taken to avoid injuring the carotid artery and vagus nerve, which lie in close proximity to the IJV. A total conduit length of approximately 5-8cm of vein was prepared. The proximal and distal ends of this length were clamped, ligated with Vicryl ties and divided. The EJV conduit was removed carefully inspected for defects, and reversed (Figure 2)
Arterial anastomosis
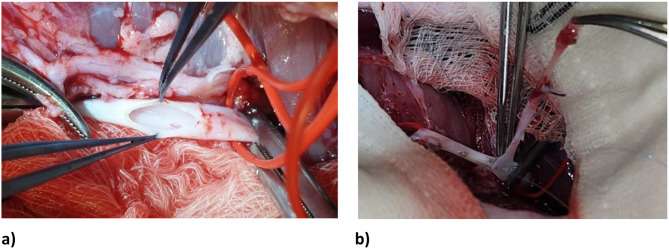
The harvested vein was then prepared for arterial anastomosis. An oblique cut was made at each end of the EJV conduit, in order to create a “hood”. The length of vein conduit was placed next to the SFA in order to measure appropriate sites of arterial anastomosis. Intravenous heparin 100 units/kg was given to the animal and allowed two minutes to circulate. Angled DeBakey clamps were applied to the SFA. First, an arteriotomy was made in the CFA using a no. 11 blade scalpel, and extended using fine Potts scissors. The arterial lumen was gently flushed with heparinized saline and inspected (Fig. 3).
Fig. 3.
a) Right femoral artery dissected, looped and clamped, with arteriotomy performed. The arterial lumen was flushed with heparinized saline and inspected prior to graft anastomosis. B) Proximal anastomosis of EJV conduit to femoral artery
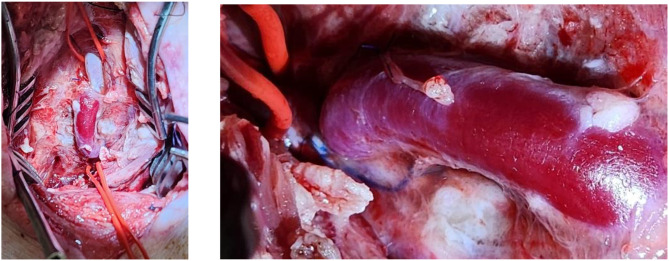
The EJV was placed adjacent to the arteriotomy site. One end of the EJV was anastomosed to the arteriotomy using a continuous 6 − 0 polypropylene suture, in parachute fashion. Prior to the final sutures being placed, the distal clamp was removed briefly to test backbleeding and flush the anastomosis free of potentially embolic debris. The proximal clamp was briefly removed to test inflow, then replaced. If flow through the graft was satisfactory, the final sutures were placed within the anastomosis, a final irrigation of the anastomosis with heparinized saline was performed, and the sutures were tied. These steps for the proximal anastomosis were then repeated in identical fashion for the distal SFA arteriotomy and and remaining end of EJV conduit (Fig. 4).
Fig. 4.
(a) Completed anastomosis. (b) Patent bypass graft at postoperative day three. Patency was confirmed by a palpable pulsatile graft, and verified with transit time flowmetry
Following the completion of the distal anastomosis and removal of distal and then proximal clamps, careful observation for hemostasis was performed. If necessary, additional 6 − 0 or 7 − 0 polypropylene sutures were applied to achieve hemostasis. The graft was observed for pulsatility, and palpated for the same. The native SFA between the graft anastomoses was permanently interval ligated with 3 − 0 braided ties to divert all arterial flow through the graft. Once satisfactory hemostasis was achieved, the wound was closed in layers using 2 − 0 braided interrupted sutures to the fascia, and 3 − 0 nylon vertical mattress sutures to the skin. TTFM measurements were performed at this point to check adequacy of graft performance, using mean graft flow (MGF) and pulsatility index (PI) parameters. An MGF of > 15mL/min and PI < 5 were deemed acceptable, with PI < 3 considered ideal, according to current literature [10].
The surgical procedure described from section 5a) above was then repeated on the contralateral limb in order to complete a total of two hindlimb bypass grafts.
Recovery
The animal was then recovered within the operating room, extubated, and transported to a designated warmed recovery area and provided analgesia. Routine postoperative monitoring using standardized monitoring charts was performed by accredited personnel and Consultant Veterinarian staff. Postoperative medications provided included aspirin 6 mg/kg q24h PO, methadone 0.1 mg IV PRN, fentanyl transdermal 50 µg/hr, meloxicam 0.4 mg/kg IV q24h and paracetamol 10 mg/kg q24h PO.
Postoperative graft interrogation and euthanasia
At postoperative day three, the pig was returned to the operating room for graft evaluation and explantation. Following premedication, induction of general anesthesia and sterile surgical preparation in the same fashion as for the initial operation (i.e. steps 2–5 repeated), the femoral surgical wounds were re-opened and the bypass graft was dissected out and looped for control. Bypass graft flows were interrogated bilaterally using the Medistim MiraQ™ system (Medistim, Oslo, Norway). Angled DeBakey clamps were applied to the native femoral artery proximal and distal to the graft, and the graft was sharply excised using a scalpel to facilitate luminal inspection, or other investigative methods as required. Following graft interrogation, the pig was euthanized with pentobarbitone sodium (150 mg/kg).
Results
Six out of six (100%) successful bilateral EJV to femoral artery bypass grafts were performed. All pigs were successfully recovered, and returned to the operating room at postoperative day 3. The wounds were re-opened and the grafts were inspected. Postoperative graft assessment was performed with TTFM using the Medistim MiraQ™ system (Medistim, Oslo, Norway) (Figs. 5 and 6), demonstrating all grafts were patent (100%).
Fig. 5.
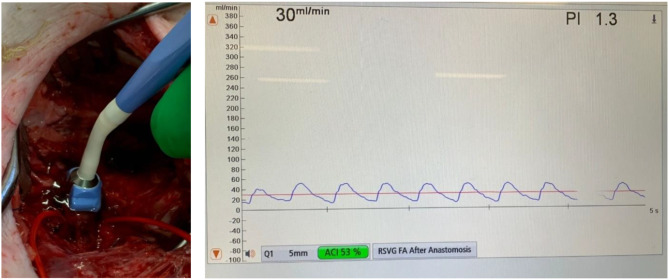
Graft performance was objectively assessed both intraoperatively and at postoperative day 3 using a Medistim MiraQ™ probe (left). In this figure demonstrating a right femoral bypass graft, a 5 mm probe was used, showing a mean graft flow rate of 30mL/min with a pulsatility index of 1.3, which are both within acceptable limits for a vein bypass graft
Fig. 6.
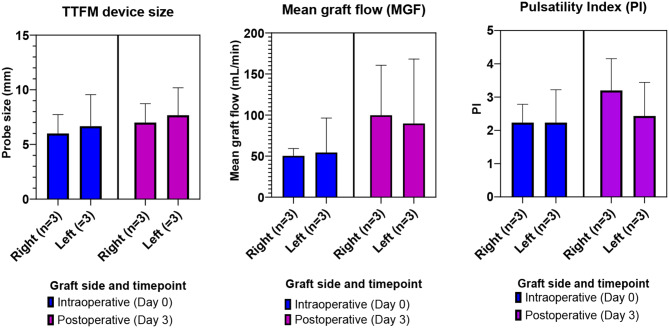
TTFM probe size, mean graft flow, and pulsatility index for each graft, immediately following anastomosis (Day 0) and postoperative day 3 (Day 3)
Discussion
The present study describes a novel method of bilateral, peripheral femoral end-to-side bypass model in a pig, using external jugular vein and femoral artery. This model offers novelty for two reasons. Firstly, the completion of bilateral grafts allows a within-subject method of comparison, which can be evaluated both immediately following anastomosis, as well as at serial timepoints as deemed necessary. This may be potentially useful for evaluating therapeutic interventions aimed at reducing graft failure, for example. Secondly, the incorporation of TTFM means that the model integrates the current gold standard for objective, on-table graft interrogation. TTFM has been widely accepted as a validation tool, at both intraoperative and postoperative timepoints [11]. The combination of these two points offers a more robust model of peripheral bypass graft surgery which can then be used to objectively evaluate further therapeutic interventions to combat graft failure. Whilst the present study demonstrates an early follow up point of postop day three, this could theoretically be extended to many weeks or months, as required.
Graft failure remains one of the key problems in cardiovascular bypass surgery. Vein graft failure has been previously attributed to acute thrombosis within the first month after surgery, followed by intimal hyperplasia up to one year, and atherosclerosis beyond one year postoperatively. Thrombosis itself may be driven by technical factors (e.g. anastomotic quality) conduit factors (such as size mismatch size or preexisting graft pathology) or because of extrinsic factors, such as a hypercoagulable state [12]. Properties of the target vessel also have the potential to influence graft failure risk, such as the severity of stenosis, the diameter, the extent of atherosclerotic burden, and previous endovascular interventions [12]. Furthermore, intraoperative endothelial injury within the graft conduit may also contribute to graft failure. Harskamp et al. demonstrated within the PREVENT IV that patients undergoing CABG whose vein grafts were preserved in a buffered saline solution had lower VGF rates and trends toward better long-term clinical outcomes compared with patients whose grafts were preserved in saline- or blood-based solutions [13].
Whilst the peri- and post-operative use of aspirin and high dose statin have arguably improved secondary prevention outcomes after CABG [14], an effective intervention to definitively prevent graft failure is severely lacking despite many years of intense preclinical research. More mechanistic insight, which can be derived from animal models, is clearly required in this field to better understand pathobiological mechanisms of graft failure, and to identify potential therapeutic targets for durable and effective intervention.
Animal models are ideal for this purpose, given their ability to offer repeated analysis of a surgical intervention within a controlled environment under very similar physiological parameters to humans (especially cardiovascular, hematological and coagulation responses). Less than 10% of CABG operations in the United States incorporate multiple arterial grafts [1], which is why the present study incorporates a venous conduit.
Several important points underpin the rationale for this model. Firstly, in any surgical model, a crucial study design parameter is the choice of animal, which may be small (e.g. rodents) or large (ovine, porcine). Whilst rodent models are inexpensive and offer ease of handling and high throughput, large animal models offer greater resemblance to human cardiovascular physiology [15], and therefore, translatability of findings. Previously, canines were some of the first large animals endorsed for preclinical testing of arterial grafts for cardiovascular research [16]. Over the last two decades, porcine models have supplanted canine models as the general surgical model in the international arena for both training and research [15]. This is partially due to socioeconomic reasons, and due to much more rigorous ethical standards being upheld for their use in research. Canines are reported to be particularly hypercoagulable, and are more distant from human hemophysiological parameters than both porcine and ovine species [17]. In a study of platelet and coagulation function between humans, bovine, ovine and canine species by Sato and Harasaki, canine species showed distinctively shorter time values for platelet mediated hemostasis time (PHT), collagen induced thrombus formation time (CITF), and clotting time (CT) than other species, including humans [18]. For these reasons, the present study is designed as a porcine model.
Secondly, the type of graft performed in the model must be clinically translatable. In humans, graft patency is greatly affected by technical factors, such as choice of conduit and its size, the choice of target vessel and its size, the quality of vascular inflow and outflow, and method of anastomosis. Therefore, these same technical considerations are of utmost importance in an animal model, in order to correctly capture the same pathophysiological processes to be studied. Previously reported models have incorporated different sources of conduit and target vessel. In previous rodent models, a substantial number report interposition techniques, including inferior epigastric vein to femoral artery [19], inferior vena cava to carotid [20, 21], inferior vena cava to abdominal aorta [22, 23], femoral vein to abdominal aorta [24], and jugular vein to abdominal aorta techniques [23, 25]. With regard to large animal models, a substantial number have concentrated on simulating coronary bypass surgery (CABG) [26–28], with fewer reports of peripheral bypass techniques, such as in the present study. Of those reporting peripheral bypass techniques, once again an interposition technique is heavily represented (such as jugular vein to carotid [29], saphenous vein to carotid [30], and jugular vein to femoral artery techniques [31]). Whilst these are acceptable, in order to function as a true graft rather than a patch, and therefore generate similar shear-stress derived endothelial remodelling responses seen in human grafts, the venous conduit must be substantially longer than it is wide (i.e. a cylinder). Furthermore, interposition techniques in humans are usually reserved for carrying out repairs of short resections of arterial tissue (e.g. traumatic transection, aneurysm repair).
Limited previous large animal studies have reported end to side approaches, such as external jugular vein to femoral bypass grafts, although one study reported multiple events of fatal exsanguination [32]. Other papers report a porcine jugular to carotid anastomosis [33], however, if a bilateral procedure is sought then risk of vagus or sympathetic nerve injury is greater, which could be highly comorbid or fatal [34]. Since most revascularization approaches in humans involve end to side anastomoses, such as a left internal mammary to left anterior descending arterial anastomosis, or a saphenous vein to superficial femoral arterial anastomosis, this approach is most desirable to replicate in a model to maximize translatability of findings. Furthermore, a mismatch between vein conduit and target artery diameters has been shown to affect vein graft patency [35], with intimal hyperplasia and thrombosis triggered by turbulence [36]. In the model setting, grafts which occlude due to processes other than those being studied are not constructive. In this model, by incorporating external jugular vein as conduit, and femoral artery as inflow, the risk of vessel mismatch (a leading cause of occlusion) as well as risk of morbidity is minimized.
Furthermore, our integration of transit time flowmetry (TTFM) with a Medistim MiraQ™ system (Medistim, Oslo, Norway) allows objective graft assessment both intraoperatively and at postoperative timepoints. Under conditions of repeated measures, such as serial evaluation of a graft over time, this aspect allows comparison of flow conditions both within the same animal at different points in time (within-subject comparison), as well as for the same timepoint between different animals (between-subject) comparison. TTFM has been adopted in many leading centres as standard of care, and we believe its incorporation in this model offers a layer of both novelty and validity, as well as translatability to real world application. Typically, flow probe sizes of 4–5 mm for the radial artery and the great saphenous vein have been reported. In the present model, on average, similarly sized probes were required, and our observed TTFM metrics of mean graft flow and PI were within accepted criteria for an adequately functioning graft. AHA guidelines recommend a pulsatility index (PI) < 5 for an acceptable graft, and ideally PI < 3 for a left system graft [8]. EACTS/ESC Guidelines recommend an MGF of ≥ 20 ml/min and a PI of ≤ 5 as acceptable perioperative results for TTFM [10]. Intraoperative TTFM may have prognostic role in identifiying grafts at risk of early failure. One study cited a cut-off value in the overall and left coronary target territories of 3.4, and the cut-off value in the right coronary target territories of 3.6 as being associated with early graft failure [37]. Whilst no specific similar guideline exists for peripheral bypass surgery, we reported our TTFM values for the sake of objectivity.
This model has limitations in that it is conducted in a disease-free animal, and was primarily intended to show proof of concept. However, it could be used as a platform upon which more sophisticated animal studies may be built. For example, porcine models of coronary and peripheral atherosclerosis offer several advantages over rodent models, including similar anatomical size to humans, as well as genetic expression and development of high-risk atherosclerotic lesions which resemble those in humans [6]. Follow up studies using this model may incorporate atherosclerotic lesions affecting target vessels in order to increase translatability to human clinical practice.
Conclusion
A novel porcine model of bilateral hindlimb bypass graft surgery has been described. This model allows longitudinal, within-subject and between-subject investigation and comparison of pathobiological processes which affect bypass grafts used in cardiovascular surgery. The model is objectively supported by transit time flowmetry, which is a widely accepted validation tool of graft performance.
Acknowledgements
The authors acknowledge the support of Medistim (Oslo, Norway), who kindly provided the Medistim MiraQ™ system used in the study.
Author contributions
ABH conceptualized the study, the surgical approach, carried out all operations, collected and analyzed all data, and prepared the manuscript. CL, CC and SOL delivered preoperative, perioperative, and postoperative care of the animals, including induction and maintenance of general anaesthesia, and performed critical review of the manuscript. NVP assisted with study design. DCM, MPV, JYS and JFF performed critical review of the manuscript.
Funding
The authors gratefully acknowledge the support of The Prince Charles Hospital Foundation Innovation Grant INN2021-52.
Data availability
Available on reasonable request to the corresponding author.
Declarations
Animal ethics statement
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the University of Queensland, St Lucia, Brisbane, QLD, Australia.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xenogiannis I, et al. Saphenous Vein Graft failure: from pathophysiology to Prevention and Treatment strategies. Circulation. 2021;144(9):728–45. [DOI] [PubMed] [Google Scholar]
- 2.Singh N, et al. Factors associated with early failure of infrainguinal lower extremity arterial bypass. J Vasc Surg. 2008;47(3):556–61. [DOI] [PubMed] [Google Scholar]
- 3.Chesebro JH, et al. A platelet-inhibitor-drug Trial in Coronary-Artery Bypass operations. N Engl J Med. 1982;307(2):73–8. [DOI] [PubMed] [Google Scholar]
- 4.Yu P, et al. Rationale and practical techniques for mouse models of early vein graft adaptations. J Vasc Surg. 2010;52(2):444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swartz DD, Andreadis ST. Animal models for vascular tissue-engineering. Curr Opin Biotechnol. 2013;24(5):916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamamdzic D, Wilensky RL. Porcine models of accelerated coronary atherosclerosis: role of diabetes mellitus and hypercholesterolemia. J Diabetes Res. 2013;2013:p761415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu RH, et al. Review of vascular graft studies in large animal models. Tissue Eng Part B Rev. 2018;24(2):133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudino M, et al. The Use of Intraoperative Transit Time Flow Measurement for coronary artery bypass surgery: systematic review of the evidence and Expert Opinion statements. Circulation. 2021;144(14):1160–71. [DOI] [PubMed] [Google Scholar]
- 9.Furbeyre H, Labussiere E. A minimally invasive catheterization of the external jugular vein in suckling piglets using ultrasound guidance. PLoS ONE. 2020;15(10):e0241444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niclauss L. Techniques and standards in intraoperative graft verification by transit time flow measurement after coronary artery bypass graft surgery: a critical review. Eur J Cardiothorac Surg. 2016;51(1):26–33. [DOI] [PubMed] [Google Scholar]
- 11.Takami Y, Takagi Y. Roles of Transit-Time Flow Measurement for coronary artery bypass surgery. Thorac Cardiovasc Surg. 2018;66(6):426–33. [DOI] [PubMed] [Google Scholar]
- 12.Gaudino M, et al. Mechanisms, consequences, and Prevention of Coronary Graft failure. Circulation. 2017;136(18):1749–64. [DOI] [PubMed] [Google Scholar]
- 13.Harskamp RE, et al. Vein graft preservation solutions, patency, and outcomes after coronary artery bypass graft surgery: follow-up from the PREVENT IV randomized clinical trial. JAMA Surg. 2014;149(8):798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulik A, et al. Secondary Prevention after coronary artery bypass graft surgery. Circulation. 2015;131(10):927–64. [DOI] [PubMed] [Google Scholar]
- 15.Swindle MM, et al. Swine as models in Biomedical Research and Toxicology Testing. Vet Pathol. 2012;49(2):344–56. [DOI] [PubMed] [Google Scholar]
- 16.Abbott WM, et al. Evaluation and performance standards for arterial prostheses. J Vasc Surg. 1993;17(4):746–56. [DOI] [PubMed] [Google Scholar]
- 17.Chemonges S, et al. Optimal management of the critically ill: anaesthesia, monitoring, data capture, and point-of-care technological practices in ovine models of critical care. 2014. 2014: p. 468309. [DOI] [PMC free article] [PubMed]
- 18.Sato M, Harasaki H. Evaluation of platelet and coagulation function in different animal species using the xylum clot signature analyzer. Asaio j. 2002;48(4):360–4. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, et al. Vein Interposition Model: a suitable model to Study Bypass Graft Patency. J Vis Exp, 2017(119). [DOI] [PMC free article] [PubMed]
- 20.Yu P, et al. Diet-induced obesity drives negative mouse vein graft wall remodeling. J Vasc Surg. 2014;59(6):1670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen BT, et al. Perivascular innate immune events modulate early murine vein graft adaptations. J Vasc Surg. 2013;57(2):486–e4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng C-N, et al. Contribution of endothelial Injury and inflammation in early phase to Vein Graft failure: the causal factors impact on the development of Intimal Hyperplasia in Murine models. PLoS ONE. 2014;9(6):e98904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehsan A, et al. Mineralocorticoid receptor antagonism inhibits vein graft remodeling in mice. J Thorac Cardiovasc Surg. 2013;145(6):1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, et al. Pioglitazone preserves vein graft integrity in a rat aortic interposition model. J Thorac Cardiovasc Surg. 2010;140(2):408–e4161. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, et al. The role of RAGE in aminoguanidine-induced suppression of venous intimal hyperplasia in diabetic rats. Ann Vasc Surg. 2009;23(2):246–54. [DOI] [PubMed] [Google Scholar]
- 26.Li X et al. Establishment and evaluation of a Porcine Vein Graft Disease Model. J Vis Exp, 2022(185). [DOI] [PubMed]
- 27.Bonatti J, et al. The axillocoronary bypass. Blood flow and short-term graft histology in a porcine model. J Cardiovasc Surg (Torino). 2002;43(5):625–31. [PubMed] [Google Scholar]
- 28.Radwan MM, Siddique A, Thankam FG. Translational model of vein graft failure following coronary artery bypass graft in atherosclerotic microswine. 2022. 70(5): pp. 445–54. [DOI] [PubMed]
- 29.Thim T, et al. Oversized vein grafts develop advanced atherosclerosis in hypercholesterolemic minipigs. BMC Cardiovasc Disord. 2012;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, et al. Remodeling of autologous saphenous vein grafts. Circulation. 1997;95(12):2684–93. [DOI] [PubMed] [Google Scholar]
- 31.Cho WH, et al. Effect of AP-1 decoy using hemagglutinating virus of Japan-Liposome on the intimal hyperplasia of the autogenous vein graft in mongrel dogs. Transpl Proc. 2006;38(7):2161–3. [DOI] [PubMed] [Google Scholar]
- 32.Benson RW, Payne DD, DeWeese JA. Evaluation of prosthetic grafts of different porosity for arterial reconstruction. Am J Surg. 1975;129(6):665–9. [DOI] [PubMed] [Google Scholar]
- 33.Schachner T, Laufer G, Bonatti J. Vivo (animal) models of vein graft disease☆. Eur J Cardiothorac Surg. 2006;30(3):451–63. [DOI] [PubMed] [Google Scholar]
- 34.Musk GC, King M, He B. Horner Syndrome in 2 pigs (Sus scrofa) after vascular grafting of the carotid artery and jugular vein. Comp Med. 2017;67(6):518–23. [PMC free article] [PubMed] [Google Scholar]
- 35.Yamane Y, et al. Impact of the size mismatch between saphenous vein graft and coronary artery on graft patency. Gen Thorac Cardiovasc Surg. 2017;65(1):25–31. [DOI] [PubMed] [Google Scholar]
- 36.Une D, et al. Correlates of Saphenous Vein Graft Hyperplasia and occlusion 1 year after coronary artery bypass grafting. Circulation. 2013;128(11suppl1):S213–8. [DOI] [PubMed] [Google Scholar]
- 37.Zhang G, et al. The predictive value of intraoperative transit-time flow measurement parameters for early graft failure in different target territories. J Cardiol. 2021;77(2):201–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on reasonable request to the corresponding author.