Abstract
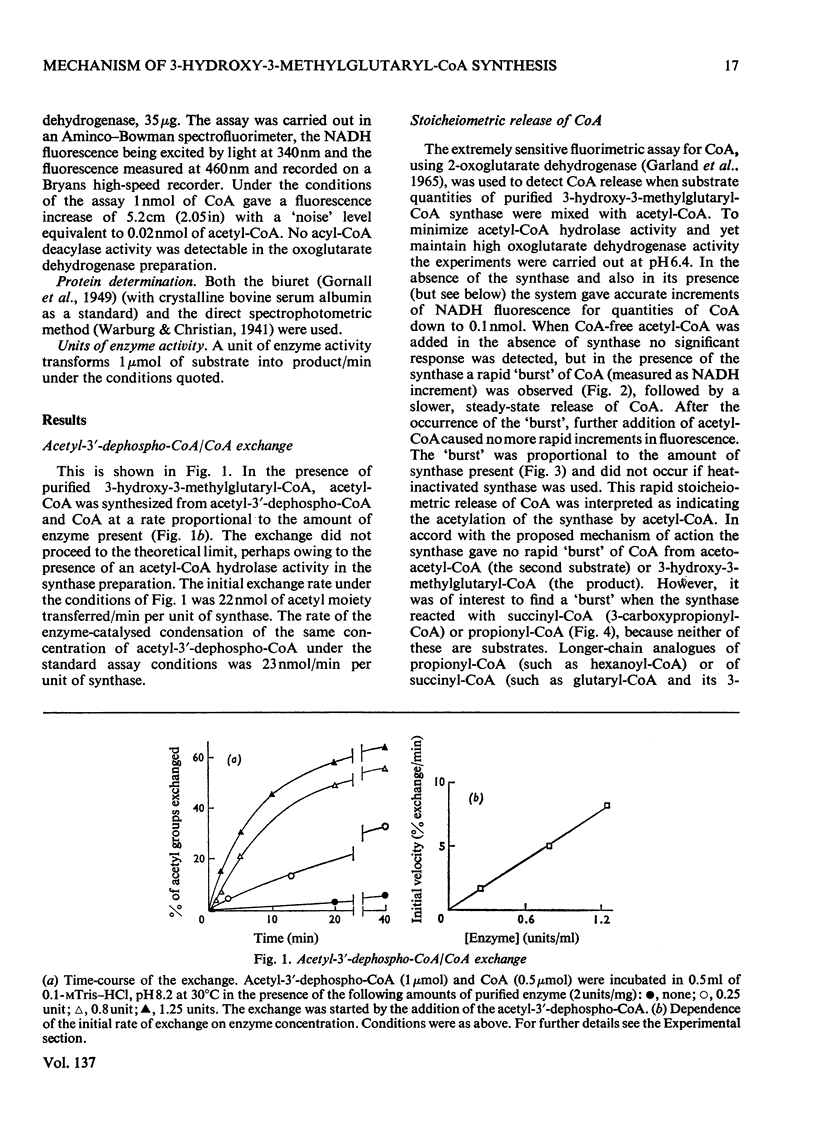
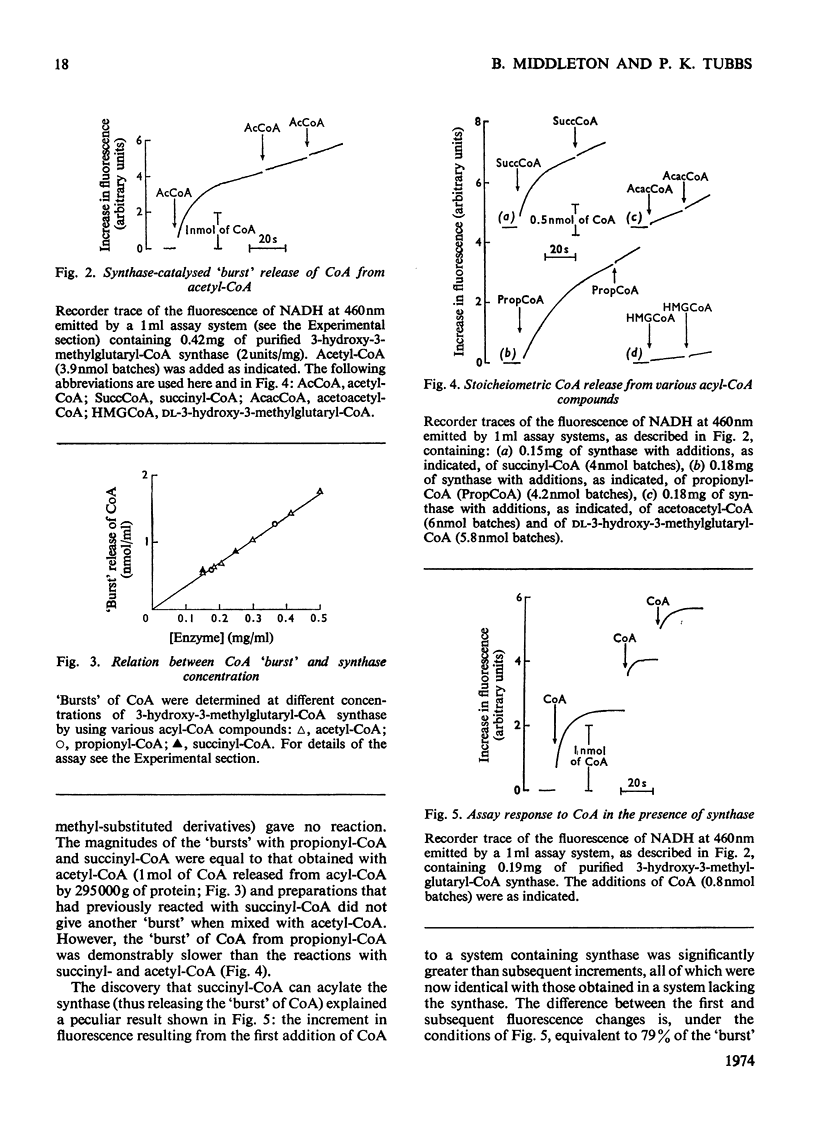
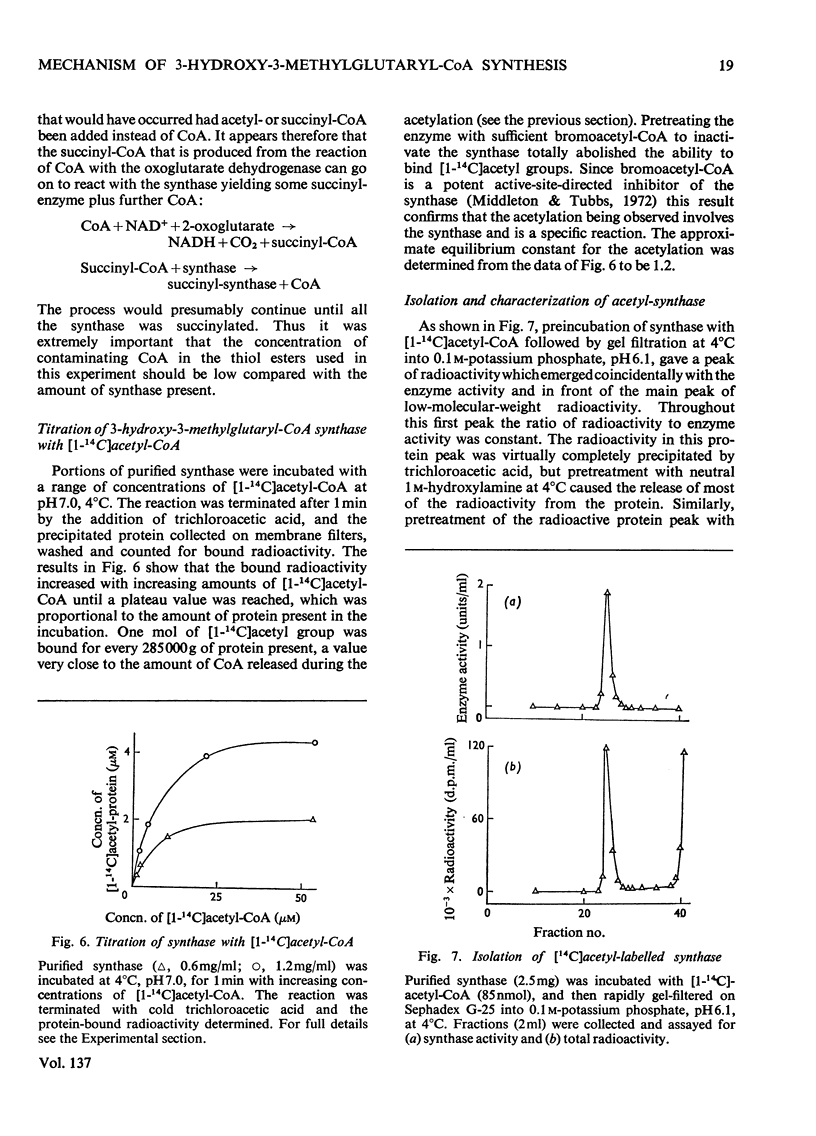
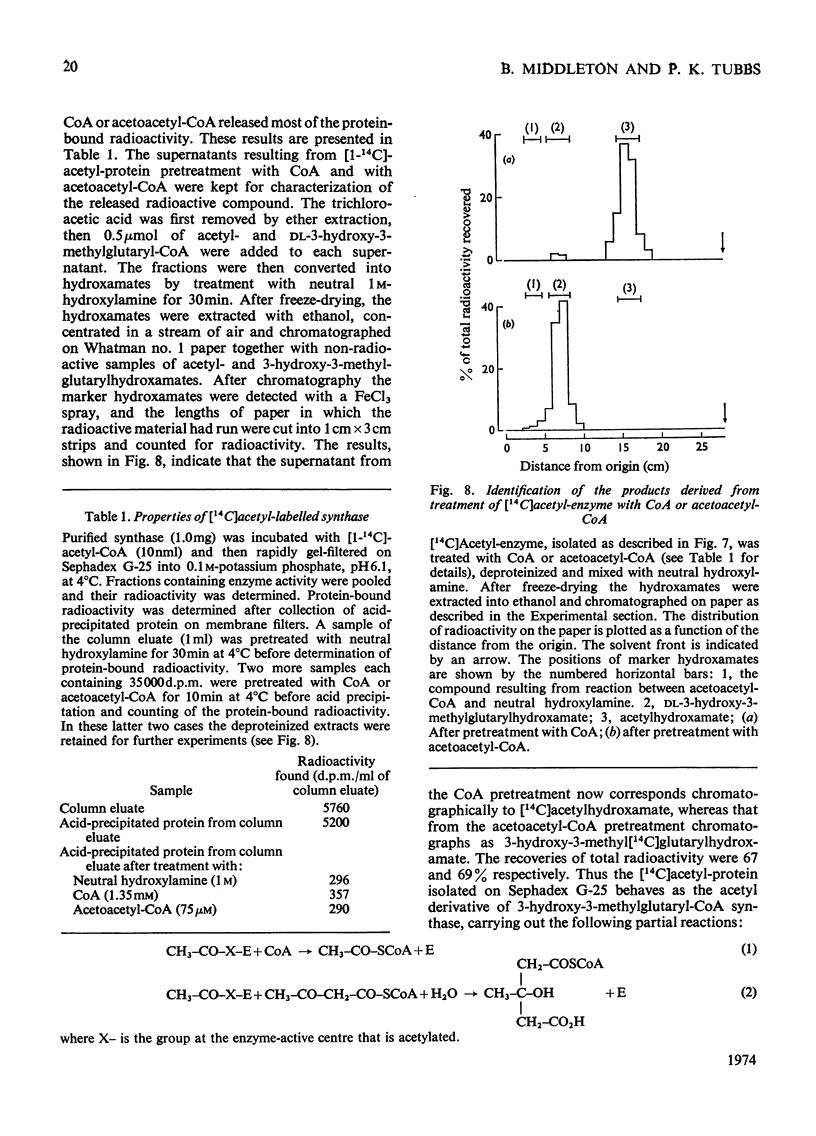
1. Purified 3-hydroxy-3-methylglutaryl-CoA synthase from baker's yeast (free from acetoacetyl-CoA thiolase activity) catalysed an exchange of acetyl moiety between 3′-dephospho-CoA and CoA. The exchange rate was comparable with the overall velocity of synthesis of 3-hydroxy-3-methylglutaryl-CoA. 2. Acetyl-CoA reacted with the synthase, giving a rapid `burst' release of CoA proportional in amount to the quantity of enzyme present. The `burst' of CoA was released from acetyl-CoA, propionyl-CoA and succinyl-CoA (3-carboxypropionyl-CoA) but not from acetoacetyl-CoA, hexanoyl-CoA, dl-3-hydroxy-3-methylglutaryl-CoA, or other derivatives of glutaryl-CoA. 3. Incubation of 3-hydroxy-3-methylglutaryl-CoA synthase with [1-14C]acetyl-CoA yielded protein-bound acetyl groups. The Keq. for the acetylation was 1.2 at pH7.0 and 4°C. Acetyl-labelled synthase was isolated free from [1-14C]acetyl-CoA by rapid gel filtration at pH6.1. The [1-14C]acetyl group was removed from the protein by treatment with hydroxylamine, CoA or acetoacetyl-CoA but not by acid. When CoA or acetoacetyl-CoA was present the radioactive product was [1-14C]acetyl-CoA or 3-hydroxy-3-methyl-[14C]glutaryl-CoA respectively. 4. The isolated [1-14C]acetyl-enzyme was slowly hydrolysed at pH6.1 and 4°C with a first-order rate constant of 0.005min−1. This rate could be stimulated either by raising the pH to 7.0 or by the addition of desulpho-CoA. 5. These properties are interpreted in terms of a mechanism in which 3-hydroxy-3-methyl-glutaryl-CoA synthase is acetylated by acetyl-CoA to give a stable acetyl-enzyme, which then condenses with acetoacetyl-CoA yielding a covalent derivative between 3-hydroxy-3-methylglutaryl-CoA and the enzyme which is then rapidly hydrolysed to free enzyme and product.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN E. G. PURINE AND PYRIMIDINE DERIVATIVES IN MATURE PEA SEEDS. Biochem J. 1963 Sep;88:498–504. doi: 10.1042/bj0880498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F., Middleton B., Tubbs P. K. A coenzyme A analogue, desulpho-coA; preparation and effects on various enzymes. Biochem Biophys Res Commun. 1966 Apr 19;23(2):208–213. doi: 10.1016/0006-291x(66)90529-8. [DOI] [PubMed] [Google Scholar]
- Das N., Cottam G. L., Srere P. A. The citrate cleavage enzyme: release of phosphate from the phosphoenzyme. Arch Biochem Biophys. 1971 Apr;143(2):602–608. doi: 10.1016/0003-9861(71)90245-1. [DOI] [PubMed] [Google Scholar]
- FOSTER R. J. Specificity of alpha-chymotrypsin. I. Promotion of the solvolysis of acetyl alpha-chymotrypsin by indole. J Biol Chem. 1961 Sep;236:2461–2466. [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Yates D. W. Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem J. 1965 Nov;97(2):587–594. doi: 10.1042/bj0970587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U., Riepertinger C., Lynen F. Reinigung und Kristallisation der Thiolase, Untersuchungen zum Wirkungsmechanismus. Eur J Biochem. 1968 Nov;6(2):264–280. doi: 10.1111/j.1432-1033.1968.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Lynen F. The role of biotin-dependent carboxylations in biosynthetic reactions. Biochem J. 1967 Feb;102(2):381–400. doi: 10.1042/bj1020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAL G., BERGMEYER H. U. [The enzymatic analysis of coenzyme A]. Biochim Biophys Acta. 1963 Apr 9;67:599–616. doi: 10.1016/0006-3002(63)91870-5. [DOI] [PubMed] [Google Scholar]
- Middleton B. The kinetic mechanism of 3-hydroxy-3-methylglutaryl-coenzyme A synthase from baker's yeast. Biochem J. 1972 Jan;126(1):35–47. doi: 10.1042/bj1260035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton B., Tubbs P. K. The purification and some properties of 3-hydroxy-3-methylglutaryl-coenzyme A synthase from Baker's yeast. Biochem J. 1972 Jan;126(1):27–34. doi: 10.1042/bj1260027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R. The purification and properties of phosphotransacetylase. J Biol Chem. 1952 May;196(2):527–534. [PubMed] [Google Scholar]
- Stewart P. R., Rudney H. The biosynthesis of beta-hydroxy-beta-methylglutaryl coenzyme A in yeast. 3. Purification and properties of the condensing enzyme thiolase system. J Biol Chem. 1966 Mar 10;241(5):1212–1221. [PubMed] [Google Scholar]
- Stewart P. R., Rudney H. The biosynthesis of beta-hydroxy-beta-methylglutaryl coenzyme A in yeast. IV. The origin of the thioester bond of beta-hydroxy-beta-methylglutaryl coenzyme A. J Biol Chem. 1966 Mar 10;241(5):1222–1225. [PubMed] [Google Scholar]


