Abstract
Background
Anemia and blood transfusions are recognized as risk factors for periprosthetic joint infections (PJI). Tranexamic acid (TXA) is established in reducing perioperative blood loss and transfusion requirements. Our study investigates the impact of perioperative TXA administration on the incidence of PJI in patients undergoing total joint arthroplasty (TJA) and evaluates the association of intravenous (IV) and topical applications with PJI occurrence.
Methods
A retrospective review was performed on 8042 patients who underwent primary total hip arthroplasty (THA) and knee arthroplasty (TKA) from January 2009 to December 2020, with a minimum one-year follow-up at our institution. We compared patients who received TXA (n = 3664, with 2345 receiving it IV and 1319 topically) to those who did not (n = 4378). 0.5–1.25 g of IV TXA was administered before skin incision, and 1.5–3 g of topical TXA was injected intra-articularly or into the drainage tube during surgery. The primary outcome was PJI development within one year, defined by the 2013 International Consensus Meeting criteria. Secondary outcomes included blood transfusion, hospital length of stay (LOS), venous thromboembolism (VTE), and 90-day readmission. We employed multivariate logistic regression and propensity score weighting to adjust for potential confounders and conducted subgroup analyses to assess PJI odds in TKA and THA patients treated with IV and topical TXA.
Results
The TXA group demonstrated a lower PJI occurrence (1.1% vs. 2.1%, p < 0.001), less blood transfusion (14.4% vs. 22.7%, p < 0.001) and shorter LOS (5.6 ± 1.6 vs. 6.5 ± 2.5, p < 0.001) compared to those without TXA. There was no difference between the two groups with regards to VTE and 90-day readmission. Perioperative TXA administration demonstrated lower PJI in multivariate analysis (OR 0.54, 95% CI 0.36–0.80, p = 0.002), and in propensity score weighting (OR 0.53, 95% CI 0.36–0.80, p = 0.002). In the subgroup analysis, both IV and topical administration of TXA resulted in decreased PJI (IV group: OR 0.53, 95% CI, 0.33–0.84, p = 0.007, topical group: OR 0.51, 95% CI, 0.29–0.89, p = 0.018), especially in primary TKA (IV TXA, OR 0.49, 95% CI, 0.29–0.83, p = 0.008; Topical TXA, OR, 0.56, 95% CI, 0.32–0.98, p = 0.042).
Conclusion
Perioperative TXA administration in primary hip and knee arthroplasty is significantly associated with a reduced PJI occurrence. Both IV and topical TXA routes showed similar association with reduced PJI occurrence, with a notable correlation observed in primary TKA.
Keywords: Tranexamic acid, Periprosthetic joint infection, Primary total joint arthroplasty, TKA, THA
Introduction
Periprosthetic joint infection (PJI) represents a severe and often catastrophic outcome of total joint arthroplasty (TJA), leading the causes for revision surgeries [1, 2]. Data from national registries indicate that PJIs occur in approximately 0.76–1.24% of total hip arthroplasty (THA) cases and 0.88–1.25% of total knee arthroplasty (TKA) cases [3]. With projections estimating a significant increase in primary TKA and THA by 508.2% and 69.7%, respectively, by 2030 [4], the economic impact is poised to be considerable [5]. Consequently, mitigating the incidence of PJI is of paramount importance.
Risk factors for PJI are multifactorial and include preoperative anemia, intraoperative blood loss, hematoma, and the need for allogeneic blood transfusion [6, 7]. Tranexamic acid (TXA), a well-established antifibrinolytic agent, has demonstrated safety and efficacy in curtailing blood loss and reducing the necessity for transfusions during total joint arthroplasty [8, 9]. Evidence suggests that TXA, regardless of its oral, intravenous, or topical form, effectively preserves hemoglobin levels, limits blood loss, and curtails transfusion needs, all without increasing the risk of adverse events [10]. The literature has documented a correlation between the intravenous administration of TXA and a decrease in PJI rates post-primary THA [11, 12]. The correlation between TXA use and PJI reduction in primary TKA, however, remains underexplored in cohort studies. Moreover, the effectiveness of different TXA administration routes in mitigating PJI risk following primary TJA continues to be a subject of clinical debate.
This study endeavors to elucidate the relationship between perioperative TXA use and PJI occurrence following primary TJA. Additionally, in our subgroup analysis, we separated intravenous and topical TXA to determine whether each administration route is independently associated with PJI occurrence.
Methods
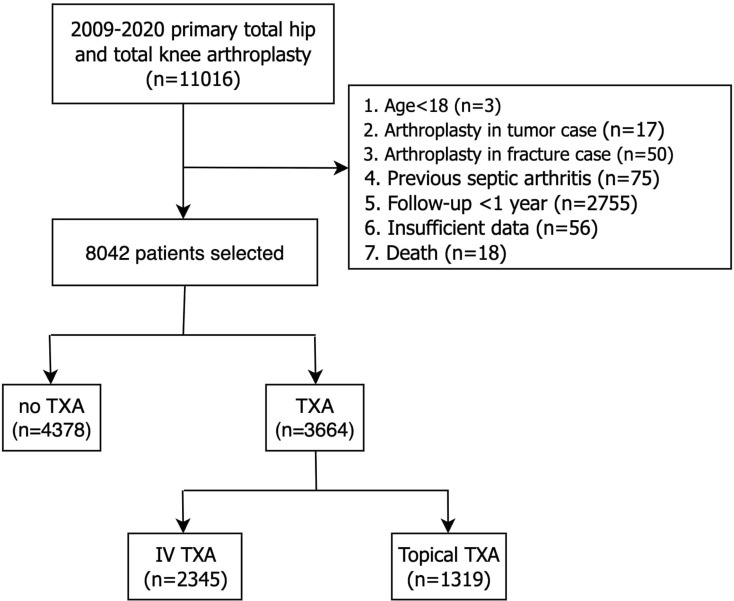
After Institutional Review Board’s approval, we retrospectively reviewed patients undergoing primary THA or TKA from January 1, 2009, to December 31, 2020, at a single institution. Patients with multiple primary joint replacements for different joints were assessed as separate cases. Patients under 18 years old, primary arthroplasty for tumor or fracture etiologies, previous septic arthritis, follow-up less than 1 year, and those with insufficient patient characteristics data were excluded. A total of 11,016 patients were initially acquired. Patients, who were less than 18 years old (n = 3), primary arthroplasty in tumor (n = 17) or fracture cases (n = 50), previous septic arthritis (n = 75), follow-up less than 1 year (n = 2755), death (n = 18), and those with insufficient data (n = 56) were excluded. After the above exclusion criteria, a final cohort of 8042 patients were included in this study. Of these, 3664 (45.6%) patients received perioperative TXA including 2345 with the intravenous route and 1319 with the topical route (Fig. 1).
Fig. 1.
Flow chart detailing patient inclusion, exclusion, and grouping
Patients were grouped based on whether or not perioperative TXA was administered. The perioperative TXA included patients who received TXA on the day of surgery. The TXA group was further sub-grouped based on intravenous or topical intra-articular TXA administration. The intravenous TXA (Tranexamin 50 mg/mL®; China Chemical and Pharmaceutical Co, Taiwan, ROC) dose was administered as a single dose of 10 mg/kg 10 min before skin incision. For topical application, 1.5–3 g of TXA was injected into the joint capsule or infused into the drainage tube, following the protocol established by Wong et al. [13], which demonstrated high efficacy in blood conservation in total joint arthroplasty. Both THA and TKA procedures followed this protocol. According to the TXA protocol at our institute, the intravenous group received 0.25–1.25 g TXA, and the topical intra-articular TXA group received greater or equal to 1.5 g TXA on the surgical day.
The preoperative characteristics of the patients include age, sex, body mass index (BMI), type of procedure (TKA vs. THA), preoperative hemoglobin (Hb) level, surgical time, Charlson Comorbidity Index (CCI), comorbidities, American Society of Anesthesiologists (ASA) score, anesthesia type (general or regional), were recorded and compared across between groups. The primary outcome was the incidence of PJI at 1 year. PJI, as defined by 2013 International Consensus Meeting, was diagnosed if either a sinus tract communicates with the prosthesis, or the same pathogen is found in two separate cultures. Alternatively, PJI can be diagnosed if three of five minor criteria are present: elevated C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR), elevated synovial fluid white blood cell (WBC) count or PMN%, positive synovial fluid culture, and histopathological signs of acute inflammation [14]. Secondary outcomes included examining postoperative blood transfusion, length of stay, thromboembolism event, and 90-day readmission. The indication for blood transfusion was set at Hb concentration less than 8 g/dl in healthy patients or between 8 and 9 g/dl with clinical symptoms of anemia (dizziness, dyspnea, or palpitation). The transfusion threshold in patients with cardiovascular disease was adjusted to a Hb level of 9 g/dL [15].
Statistical analysis
Statistical analysis was performed using the IBM Statistical Package for Social Science (SPSS) software, version 26.0 for Windows and R language. Chi-square and Fisher’s exact tests were used to compare the categorical data of both groups. The independent t-tests was used to compare the continuous data between groups.
Univariate and multivariate logistic regression was conducted for all covariates of interest to investigate the potential association between the use of TXA and PJI. Additionally, subgroup analyses were performed for PJI, taking into account the type of surgery (total hip and knee arthroplasty). To balance the covariates, we used propensity score weighting with the average treatment effect on the treated. The balance of covariates was assessed using the standardized mean difference (SMD) approach, with an SMD exceeding 10% indicating a significant imbalance in factors between the two groups. Adjusted regressions were subsequently conducted for outcome analysis. Odds ratios with their corresponding 95% confidence intervals (CIs) were calculated. A post-hoc power analysis showed sufficient power (94%) to detect the reduction in PJI between two independent proportions at an alpha of 0.05 in patients with and without TXA. All reported p-values are two-sided, and statistical significance was defined as p < 0.05.
Results
Demographics of the study participants were described (Table 1). Of all total joint replacement patients, 45% received TXA. There were no differences in sex, age, BMI, diagnosis, laterality, or the incidence of heart disease, chronic obstructive pulmonary disease, and diabetes. Patients who received TXA exhibited a higher proportion of preoperative anemia, shorter surgical time, a lower proportion of individuals undergoing TKA, lower CCI score, lower incidence of renal failure, liver disease, and rheumatoid arthritis, and a higher proportion of surgeries performed under spinal anesthesia. (all p < 0.05).
Table 1.
Patient demographics data between patients with and without tranexamic acid
| Total (n = 8042) | TXA (n = 3664) | no TXA (n = 4378) | p-value | ||||
|---|---|---|---|---|---|---|---|
| Age, year (mean ± SD) | 67.9 | ± 10.3 | 67.7 | ± 10.6 | 68.1 | ± 10.0 | 0.241 |
| Male (n, %) | 2236 | (27.8) | 1047 | (28.6) | 1189 | (27.2) | 0.158 |
| BMI, kg/m2 (mean ± SD) | 26.9 | ± 4.3 | 26.9 | ± 4.5 | 26.9 | ± 4.1 | 0.229 |
| Preoperative Hb, g/dL (mean ± SD) | 12.5 | ± 1.7 | 12.2 | ± 1.8 | 12.7 | ± 1.6 | 0.000 |
| Surgical time, min (mean ± SD) | 148 | ± 31 | 142 | ± 30 | 154 | ± 31 | 0.000 |
| Joint (n. %) | < 0.001 | ||||||
| Hip | 1766 | (22.0) | 967 | (26.5) | 796 | (18.2) | |
| Knee | 6276 | (78.0) | 2697 | (73.5) | 3582 | (81.8) | |
| Diagnosis | 0.478 | ||||||
| Osteoarthritis | 6894 | (85.7) | 3086 | (84.3) | 3808 | (87.0) | |
| Osteonecrosis | 699 | (8.7) | 358 | (9.8) | 341 | (7.8) | |
| Rheumatoid arthritis | 195 | (2.4) | 116 | (3.2) | 79 | (1.8) | |
| Ankylosing spondylitis | 24 | (0.3) | 10 | (0.3) | 14 | (0.3) | |
| Posttraumatic osteoarthritis | 153 | (1.9) | 71 | (1.9) | 82 | (1.9) | |
| Hip dysplasia | 70 | (0.9) | 18 | (0.5) | 52 | (1.2) | |
| Gouty arthritis | 5 | (0.1) | 3 | (0.1) | 2 | (0.0) | |
| Lateral (n, %) | 0.834 | ||||||
| Right | 4198 | (52.2) | 1913 | (52.2) | 2285 | (52.2) | |
| Left | 3837 | (47.7) | 1749 | (47.7) | 2088 | (47.7) | |
| Bilateral | 6 | (0.1) | 2 | (0.1) | 4 | (0.1) | |
| CCI > 2 (n, %) | 5222 | (64.9) | 2258 | (61.7) | 2964 | (67.7) | < 0.001 |
| Comorbidities (n, %) | |||||||
| Heart disease | 368 | (4.6) | 165 | (4.5) | 203 | (4.6) | 0.775 |
| COPD | 192 | (2.4) | 100 | (2.7) | 92 | (2.1) | 0.066 |
| Diabetes | 1738 | (21.6) | 760 | (20.7) | 978 | (22.3) | 0.083 |
| Renal failure | 1629 | (20.3) | 430 | (11.8) | 1199 | (27.4) | < 0.001 |
| Liver disease | 164 | (2.0) | 57 | (1.6) | 107 | (2.4) | 0.005 |
| Rheumatoid Arthritis | 266 | (3.3) | 97 | (2.6) | 169 | (3.9) | 0.002 |
| ASA (n, %) | < 0.001a | ||||||
| 1 | 146 | (1.8) | 42 | (1.1) | 105 | (2.4) | |
| 2 | 4810 | (59.8) | 2146 | (58.6) | 2664 | (60.8) | |
| 3 | 3085 | (38.4) | 1476 | (40.3) | 1609 | (36.8) | |
| 4 | 1 | (0) | 1 | (0) | 0 | (0) | |
| Anesthesia (n, %) | < 0.001a | ||||||
| General | 7253 | (90.2) | 3227 | (88.1) | 4026 | (92.0) | |
| Regional | 789 | (9.8) | 437 | (11.9) | 352 | (8.0) | |
a fisher’s exact test
TXA, tranexamic acid; BMI, body mass index; Hb, hemoglobin; TKA, total knee arthroplasty; CCI, Charlson Comorbidity Index; ASA, American Society of Anesthesiology; COPD, chronic obstructive pulmonary disease; IQR, interquartile range
The total periprosthetic joint infection incidence was 1.7%. Patients with TXA had a lower PJI incidence compared to those did not receive TXA (1.1% vs. 2.1%, p < 0.001). Additionally, patients with TXA had fewer postoperative blood transfusion events (14.4% vs. 22.7%, p < 0.001) and a shorter length of hospital stay (mean 5.6 ± 1.6 days vs. 6.5 ± 2.5 days, p < 0.001) compared to patient without TXA. There were no significant differences in the incidence of thromboembolic events and 90-day readmission between the two groups (Table 2).
Table 2.
The outcomes in patients with and without tranexamic acid
| Outcomes | Total (n = 8042) |
TXA (n = 3664) |
no TXA (n = 4378) |
p-value | |||
|---|---|---|---|---|---|---|---|
| PJI (n, %) | 133 | (1.7) | 39 | (1.1) | 94 | (2.1) | < 0.001 |
| Transfusion (n, %) | 1519 | (18.9) | 527 | (14.4) | 992 | (22.7) | < 0.001 |
| Length of stay, day (mean ± SD) | 6.1 | ± 2.2 | 5.6 | ± 1.6 | 6.5 | ± 2.5 | < 0.001 |
| DVT/PE (n, %) | 62 | (0.8) | 31 | (0.8) | 31 | (0.7) | 0.481 |
| 90-day re-admission (n, %) | 100 | (1.2) | 50 | (1.4) | 50 | (1.1) | 0.370 |
TXA, tranexamic acid; PJI, periprosthetic joint infection; DVT, deep vein thrombosis; PE, pulmonary embolism; SD; standard deviation
n univariate analysis, use of TXA (OR, 0.49; 95% CI, 0.34–0.71; p < 0.001), TKA (OR, 2.08; 95% CI, 1.23–3.51, p = 0.006) and longer surgical time (OR, 1.01; 95% CI, 1.00-1.01; p = 0.011) showed a statistically significant association with PJI. In multivariate analysis, after adjusting for potential confounders listed, the association between the use of TXA and PJI remained significant (OR 0.54, 95% CI 0.37–0.80, p = 0.002, Table 3). Covariates associated with higher odds of PJI included male (OR 1.53, 95%CI 1.03–2.28, P = 0.039) and TKA (OR 2.23, 95%CI 1.24-4.00, p = 0.008). No other covariates resulted in a significant odds ratio against PJI.
Table 3.
Univariate and multivariate analysis for periprosthetic joint infection
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | Adjust OR | 95%CI | p-value | |
| TXA | 0.49 | 0.34–0.71 | < 0.001 | 0.54 | 0.36–0.80 | 0.002 |
| Age (per 1-year increment) | 1.00 | 0.99–1.02 | 0.644 | 1.00 | 0.98–1.02 | 0.680 |
| Male | 1.34 | 0.93–1.92 | 0.119 | 1.53 | 1.03–2.28 | 0.039 |
| Surgery: TKA | 2.08 | 1.23–3.51 | 0.006 | 2.23 | 1.24-4.00 | 0.008 |
| BMI (per 1-kg/m2 increment) | 1.03 | 0.99–1.07 | 0.108 | 1.02 | 0.98–1.07 | 0.289 |
| Laterality: Right | 0.84 | 0.60–1.19 | 0.335 | 0.84 | 0.59–1.19 | 0.304 |
| Preoperative Hb (per 1- g/dL increment) | 1.05 | 0.95–1.15 | 0.390 | 0.97 | 0.87–1.08 | 0.601 |
| Surgical time (per 1- min increment) | 1.01 | 1.00-1.01 | 0.011 | 1.00 | 0.99–1.01 | 0.153 |
| CCI > 2 | 1.17 | 0.81–1.70 | 0.396 | - | - | -^ |
| Heart disease | 0.81 | 0.33-2.00 | 0.650 | 0.73 | 0.29–1.82 | 0.499 |
| COPD | 1.27 | 0.47–3.48 | 0.637 | 1.23 | 0.44–3.42 | 0.695 |
| Diabetes | 1.10 | 0.74–1.66 | 0.632 | 0.95 | 0.62–1.45 | 0.809 |
| Renal failure | 1.25 | 0.84–1.87 | 0.272 | 1.07 | 0.71–1.62 | 0.742 |
| Liver disease | 1.90 | 0.77–4.72 | 0.164 | 1.79 | 0.71–4.47 | 0.215 |
| Rheumatoid Arthritis | 1.39 | 0.61–3.18 | 0.436 | 1.47 | 0.62–3.50 | 0.377 |
| ASA > 3 | 0.75 | 0.53–1.06 | 0.108 | 0.73 | 0.51–1.07 | 0.104 |
| General anesthesia | 0.66 | 0.34–1.31 | 0.237 | 0.62 | 0.31–1.25 | 0.184 |
^CCI > 2 is not involved into analysis due to collinearity with following comorbidities
TXA, tranexamic acid; TKA, total knee arthroplasty; BMI, body mass index; Hb, hemoglobin; CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; ASA, American Society of Anesthesiology; OR, odds ratio; CI, confidence interval
In three subgroup analysis of IV and topical TXA, both intravenous TXA and topical TXA demonstrated lower odds of PJI compared to control group (IV group: OR 0.53, 95% CI, 0.33–0.84, p = 0.007, topical group: OR 0.51, 95% CI, 0.29–0.89, p = 0.018; Table 4). No significant difference was observed between IV and topical TXA in terms of PJI occurrence (OR 0.96, 95% CI, 0.50–1.85, p = 0.907). When stratified by hip or knee arthroplasty, both IV TXA and topical TXA were associated with significantly lower odds of PJI among TKA patients (IV TXA, OR 0.49, 95% CI, 0.29–0.83, p = 0.008; Topical TXA, OR, 0.56, 95% CI, 0.32–0.98, p = 0.042).
Table 4.
Subgroup analysis for association between TXA and PJI
| Variable | PJI number/ total | PJI rate | Adjust OR | 95%CI | p value |
|---|---|---|---|---|---|
| Overall (n = 8042) | |||||
| No TXA | 94 / 4378 | 2.1% | reference | ||
| With TXA | 39 / 3664 | 1.1% | 0.54 | 0.36–0.80 | 0.002 |
| IV TXA | 24 / 2345 | 1.0% | 0.53 | 0.33–0.84 | 0.007 |
| Topical TXA | 15 / 1319 | 1.1% | 0.51 | 0.29–0.89 | 0.018 |
| IV TXA - Topical TXA | - | 0.96 | 0.50–1.85 | 0.907 | |
| Hip (n = 1766) | |||||
| No TXA | 10 / 797 | 1.3% | reference | ||
| With TXA | 6 / 969 | 0.6% | 0.51 | 0.17–1.47 | 0.209 |
| IV TXA | 6 / 777 | 0.8% | 0.65 | 0.22–1.89 | 0.426 |
| Topical TXA | 0 / 192 | 0% | - | - | 0.995$ |
| IV TXA - Topical TXA | - | - | - | 0.995$ | |
| Knee (n = 6276) | |||||
| No TXA | 84 / 3581 | 2.3% | reference | ||
| With TXA | 33 / 2695 | 1.2% | 0.52 | 0.34–0.79 | 0.002 |
| IV TXA | 18 / 1568 | 1.1% | 0.49 | 0.29–0.83 | 0.008 |
| Topical TXA | 15 / 1127 | 1.3% | 0.56 | 0.32–0.98 | 0.042 |
| IV TXA - Topical TXA | - | 1.13 | 0.57–2.25 | 0.731 | |
$The model failed because of the small sample size
TXA, tranexamic acid; PJI, periprosthetic joint infection; OR: odds ratio; CI, confidence interval
After applying the weighting, there were no significant differences observed between two groups in terms of age, sex, type of surgery, BMI, laterality, preoperative hemoglobin, surgical time, anesthesia, ASA grade, or CCI scores (Table 5). The outcome remains consistent after weighting. The use of TXA was found to be associated with lower occurrence of PJI (OR 0.53, 95% CI, 0.36–0.80, p = 0.002) and shorter hospital day, average 0.86 days less (95% CI,0.77 to 0.95 days, p = 0.017). There were no significant differences observed in the occurrence of thromboembolism events, 90-day admission, or transfusion requirements (Table 6).
Table 5.
Characteristics assessed for unweighted and weighted standardized mean difference (SMD) following propensity score weighting, using the average treatment effect for the treated (ATT) estimation
| Variable | Unweighted cohort | Propensity score weighting cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TXA | non-TXA | SMD (%) | TXA | non-TXA | SMD (%) | ||||||
| (n = 3664) | (n = 4377) | (n = 3664) | (n = 3637) | ||||||||
| Age, year (mean ± SD) | 67.6 | ± 10.6 | 68.1 | ± 10.0 | 4.2 | 67.6 | ± 10.6 | 67.9 | ± 10.3 | 2.4 | |
| Male (n, %) | 1047 | (28.6) | 1189 | (27.2) | 3.2 | 1047 | (28.6) | 1067 | (29.3) | 1.7 | |
| Surgery: TKA (n, %) | 2695 | (73.6) | 3581 | (81.8) | 19.9 | 2695 | (73.6) | 2707 | (74.4) | 2.0 | |
| BMI, kg/m2 (mean ± SD) | 26.9 | ± 4.5 | 26.9 | ± 4.1 | 0.3 | 26.9 | ± 4.5 | 26.9 | ± 4.3 | 0.4 | |
| Laterality (n, %) | 1.4 | 0.9 | |||||||||
| Left | 1913 | (52.2) | 2285 | (52.2) | 1913 | (52.2) | 1896 | (52.1) | |||
| Right | 1749 | (47.7) | 2088 | (47.7) | 1749 | (47.7) | 1740 | (47.8) | |||
| Bilateral | 2 | (0.1) | 4 | (0.1) | 2 | (0.1) | 1 | (0.1) | |||
| Preoperative Hb, g/dL (mean ± SD) | 12.2 | ± 1.8 | 12.8 | ± 1.7 | 34.3 | 12.2 | ± 1.8 | 12.1 | ± 1.8 | 1.4 | |
| Surgical time, min (mean ± SD) | 142 | ± 31 | 154 | ± 31 | 40.0 | 142 | ± 31 | 143 | ± 25 | 4.6 | |
| CCI > 2 (n, %) | 2258 | (61.7) | 3964 | (67.7) | 12.7 | 2258 | (61.6) | 2263 | (62.2) | 1.3 | |
| ASA > 3 (n, %) | 1476 | (40.3) | 3687 | (16.9) | 7.3 | 1477 | (40.3) | 1469 | (40.4) | 0.2 | |
| General anesthesia (n, %) | 3227 | (88.1) | 4026 | (92.0) | 13.0 | 437 | (11.9) | 451 | (12.4) | 1.4 | |
SMD, standardized mean difference; TXA, tranexamic acid; TKA, total knee arthroplasty; CCI, Charlson Comorbidity Index; ASA, American Society of Anesthesiology score
Table 6.
Outcomes associated with the use of TXA through propensity score weighting
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| PJI | 0.53 | 0.36–0.80 | 0.002 |
| Transfusion | 0.43 | 0.13–1.44 | 0.173 |
| 90-day readmission | 1.15 | 0.35–3.72 | 0.800 |
| DVT/PE | 1.21 | 0.37–3.93 | 0.745 |
| Length of stay estimate (days) | 0.86 | 0.77–0.95 | 0.017 |
|
PJI, periprosthetic joint infection; DVT, deep vein thrombosis; PE, pulmonary embolism; OR: odds ratio; CI, confidence interval | |||
Discussion
This study found that perioperative TXA was associated with a decreased PJI occurrence after primary TJA. In the subgroup analysis, different routes of TXA administration were all associated with decreased odds of PJI, especially after primary TKA. Our findings align with previous studies that have shown a correlation between the use of TXA and a reduced PJI occurrence following primary total joint replacement [11, 12]. Similar results were observed in the cohort of Heckmann et al., which included over one million patients and demonstrated that TXA administration was associated with decreased blood loss, anemia, transfusion, and PJI event, without an increase of thromboembolic complications [16] There are several hypotheses explaining how TXA may contribute to a lower the risk of PJI. First, TXA effectively reduces blood loss during surgery, thereby reducing the risk of anemia, blood transfusion, and the formation of hematomas, all of which are established risk factors for PJI [7]. In our study, patients who received TXA had a decreased need for blood transfusions, shorter hospital stays, and lower PJI occurrence.
In addition to its impact on blood loss, there may be other mechanisms by which TXA reduces the risk of PJI. One study suggested that TXA possesses an inhibitory effect on biofilm formation in infected tissue, thereby providing protection against PJI [17]. Furthermore, TXA has been shown to modulate the immune response through its action on plasmin, which may contribute to reduced postsurgical infection rates [18]. Another in vitro study demonstrated that TXA exhibits an antibiotic effect by suppressing bacterial growth in a planktonic state [19]. These findings collectively support the beneficial effects of TXA in reducing the risk of PJI through multiple pathways, including the reduction of blood loss, transfusion requirements, and potential antimicrobial and immunomodulatory properties.
Our second important finding revealed that both intravenous and topical administration of TXA was related to a decreased PJI occurrence in primary total knee arthroplasty. This result differs from the study conducted by Yazdi et al. [12], which showed a significant association between TXA and lower odds of PJI in primary total hip arthroplasty. Although not statistically significant, there was a 49% decrease in PJI in THA with TXA, a 35% decrease in PJI in THA with IV TXA, and no PJI events in cases of THA patients with topical TXA. This result may be indicative of a type I error in the THA group, or it’s possible that the study was underpowered to detect a difference in the THA group. Our study’s power calculation was based on all TJAs, encompassing both THA and TKA. A larger-scale study is necessary to comprehensively assess the effectiveness of TXA in THA patients.
The factors associated with increased PJI occurrence in our studies are male and undergoing total knee arthroplasty (TKA), which aligns with findings from previous literature [20]. Yazdi et al. provided several explanations for increased PJI risk in males, including potential confounders such as smoking and alcohol abuse. Factors such as skin metabolism, hair growth, sebum production, skin pH, and skin thickness may also contribute to this predisposition [21]. Another study by Beam et al. speculated that higher PJI occurrence in TKA patients could be attributed to less protective soft tissue coverage and increased stress during joint movement [22].
Our analysis revealed a higher transfusion rate, with 14.4% in the TXA group and 22.7% in the control group. We hypothesized that the elevated blood transfusion rate could be attributed to several factors, including older age, a higher proportion of female patients, increased prevalence of anemia, presence of comorbidities (higher CCI scores), and the majority of surgeries being performed under general anesthesia. It is important to note that these factors may differ from those in the study conducted by Yazdi et al., potentially contributing to the differences in transfusion rates observed between the two studies [23].
There is a limited number of studies in the reviewed literature that directly compares the efficacy of different routes of TXA administration in patients undergoing primary joint arthroplasty with a sub-group analysis of hip and knee. One strength of our study is the longer follow-up time of at least one year [24], which sets it apart from other studies that typically limit their assessment of PJI to a 90-day period [11, 12]. This extended follow-up period allows us to gather data from a significant proportion of PJI patients, providing a more comprehensive understanding of the outcomes.
This study, while providing important insights, is subject to several limitations. Firstly, as a single-center study employing a uniform protocol for TXA administration, the external validity and applicability of our findings to varied clinical settings may be constrained. Secondly, the retrospective design of our research inherently carries limitations typical of such methodologies. The higher proportion of knee arthroplasties (TKA) and longer surgical times complicated baseline adjustments in the non TXA group. Although univariate analysis showed a higher odds ratio for PJI related to TKA and longer durations, odds ratio for PJI regarding surgical time was not significant in the stepwise multivariate analysis, indicating that surgical time’s effect was mitigated by controlling for other factors. While propensity score weighting (PSW) aimed to balance the influence of TKA and surgical time, collinearity in the no TXA group may limit definitive conclusions. There are also potential biases due to unobserved confounders. While we have attempted to mitigate this through comprehensive multivariate regression and propensity score weighting (PSW), some degree of residual bias cannot be entirely ruled out. While propensity score matching (PSM) could have been employed, it often reduces the sample size to below 2000 participants, leading to an effect size lacking clinical significance. Therefore, we chose PSW to retain all samples, enhancing statistical power and reducing bias. However, while PSW controls for known confounding variables, the potential impact of unknown confounders remains a concern. Despite this, we believe PSW offers a more accurate comparison of treatment effects in this study. Our findings on TXA’s association with reduced PJI rates should be considered with potential protocol deviations. Patients with contraindications, such as prior thromboembolic events, renal impairment or allergy, were excluded from TXA administration, possibly underestimating TXA’s effect. Variability in TXA dosing and timing, especially with IV administration influenced by weight and surgical delays, may also impact outcomes. Incomplete TXA administration due to intraoperative reactions introduces further bias. Despite these limitations, the consistent association observed between TXA use and reduced PJI occurrence underscores the significance of TXA as a key factor in PJI reduction. However, further research addressing these limitations would be invaluable in solidifying the role of TXA in orthopedic surgery.
In conclusion, perioperative TXA administration in primary hip and knee arthroplasty is significantly associated with lower PJI occurrence after controlling for potential confounders. Both IV and topical TXA routes showed similar association with reduced PJI occurrence. These findings suggest that TXA may effectively prevent PJI, likely due to its blood-conserving properties. Further research is needed to explore the mechanisms underlying TXA’s protective effects in orthopedic surgery.
Acknowledgements
We acknowledge and thank the Biostatistics Center at Kaohsiung Chang Gung Memorial Hospital and Mr. Chun-Sheng Lin for their statistical work. This work was partially supported by Kaohsiung Chang Gung Memorial Hospital, Taiwan (Grant numbers: CORPG8M0011 to FC Kuo and CMRPG8K1551, CMRPG8K1552, CMRPG8K1553 to JW Wang).
Abbreviations
- TXA
Tranexamic acid
- PJI
Periprosthetic Joint Infection
- TJA
Total Joint Arthroplasty
- TKA
Total Knee Arthroplasty
- THA
Total Hip Arthroplasty
- BMI
Body Mass Index
- Hb
Hemoglobin
- CCI
Charlson Comorbidity Index
- ASA
American Society of Anesthesiologists
- SMD
Standardized Mean Difference
- ORs
Odds Ratios
Author contributions
All authors made a significant contribution to the research concept, design, data collection, analysis and interpretation of data, and critical correction of important intellectual content. All authors have read and approved the final submitted version of the manuscript. Specific contributions are: (1) Conception and design of research: YC Hsu, FC Kuo (2) Data acquisition: YC Hsu, FC Kuo (3) Data analysis and interpretation: Allen HS Hsu and CT Wu (4) Draft article: Y.C. Hsu and TL Tan (5) Final approval of submitted version: JW Wang and FC Kuo.
Funding
This work was partially supported by Kaohsiung Chang Gung Memorial Hospital, Taiwan (Grant numbers: CORPG8M0011 to FC Kuo and CMRPG8K1551, CMRPG8K1552, CMRPG8K1553 tos JW Wang).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The need for informed consent was waived by the Ethics Committee/Institutional Review Board of the Institutional Review Board of Chang Gung Medical Foundation has approved this study (IRB No. 202201588B0).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jun-Wen Wang, Email: wangjw@adm.cgmh.org.tw.
Feng-Chih Kuo, Email: fongchikuo@cgmh.org.tw.
References
- 1.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–33. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Springer BD, Cahue S, Etkin CD, Lewallen DG, McGrory BJ. Infection burden in total hip and knee arthroplasties: an international registry-based perspective. Arthroplast Today. 2017;3:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Tsai W-C, Tan T-S, Kung P-T, Chiu L-T, Ku M-C. Temporal trends in primary and revision total knee and hip replacement in Taiwan. J Chin Med Association. 2015;78:538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Premkumar A, Kolin DA, Farley KX, Wilson JM, McLawhorn AS, Cross MB, et al. Projected Economic Burden of Periprosthetic Joint Infection of the hip and knee in the United States. J Arthroplast. 2021;36:1484–e14893. [DOI] [PubMed] [Google Scholar]
- 6.Greenky M, Gandhi K, Pulido L, Restrepo C, Parvizi J. Preoperative anemia in total joint arthroplasty: is it associated with periprosthetic joint infection? Clin Orthop Relat Res. 2012;470:2695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96:272–8. [DOI] [PubMed] [Google Scholar]
- 8.Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, et al. The efficacy of Tranexamic Acid in Total Hip Arthroplasty: A Network Meta-analysis. J Arthroplasty. 2018;33:3083–e30894. [DOI] [PubMed] [Google Scholar]
- 9.Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, et al. The Safety of Tranexamic Acid in Total Joint Arthroplasty: a direct Meta-analysis. J Arthroplasty. 2018;33:3070–e30821. [DOI] [PubMed] [Google Scholar]
- 10.Luo Z-Y, Wang H-Y, Wang D, Zhou K, Pei F-X, Zhou Z-K. Oral vs intravenous vs topical tranexamic acid in primary hip arthroplasty: a prospective, randomized, Double-Blind, controlled study. J Arthroplasty. 2018;33:786–93. [DOI] [PubMed] [Google Scholar]
- 11.Hong GJ, Wilson LA, Liu J, Memtsoudis SG. Tranexamic Acid Administration is Associated with a decreased odds of Prosthetic Joint Infection Following Primary Total Hip and primary total knee arthroplasty: a National Database Analysis. J Arthroplasty. 2021;36:1109–13. [DOI] [PubMed] [Google Scholar]
- 12.Yazdi H, Klement MR, Hammad M, Inoue D, Xu C, Goswami K, et al. Tranexamic acid is Associated with reduced periprosthetic joint infection after primary total joint arthroplasty. J Arthroplast. 2020;35:840–4. [DOI] [PubMed] [Google Scholar]
- 13.Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92:2503–13. [DOI] [PubMed] [Google Scholar]
- 14.Parvizi J, Gehrke T, Chen AF. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J. 2013;95-B:1450–2. [DOI] [PubMed]
- 15.Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86:970–3. [DOI] [PubMed] [Google Scholar]
- 16.Heckmann ND, Haque TF, Piple AS, Mayfield CK, Bouz GJ, Mayer L et al. Tranexamic acid and prothrombotic complications following total hip and total knee arthroplasty: a Population-wide Safety Analysis Accounting for Surgeon Selection Bias. J Arthroplast. 2022;0. [DOI] [PubMed]
- 17.Wang J, Zhang Z, Li J, Huang B, Jiang Z, Pan Y, et al. Tranexamic acid protects against implant-associated infection by reducing biofilm formation. Sci Rep. 2022;12:4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draxler DF, Yep K, Hanafi G, Winton A, Daglas M, Ho H, et al. Tranexamic acid modulates the immune response and reduces postsurgical infection rates. Blood Adv. 2019;3:1598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjumea A, Díaz-Navarro M, Hafian R, Sánchez-Somolinos M, Vaquero J, Chana F, et al. Effect of Tranexamic Acid against Staphylococcus spp. and Cutibacterium acnes Associated with Peri-implant infection: results from an in vitro study. Microbiol Spectr. 2023;10:e01612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong L, Cao J, Zhang Y, Ding W, Shen Y. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: a meta-analysis. Int Wound J. 2017;14:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panula VJ, Alakylä KJ, Venäläinen MS, Haapakoski JJ, Eskelinen AP, Manninen MJ, et al. Risk factors for prosthetic joint infections following total hip arthroplasty based on 33,337 hips in the Finnish Arthroplasty Register from 2014 to 2018. Acta Orthop. 2021;92:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beam E, Osmon D. Prosthetic joint infection update. Infect Dis Clin North Am. 2018;32:843–59. [DOI] [PubMed] [Google Scholar]
- 23.Hart A, Khalil JA, Carli A, Huk O, Zukor D, Antoniou J. Blood transfusion in primary total hip and knee arthroplasty. Incidence, risk factors, and thirty-day complication rates. J Bone Joint Surg Am. 2014;96:1945–51. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Tan TL, Li WT, Goswami K, Parvizi J. Reporting outcomes of treatment for Periprosthetic Joint Infection of the knee and hip together with a Minimum 1-Year Follow-Up is Reliable. J Arthroplasty. 2020;35:1906–e19115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.