Abstract
Background
Accumulating evidence suggests that the radiographic phenotypes of patients with knee osteoarthritis (KOA) often do not correlate with their clinical findings, which are the primary reason for seeking medical care. Therefore, at OARSI 2024, we proposed a clinical finding staging system—Chinese medicine staging (CMS)—to guide the treatment of KOA. However, the clinical effectiveness and application characteristics of CMS in guiding non-surgical treatment of KOA remain unclear.
Methods
A total of 14,985 KOA patients were included in the study. Data from 13,983 patients were used to analyze the characteristics of CMS application, while 1465 patients were used to evaluate CMS-guided clinical effectiveness, and 152 patients were included in a comparative analysis of clinical effectiveness without CMS guidance. The demographic characteristics of the CMS-using population were examined, and the correlation between CMS and treatment modalities was analyzed to clarify CMS application characteristics. VAS and WOMAC scores were compared between the CMS-guided and non-CMS-guided groups both before treatment and at week 8 of treatment, using the minimal clinically significant difference as the benchmark.
Results
In application characteristics, regarding nonsurgical treatments, an increase in the CMS led to a decrease in basic treatment and an increase in nonpharmacological and pharmacological treatments (P < 0.001). Regarding surgical treatments, no change in the proportion of surgical interventions was observed with worsening CMS (P > 0.05). In clinical effectiveness, at week 8 of treatment, VAS scores and WOMAC scores were significantly lower in CMS-guided group (VSA: 3.51; 95% CI, 3.42–3.60, Pain: 3.55; 95% CI, 3.40–3.70, Stiffness: 1.18; 95% CI, 1.11–1.25, Function: 12.57; 95% CI, 12.07–13.08, Total: 17.31; 95% CI, 16.63–17.99), which had a higher net difference than non-CMS-guided group (VSA: 3.30; 95% CI, 3.05–3.54, Pain: 3.14; 95% CI, 2.68–3.60, Stiffness: 0.95; 95% CI, 0.76–1.15, Function: 11.36; 95% CI, 9.80–12.91, Total: 15.45; 95% CI, 13.40–17.49). The net differences in CMS-guided group were all higher than in MCID.
Conclusion
CMS is consistent with the patient’s clinical finding, is suitable for guiding non-surgical treatment of KOA and can achieve clinically significant therapeutic effects.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-024-05374-8.
Keywords: Osteoarthritis, Chinese medicine staging, Clinical symptom, Clinical decision, Clinical treatment
Introduction
Knee osteoarthritis (KOA) is a chronic disease that affects a significant number of middle-aged and elderly individuals [1, 2]. It impacts both their physical and psychological well-being to varying extents [3, 4], while also imposing a substantial economic burden on patients, their families, and society [5, 6]. Radiographic and symptomatic presentations are the two main features of KOA. Radiographs typically show degeneration of articular cartilage, subchondral osteosclerosis, and bone hypertrophy [7, 8]. Kellgren–Lawrence grading (KLG), a widely used method to assess the severity of osteoarthritis, is commonly applied to monitor disease progression and inform surgical decision-making in KOA [9–11].
Pain, the primary clinical manifestation of KOA [12], is a key factor contributing to functional limitations and poor quality of life, and it plays a crucial role in prompting individuals to seek medical care [13]. Previous studies have found a weak correlation between imaging features of KOA and clinical findings. Specifically, related studies have reported that 5.9–31.2% of individuals classified as KLG III or IV do not experience knee pain [14–16]. The American Academy of Orthopaedic Surgeons guidelines for KOA recommend treatment only for symptomatic KOA [17, 18]. However, in clinical practice, there remains a lack of standardized methods for assessing the severity of KOA symptoms.
At the 2023 World Congress on Osteoarthritis organized by the Osteoarthritis Research Society International, a new clinical finding-based method for classifying KOA, called Chinese medicine staging (CMS) [19], was proposed. This staging system categorizes patients into three groups based on clinical findings: CMS I, CMS II, and CMS III. However, the clinical effectiveness and application characteristics of CMS in guiding the treatment of KOA remain unclear. Therefore, the aim of this study is to analyse the application of CMS in KOA treatment using large sample data, in order to provide a scientific basis for its application in the management of KOA.
Population and methods
Study design and selection of patients
This study was led by the Third Affiliated Hospital of Beijing University of Chinese Medicine, China, integrating data from three case registry studies, one randomised controlled study and one community-based cross-sectional study on KOA. The study involving 84 hospitals and 8 community health service centers across China from September 2019 to December 2022 (Table S1), a total of 14,985 (Figure S1) KOA patients were included. Of these, data from 13,983 patients used to analyze application characteristics of the CMS, data from 1465 patients were used to assess CMS-guided clinical effectiveness, and data from 152 patients were used to analyze non-CMS-guided clinical effectiveness. This study was approved by the Scientific Research Ethics Committee of the Third Affiliated Hospital of Beijing University of Chinese Medicine, Wangjing Hospital of China Academy of Traditional Chinese Medicine, and Peking Union Medical College Hospital (BZYSY-2022KYKTPJ-04, BZYSY-2021KYKTSL-27, WJEC-KT-2018-039-P002, HS-2090, and HS-2363), and was registered in the China Clinical Trial Registry (ChiCTR2200062700, ChiCTR1900026262, ChiCTR2000034475, ChiCTR2100053812).
Diagnostic criteria
We have developed diagnostic criteria based on the American College of Rheumatology(ACR) clinical and radiological criteria [20, 21], including the following parameters: (1) recurrent knee pain in the past month; (2) age > 50 years; (3) morning stiffness lasting ≤ 30 min; (4) sensation of bone rubbing during activity; and (5) X-ray (standing or weight-bearing) showing narrowing of the joint space, subchondral sclerosis and/or cystic changes, and osteophyte formation at the edge of the joint. The diagnosis can be made if criterion 1 and any two of criteria 2, 3, 4, or 5 are met.
Inclusion and exclusion criteria
Patients were included in this study if they met the following inclusion and exclusion criteria. The inclusion criteria were (1) meeting the revised KOA diagnostic criteria of the American College of Rheumatology and (2) having complete clinical information. The exclusion criteria were (1) presence of other types of arthritis such as rheumatoid arthritis, gouty arthritis, septic arthritis, etc.; (2) presence of cerebrovascular and serious liver diseases affecting the patient’s quality of life; and (3) history of trauma to the knee joint.
CMS and KLG
Based on the “Better Management Of Knee Osteoarthritis: Chinese Medicine Treatment Guideline” [19], KOA was categorized into three clinical staging: (1) CMS I: mild knee pain(VAS < 4), mainly including soreness, accompanied by lassitude and inability to walk for a long time; (2) CMS II: moderate knee pain(VAS 4–7), mainly including vague pain, accompanied by soreness and weakness of the waist and knee, aggravated by physical activity; and (3) CMS III: severe knee pain(VAS > 7), mainly including stabbing, distending, and scorching pain, accompanied by joint heaviness, aggravated by cold and alleviated by warmth. Radiographic grading related to KOA was assessed using KLG [22]. We made a simple adjustment to CMS by referring to the order of KLG, with CMS I in the original article [19] corresponding to CMS III in this study.
Treatment and outcomes
In the clinical effectiveness analysis, with reference to the Guidelines for Chinese Medicine Diagnosis and Treatment of Knee Osteoarthritis (2020 Edition) [12], patients with CMS I were trained with Baduanjin (Chinese traditional exercise that consists of eight movements, as shown in Figure S2, 30 min/times, once/day, 5 times/week for 8 weeks), patients with CMS II was were treated with Tenghuang Jianggu Tablet (Please refer to the section on drug ingredients in the supplementary materials), and patients with CMS III were treated with Wangbi Tablet (Please refer to the section on drug ingredients in the supplementary materials). In addition, we included patients with KOA not guided by CMS who were treated with NSAIDs for comparative analyses with CMS effectiveness. Clinical effectiveness was assessed by Visual analogue scale (VAS) score (0 best-10 worst; minimal clinically important difference (MCID), 1.8 [23]), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) total score (0 best-8 worst; MCID, 8.97 [24]), WOMAC-Pain score (0 best-20 worst; MCID, 2.12 [24]), WOMAC-Function score (0 best-68 worst; MCID, 6 [23]), and WOMAC-Stiffness score (0 best-8 worst; MCID, 0.76 [24]). Meanwhile, improvement in each outcome greater than or equal to the MCID was considered clinically effective. These outcomes were measured before treatment and at week 8 of treatment.
Data collection and analysis
Clinical data from patients, including sex, age (50–59, 60–69, 70–79, and ≥ 80 years), occupation (manual labor, nonmanual labor), body mass index (BMI), living environment (high-rise without elevator, low-rise with elevator), family history, smoking habits, alcohol consumption, osteoporosis, CMS, KLG, treatment protocols, VAS score and WOMAC score were collected. The treatment modalities were based on the Guidelines for the Diagnosis and Treatment of Osteoarthritis in China (2021 edition) [25], which categorized treatments into five groups: basic treatment, nonpharmacological treatment, pharmacological treatment, restorative treatment, and reconstructive treatment. A Sankey diagram was developed to visualize the distribution and flow of clinical types and radiographic grades in patients with KOA. Correlation analysis was performed to examine the relationships between various variables and clinical typing as well as imaging grading, aiming to identify similarities and differences. Causality inference was then applied to explore potential causal relationships between variables, with data structured in the form of a raw matrix. Using the Causal Discovery Toolbox tool [26] in Python, an undirected graph was constructed based on the data characteristics. Subsequently, the graphical lasso method [27] was employed to predict the neighboring nodes of each variable, and the additive noise model [28] was used to identify pairs of causal relationships.
Statistical analysis
IBM SPSS Statistics V26.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Descriptive and frequency analyses were applied to general data, and the distribution of count data was expressed as counts (%) or median (interquartile range). The chi-square test or Fisher’s exact test was used for quantitative data analysis. For ordinal variables, Kendall’s τ-b test was used to assess correlation, while Cramer’s V test was applied to examine the strength of associations for dichotomous variables. All measured data were assessed for normality using the Shapiro–Wilk test. Data conforming to a normal distribution were compared using an independent samples t-test, while non-normally distributed data were analyzed using the Mann–Whitney U test. Differences were considered statistically significant at P < 0.05. Additionally, the results derivation of clinical studies should be considered in the context of clinical significance except for statistical significance. Therefore, MCID was used to evaluate the clinical benefit from a clinical perspective [29]. We employed the chi-square test to analyze the number of participants achieving the MCID, in order to assess the clinical benefit of CMS.
Results
Analysis of the application characteristics of CMS
Distribution of age in clinical staging and radiographic grading
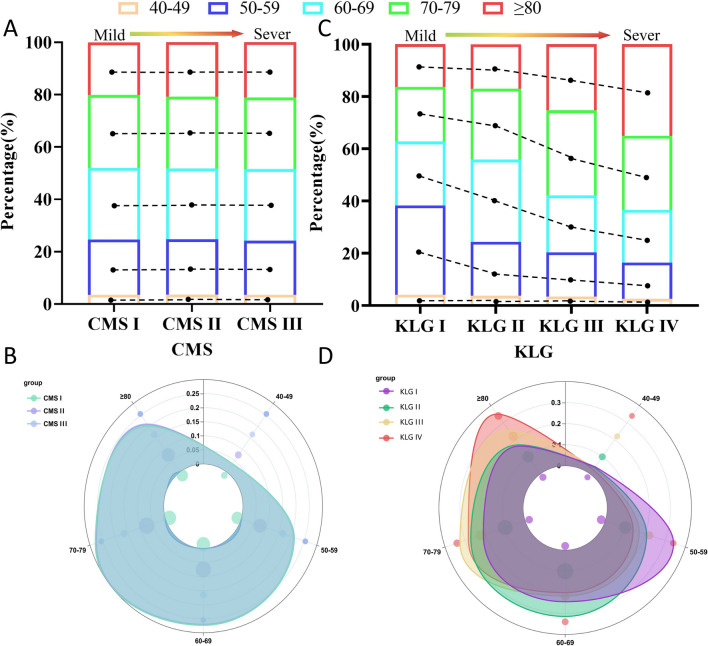
Data from 13,983 patients used to analyze CMS application characteristics, 5749 in the CMS I, 7167 in the CMS II, and 1067 in CMS III, 1948 in KLG I, 7467 in KLG II, 3274 for KLG III, and 1294 for KLG IV (Table S2). Based on the data distribution presented in the Sankey diagram (Figure S3), we compared the relationships of KLG and CMS with age (Table S3). Across CMS, there was no significant difference among age groups (χ2 = 1.250, P = 0.051), and CMS was not correlated with age (τ = 0.004, P = 0.626). However, across KLG, differences were observed among different age groups (χ2 = 628.306, P < 0.001), and KLG was correlated with age (τ = 0.149, P < 0.001). With increasing age, KLG showed an increasing trend, whereas there was no significant change in CMS (Fig. 1).
Fig. 1.
Distribution of age in CMS and KLG. The age were evenly distributed among the different CMS (A, B), while KLG showed an aggravating trend with age (C, D)
Treatment modalities
Nonsurgical treatment
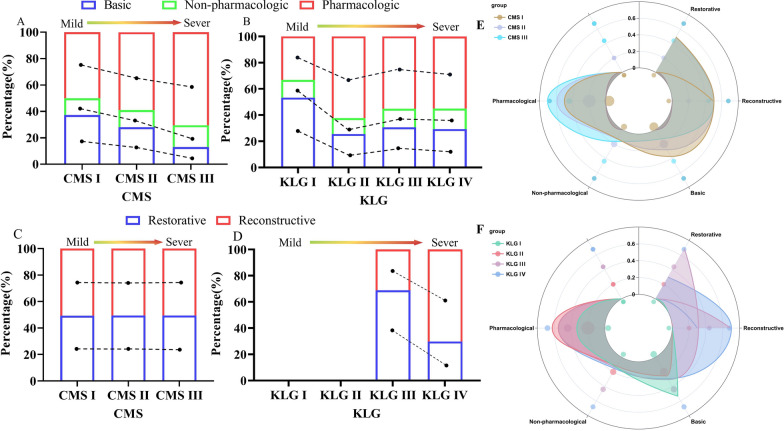
There were differences in nonsurgical treatment across different CMS (χ2 = 430.396, P < 0.001), and similar differences were observed across different KLG (χ2 = 637.272, P < 0.001). However, compared with KLG, CMS showed a stronger correlation with nonsurgical treatment (τ = 0.240 > 0.182) (Table S4). As the CMS worsened, the number of pharmacological and nonpharmacological treatments tended to increase, in addition to basic treatment. Conversely, no significant patterns were observed across KLG (Fig. 2A, B).
Fig. 2.
Distribution of treatment modalities in CMS and KLG. As CMS aggravated, there was an increasing trend in pharmacologic and non-pharmacologic treatments (A), whereas there was no significant pattern in KLG (B). There were no significant diferences in the proportions of different CMS across surgical treatments (C). KLG III has more restorative treatment while KLG IV has more reconstructive treatment (D). E, F is the percentage of CMS and KLG across treatment modalities
Surgical treatment
There were differences in surgical treatment across different KLG (χ2 = 70.920, τ = 0.391, P < 0.001) but not across different CMS (χ2 = 0.190, τ = 0.013, P > 0.05). A significant correlation was found between KLG and surgical treatment compared with CMS (Table S5). There were no significant differences in the proportions of patients across different CMS and surgical modalities, but differences were observed across KLG (Fig. 2C, D).
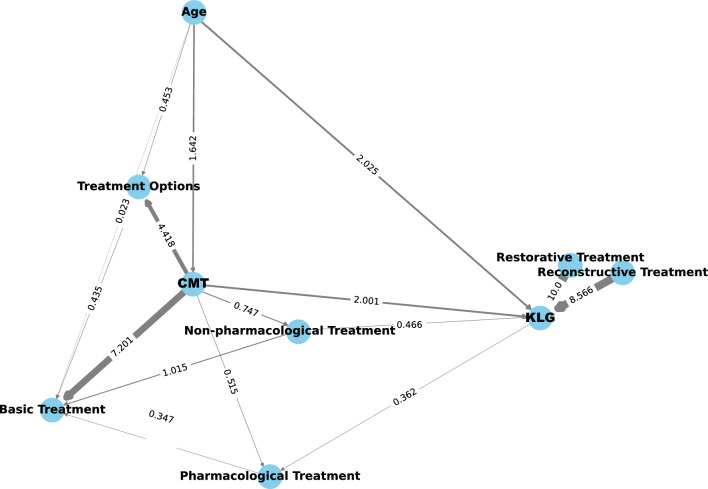
Causality analysis
The results indicated that KLG had a stronger causal relationship with age compared with CMS. Additionally, treatment options showed a strong causal relationship with CMS, especially in nonsurgical treatments (basic, nonpharmacological, and pharmacological). Conversely, surgical treatments (restorative and reconstructive) exhibited a strong causal relationship with KLG (Fig. 3).
Fig. 3.
Causality diagram illustrating the causation between CMS, KLG, and various factors. The arrows point to ‘results’. The value is the causality factor. Larger values and thicker lines indicate a more pronounced causality
Analysis of clinical outcomes
Clinical effectiveness under CMS guidance
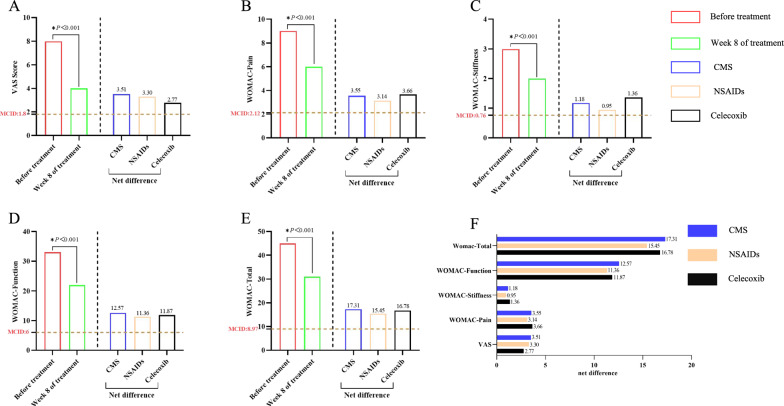
CMS included a total of 1465 patients with KOA, the net difference in VAS at week 8 of treatment was 3.51 (95% CI: 3.42, 3.60). The net difference in WOMAC total score was 17.31 (95% CI: 16.63, 17.99). The net difference in WOMAC pain was 3.55 (95% CI: 3.40, 3.70). The net difference in WOMAC stiffness was 1.18 (95% CI: 1.11, 1.25). The net difference in WOMAC function was 12.57 (95% CI: 12.07, 13.08). Data from 152 patients used to analyze clinical effectiveness that were not guided by CMS, the net difference in VAS, WOMAC pain, WOMAC stiffness and WOMAC total at week 8 of treatment was 3.30 (95% CI: 3.05, 3.54), 3.14 (95% CI: 2.68, 3.60), 0.95 (95% CI: 0.76, 1.15), 11.36 (95% CI: 9.80, 12.91), 15.45(95% CI: 13.40, 17.49) (Table S6).
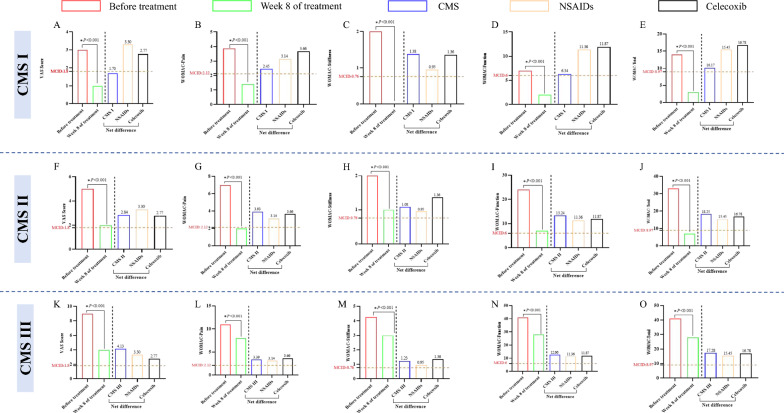
We analysed the results of the study in comparison with the study of celecoxib for KOA [30], and found that the net difference in VAS, function and WOMAC total score was significantly higher in CMS than celecoxib (3.51 vs. 2.77, 12.57 vs. 11.87, 17.31 vs. 16.78), and there was no significant difference in the net difference in pain (3.55 vs. 3.66) and stiffness (1.18 vs. 1.36). In addition, the net differences in CMS were all higher than in MCID, which has clinical therapeutic implications. The intuitive comparison trends are shown in Fig. 4.
Fig. 4.
Differences in VAS and WOMAC scores at each visit point in CMS. Net difference: difference between before and Week 8 of treatment, positive values being improvement, negative values being aggravation. Celecoxib: all results are the difference from baseline at Week 8 of treatment, and the VAS and WOMAC scores were scored in units of 100 mm, and the values were homogenised to ensure comparability of study results. A–E is the change of VAS and WOMA scores in CMS, the net difference of all outcomes is higher than MCID. F CMS has higher net differences in VAS, function, and WOMAC-Total than NSAIDs and celecoxib, and the net difference between Stiffness and Pain was higher than NSAIDs but lower than celecoxib
We also analysed the clinical effectiveness of each CMS. In 71 CMS I patients, at week 8 of treatment, the net difference in VAS was 1.70 (95% CI: 1.49, 1.92). The net difference in WOMAC total, WOMAC pain, WOMAC stiffness, and WOMAC function was 10.17 (95% CI: 7.51, 12.83), 2.45 (95% CI: 1.72, 3.18), 1.38 (95% CI: 1.00, 1.76), 6.34 (95% CI: 4.19, 8.49). In 569 CMS II patients, at week 8 of treatment, the net difference in VAS was 2.84 (95% CI: 2.72, 2.96). The net difference in WOMAC total, WOMAC pain, WOMAC stiffness, and WOMAC function was 18.25 (95% CI: 17.23, 19.28), 3.93 (95% CI: 3.71, 4.15), 1.08 (95% CI: 0.98, 1.19), 13.24 (95% CI: 12.46, 14.02). In 825 CMS III patients, at week 8 of treatment, the net difference in VAS was 4.13 (95% CI: 4.02, 4.24). The net difference in WOMAC total, WOMAC pain, WOMAC stiffness, and WOMAC function was 17.28 (95% CI: 17.23, 19.28), 3.39 (95% CI: 3.18, 3.60), 1.23 (95% CI: 1.14, 1.33), 12.66 (95% CI: 11.97, 13.34). All statistically significant differences (P < 0.001) (Tables 1, 2). Net difference of all outcomes exceeded MCID which was clinically significant. The intuitive decreased tendency were shown in Fig. 5.
Table 1.
Changes in VAS scores at CMS I–III
| CM staging | Before treatment | Week 8 of treatment | Net difference (95%CI)a | Decrease (95%CI) | P |
|---|---|---|---|---|---|
| CMS I (n = 71) | 3.00 (2.50, 3.00) | 1.00 (1.00, 1.00) | 1.70 (1.49, 1.92) | 62% (55–69) | < 0.001 |
| CMS II (n = 569) | 5.00 (4.00, 6.00) | 2.00 (1.00, 3.00) | 2.84 (2.72, 2.96) | 57% (54–59) | < 0.001 |
| CMS III (n = 825) | 9.00 (8.00, 9.00) | 4.00 (4.00, 6.00) | 4.13 (4.02, 4.24) | 47% (46–48) | < 0.001 |
Data representation: M(Q1–Q3)
aNet difference: difference between before and Week 8 of treatment, positive values being improvement, negative values being aggravation
Table 2.
Changes in WOMAC scores at CMS I–III
| CM staging | Outcome | Before treatment | Week 8 of treatment | Net difference (95%CI)a | Decrease (95%CI) | P |
|---|---|---|---|---|---|---|
| CMS I (n = 71) | WOMAC-pain | 4.00 (2.00, 5.00) | 1.00 (0.00, 2.00) | 2.45 (1.72, 3.18) | 58% (47–69) | < 0.001 |
| WOMAC-stiffness | 2.00 (0.00, 3.50) | 0.00 (0.00, 0.00) | 1.38 (1.00, 1.76) | 50% (39–62) | < 0.001 | |
| WOMAC-function | 7.00 (5.50, 16.50) | 2.00 (0.00, 5.00) | 6.34 (4.19, 8.49) | 58% (42–75) | < 0.001 | |
| Womac-total | 14.00 (9.00, 24.50) | 3.00 (0.00, 7.00) | 10.17 (7.51, 12.83) | 63% (52–73) | < 0.001 | |
| CMS II (n = 569) | WOMAC-pain | 7.00 (5.00, 8.00) | 2.00 (1.00, 4.00) | 3.93 (3.71–4.15) | 59% (57–62) | < 0.001 |
| WOMAC-stiffness | 2.00 (1.00, 3.00) | 1.00 (0.00, 2.00) | 1.08 (0.98–1.19) | 40% (36–44) | < 0.001 | |
| WOMAC-function | 24.00 (14.00, 29.00) | 7.00 (3.00, 13.00) | 13.24 (12.46–14.02) | 57% (54–60) | < 0.001 | |
| Womac-total | 33.00 (20.00, 41.00) | 9.00 (5.00, 19.00) | 18.25 (17.23–19.28) | 58% (56–61) | < 0.001 | |
| CMS III (n = 825) | WOMAC-pain | 11.00 (9.00, 13.00) | 8.00 (7.00, 10.00) | 3.39 (3.18, 3.60) | 26% (25–28) | < 0.001 |
| WOMAC-stiffness | 4.00 (3.00, 5.00) | 3.00 (2.00, 4.00) | 1.23 (1.14, 1.33) | 22% (20–24) | < 0.001 | |
| WOMAC-function | 41.00 (34.00, 47.00) | 28.00 (24.00, 34.00) | 12.66 (11.97, 13.34) | 28% (27–29) | < 0.001 | |
| Womac-total | 56.00 (47.00, 66.00) | 38.00 (34.00, 47.00) | 17.28 (16.34, 18.21) | 28% (26–29) | < 0.001 |
Data representation: M(Q1–Q3)
aNet difference: difference between before and week 8 of treatment, positive values being improvement, negative values being aggravation
Fig. 5.
A–E Differences in VAS and WOMAC scores at each visit point in CMS I. The net difference of VAS was lower than MCID, and the net difference of pain, stiffness, function, and WOMAC total scores were higher than MCID. The net difference of all outcomes was lower than Celecoxib. F–J Differences in VAS and WOMAC scores at each visit point in CMS II. The change of VAS and WOMA scores, the net difference of VAS, Pain, Stiffness, Function, and WOMAC total scores were higher than MCID. The net difference of VAS, Pain, Function, and WOMAC total scores was higher than Celecoxib. K–O Differences in VAS and WOMAC scores at each visit point in CMS III. The net difference of VAS, Pain, Stiffness, Function, and WOMAC total scores were higher than MCID. The net difference of VAS, Function, and WOMAC total scores was higher than Celecoxib
Changes in CMS and KLG before and after treatment
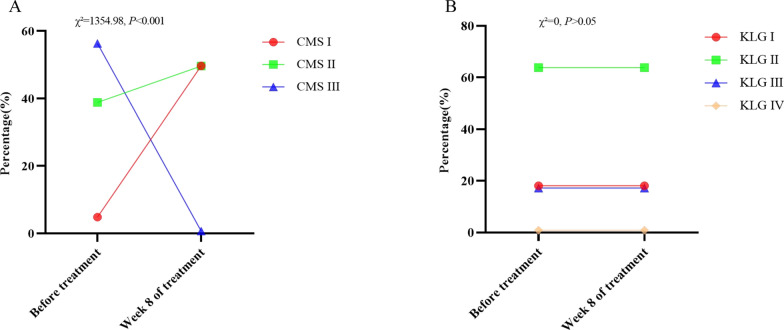
Of the 1,465 patients with KOA in CMS, the percentage of CMS I-III before treatment was 4.85%, 38.84%, 56.31%, and after treatment was 49.69%, 49.62%, and 0.68%, respectively, which is a statistically significant difference (χ2 = 1354.98, P < 0.001). However, the percentage of KLG I-IV was unchanged before and after treatment (χ2 = 0, P > 0.05) (Table S7, Fig. 6).
Fig. 6.
Changes in CMS and KLG before and after treatment
Discussion
In this study of 13,983 KOA patients analyzing the characteristics of CMS applications, over 90% experienced moderate-to-mild pain. Approximately 4.72% of patients exhibited clinical symptoms inconsistent with their radiographic findings, with 0.24% of those with KLG I in CMS III and 4.48% with KLG IV in CMS I. Previous studies have highlighted the discrepancy between knee pain and OA, particularly in cases of mild OA, and noted that a proportion of patients with severe OA report no pain (approximately 25.8%) [31]. While the findings in this survey are significantly lower than those reported in earlier studies, it is important to consider that global OA incidence was projected to reach approximately 414.7 million cases of OA by 2019 [32–34]. Even at 4.72%, this corresponds to an estimated 20 million individuals experiencing inconsistent symptoms and radiographic findings. This suggests that a significant number of patients may be receiving treatment regimens that do not align with their clinical symptoms, potentially leading to substantial waste of healthcare resources. Therefore, implementing clinically tailored treatment strategies for KOA is crucial.
The evaluation of the demographic data for patients with KOA revealed variations in age distribution based on clinical typing and radiographic grading. Advanced age is widely recognized as a significant factor in the development of KOA [34], with previous studies suggesting that the severity of OA tends to worsen with age [35, 36]. Regardless of correlation or causality, our results indicate a stronger correlation between KLG and age. Interestingly, no significant differences were observed in the proportion of patients across different age groups in the CMS. While it is well established that age correlates with osteoarthritis, it does not necessarily correlate with the severity of clinical symptoms. This explains the absence of a significant relationship between CMS (a clinical findings Staging method) and age. Therefore, this finding suggests that CMS is independent of age. Consequently, CMS can be effectively applied across different age groups, offering an objective reflection of patients' actual symptoms. As the predominant population affected by KOA, women exhibit a higher prevalence than men [37]. Previous studies have demonstrated sex differences in pain perception [38–41], with women generally exhibiting lower pain thresholds compared with men [42]. In our study, women were more prevalent in both CMS and KLG, suggesting that they may experience more severe clinical symptoms and radiographic manifestations (Table S8). Obesity, a recognized risk factor for KOA [43], is associated with chronic inflammation [44]. BMI, which is closely related to total body fat, reflects the degree of obesity in patients [45]. Our findings show that in CMS, obese and overweight individuals were predominant in CMS III, whereas normal weight and overweight individuals were more prevalent in CMS I and II. Conversely, normal weight and overweight individuals were predominant across various KLG (Table S9).This suggests that BMI may contribute to inconsistent clinical symptoms and radiographic findings among patients with KOA. Additionally, our study revealed consistent trends in the distribution of factors such as occupation (Table S10), residential environment (Table S11), family history of KOA (Table S12), osteoporosis (Table S13), alcohol history (Table S14), and smoking history (Table S15) across both CMS and KLG. This potentially suggests that, similar to the KLG, CMS may not be influenced by these factors when assessing the severity of clinical findings in patients with KOA.
This study analyzed the differences between CMS and KLG in terms of treatment options and found a correlation between CMS and treatment options. The correlation showed an increasing trend with the worsening of CMS, whereas no clear pattern was observed with KLG. Furthermore, we categorized treatment modalities into nonsurgical (basic, nonpharmacological, and pharmacological) and surgical (restorative and reconstructive) treatments to compare the treatment approaches based on CMS and KLG. We found that CMS correlated more strongly associated with nonsurgical treatment. As CMS worsened, there was a noticeable increase in the use of pharmacological and nonpharmacological treatments, alongside basic treatment. In contrast, no significant difference was observed across CMS stages with respect to surgical treatment, and no correlation was found with surgical interventions. KLG, on the other hand, demonstrated the opposite pattern. These findings suggest that while both CMS and KLG can guide the clinical treatment of KOA by providing corresponding treatment plans based on clinical symptoms or radiographic changes, symptoms can change dynamically over a relatively short period. CMS can be more useful in adjusting the treatment strategies in response to these dynamic changes. However, radiographic grading primarily assesses the severity of osteoarthritis (OA) and is less effective in evaluating symptoms. Causality analysis revealed that CMS exhibited a causal relationship with treatment options. Basic, pharmacological, and nonpharmacological treatments were strongly associated with CMS, while restorative and reconstructive treatments were more closely linked to KLG. Therefore, compared with KLG, CMS may be more appropriate for assessing the severity of symptoms to determine nonsurgical treatment options for patients with KOA, but it is not suitable for assessing the severity of OA and options for surgical treatment compared with KLG.
In this study, we developed a staging method based on the clinical findings of KOA and used CMS to guide the non-surgical treatment of KOA for the first time. The results demonstrated significant and clinically meaningful improvements in pain, function, and stiffness in KOA patients at different CMS. To enhance the reliability of the findings, we also compared the results with a previous study [29]. This comparison showed that the symptomatic improvement in KOA patients guided by CMS was superior to that of NSAID treatment without CMS guidance. Furthermore, we conducted a detailed analysis of the clinical effectiveness at each stage of CMS. In CMS I, the improvement in the VAS did not reach the MCID, which is due to the fact that the maximum VAS score in CMS I is 3, and this mild pain may not change much when scored. Additionally, we also compared and analysed the results with studies related to celecoxib for KOA and found that in CMS II and CMS III, the improvement of pain and function in KOA patients was superior to celecoxib. This also suggests that CMS can achieve satisfactory effectiveness when guiding non-surgical treatment of KOA. Moreover, CMS changed significantly before and after treatment, indicating that the CMS can be flexibly adjusted based on the clinical findings of KOA patients, ensuring its alignment with their evolving condition.
This study involved over 14,000 patients with KOA from 84 hospitals and 8 communities, representing a range of medical institutions (level I, mainly community hospitals; level II, mainly regional hospitals; and level III, dominated by hospitals above the regional level). This diverse sample enhances the generalizability of our findings. By adopting this approach, we were able to objectively and comprehensively capture the characteristics of the KOA population and the current treatment landscape. We introduced the concept of the CMS and explored the causal relationships between CMS, KLG, and various influencing factors through correlation analysis. These findings provide a clearer understanding of the scope and application of both CMS and KLG. The above results suggest that using CMS to guide the development of nonsurgical treatment strategies could offer more accurate and tailored treatment options, potentially enhancing cost-effectiveness in alleviating clinical symptoms. However, given the limitations of the current survey, further analysis will be conducted in future studies to refine these conclusions.
However, our study has some limitations. The number of patients with KOA classified as KLG I, KLG IV, and CMS III was relatively small, which aligns with the general pattern of clinical onset of KOA. Additionally, this study did not assess post-intervention treatment outcomes. While this omission does not affect the validity of our findings, the advantages of clinical typing still warrant further analysis through longitudinal studies in the future.
In conclusion, this study elucidated the application characteristics and clinical effectiveness of CMS. CMS is consistent with patients' clinical findings, suitable for guiding non-surgical treatment of KOA, and can have significant clinical effects. Our research results provide a reliable basis for the clinical application of CMS, which is conducive to improving the management of non-surgical treatment for KOA patients worldwide. Meanwhile, KLG and CMS should be used in combination in the clinical management of KOA to realize synergistic effects.
Supplementary Information
Author contributions
Weiheng Chen designed the project and supervised the research, Ling Qin and Changhai Ding supervised the research and critically revised the content of the manuscript. Yan Yan and Baohong Mi collected and analyzed the data and drafted the manuscript, Yan Yan and Jiawen Zhang collected the data. Yan Yan and Jiawen Zhang analyzed the data. Baohong Mi, Yanqiong Zhang and Na Lin interpreted the data. All listed authors have each made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; participated in drafting the manuscript or revising it critically for content; and have approved the final version of the submitted manuscript. At the same time, we would like to express our gratitude to Jun Zhou, Yawei Dong, Zelu Zheng, Xiaohan Wang, Yuxin Luo, and Zhuoyun Wu for their contributions to this study.
Funding
This study was funded by the Capital Health Research and Development Special Fund of Beijing Municipal Health Commission (No. 2022-1-7032).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by he Scientific Research Ethics Committee of the Third Affiliated Hospital of Beijing University of Chinese Medicine, Wangjing Hospital of China Academy of Traditional Chinese Medicine, and Peking Union Medical College Hospital (BZYSY-2022KYKTPJ-04, BZYSY-2021KYKTSL-27, WJEC-KT-2018-039-P002, HS-2090, and HS-2363). During the preparation of this work, all authors did not use the Generative AI and AI-assisted technologies.
Competing interests
All authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan Yan and Baohong Mi have contributed equally to this work.
References
- 1.Bruyère O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. 2019;49(3):337–50. [DOI] [PubMed] [Google Scholar]
- 2.Wen C, Xiao G. Advances in osteoarthritis research in 2021 and beyond. J Orthop Translat. 2022;22(32):A1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng S, Tu L, Cicuttini F, Zhu Z, Han W, Antony B, et al. Depression in patients with knee osteoarthritis: risk factors and associations with joint symptoms. BMC Musculoskelet Disord. 2021;22(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haan MN, Lee A, Odden MC, Aiello AE, To TM, Neuhaus JM. Gender differences in the combined effects of cardiovascular disease and osteoarthritis on progression to functional impairment in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2016;71(8):1089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthr Cartil. 2022;30(1):10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho KK, Lee WY, Griffith JF, Ong MT, Li G. Randomized control trial of mesenchymal stem cells versus hyaluronic acid in patients with knee osteoarthritis—a Hong Kong pilot study. J Orthop Translat. 2022;6(37):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho J, Mak CCH, Sharma V, To K, Khan W. Mendelian randomization studies of lifestyle-related risk factors for osteoarthritis: a PRISMA review and meta-analysis. Int J Mol Sci. 2022;23(19):11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv Z, Wang Z, Chen D, Shi D. Advances in osteoarthritis research: from diagnosis, treatment to mechanism studies. J Orthop Translat. 2024;6(44):A4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Fu S, Gong Z, Zhu Z, Zeng D, Cao P, et al. MRI-based texture analysis of infrapatellar fat pad to predict knee osteoarthritis incidence. Radiology. 2022;304(3):611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goh GS, Schwartz AM, Friend JK, Grace TR, Wickes CB, Bolognesi MP, et al. Patients who have Kellgren-Lawrence Grade 3 and 4 osteoarthritis benefit equally from total knee arthroplasty. J Arthroplast. 2023;38(9):1714–7. [DOI] [PubMed] [Google Scholar]
- 11.Neubauer M, Reinberger EM, Dammerer D, Moser LB, Neugebauer J, Gottsauner-Wolf F, et al. Unicompartmental knee arthroplasty provides superior clinical and radiological outcomes compared to high tibial osteotomy at a follow-up of 5–8 y. J Clin Med. 2023;12(16):5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WH. Guidelines for the treatment of osteoarthritis of the knee in Chinese medicine (2020 edition). J Tradit Chin Orthopedic Traumatol. 2020;32(10):1–14 ((in Chinese)). [Google Scholar]
- 13.Kim I, Kim HA, Seo YI, Song YW, Jeong JY, Kim DH. The prevalence of knee osteoarthritis in elderly community residents in Korea. J Korean Med Sci. 2010;25(2):293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vargas E, Silva NCO, Dos Anjos RL, Santana MMC, Battistella LR, Marcon AF. Discordance between radiographic findings, pain, and superficial temperature in knee osteoarthritis. Reumatologia. 2020;58(6):375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Son KM, Hong JI, Kim DH, Jang DG, Crema MD, Kim HA. Absence of pain in subjects with advanced radiographic knee osteoarthritis. BMC Musculoskelet Disord. 2020;21(1):640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65(2):363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brophy RH, Fillingham YA. AAOS clinical practice guideline summary: management of osteoarthritis of the knee (nonarthroplasty). J Am Acad Orthop Surg. 2022;30(9):e721–9. [DOI] [PubMed] [Google Scholar]
- 18.Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee—evidence-based guideline. J Am Acad Orthop Surg. 2013;21(9):577–9. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Ding C, Wang C, Ma Y, Wang Q, Wang Z, et al. Better management of knee osteoarthritis: Chinese medicine treatment guideline. Osteoarthr Cartil. 2023;31:S168–9. [Google Scholar]
- 20.Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995;38(11):1541–6. [DOI] [PubMed] [Google Scholar]
- 21.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–49. [DOI] [PubMed] [Google Scholar]
- 22.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinman RS, McCrory P, Pirotta M, Relf I, Forbes A, Crossley KM, et al. Acupuncture for chronic knee pain: a randomized clinical trial. JAMA. 2014;312(13):1313–22. [DOI] [PubMed] [Google Scholar]
- 24.Feng J, Li Z, Tian L, Mu P, Hu Y, Xiong F, et al. Efficacy and safety of curcuminoids alone in alleviating pain and dysfunction for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Med Ther. 2022;22(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guidelines for the diagnosis and treatment of osteoarthritis in China (2021 edition).Chin J Orthop. 2021;41(18):1291–314. (in Chinese).
- 26.Kalainathan D, Goudet O.Causal discovery toolbox: uncover causal relationships in python. arXiv, 2019.
- 27.Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics. 2008;9(3):432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyer PO, Janzing D, Mooij JM, Peters J, Schölkopf B. Nonlinear causal discovery with additive noise models. Adv Neural Inform Process Syst. 2008;689(2009):689–96. [Google Scholar]
- 29.Kalkman CJ. Minimal clinically important difference. Maximum Impact Anesthesiol. 2020;132(6):1296–8. [DOI] [PubMed] [Google Scholar]
- 30.Yoo WH, Yoo HG, Park SH, Baek HJ, Lee YJ, Shim SC, et al. Efficacy and safety of PG201 (Layla(®)) and celecoxib in the treatment of symptomatic knee osteoarthritis: a double-blinded, randomized, multi-center, active drug comparative, parallel-group, non-inferiority, phase III study. Rheumatol Int. 2014;34(10):1369–78. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Tang H, Lin J, Zeng R. Temporal trends in the disease burden of osteoarthritis from 1990 to 2019, and projections until 2030. PLoS ONE. 2023;18(7): e0288561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819–28. [DOI] [PubMed] [Google Scholar]
- 33.Giorgino R, Albano D, Fusco S, Peretti GM, Mangiavini L, Messina C. Knee osteoarthritis: epidemiology, pathogenesis, and mesenchymal stem cells: What else is new? An update. Int J Mol Sci. 2023;24(7):6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang L, Xia C, Xu H, Ge Q, Shi Z, Kong L, et al. Defining disease progression in Chinese mainland people: association between bone mineral density and knee osteoarthritis. J Orthop Translat. 2020;6(26):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Courties A, Kouki I, Soliman N, Mathieu S, Sellam J. Osteoarthritis year in review 2024: epidemiology and therapy. Osteoarthr Cartil. 2024;32(11):1397–404. [DOI] [PubMed] [Google Scholar]
- 36.Althomali OW, Amin J, Acar T, Shahanawaz S, Talal Abdulrahman A, Alnagar DK, et al. Prevalence of symptomatic knee osteoarthritis in Saudi Arabia and associated modifiable and non-modifiable risk factors: a population-based cross-sectional study. Healthcare. 2023;11(5):728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji S, Liu L, Li J, Zhao G, Cai Y, Dong Y, et al. Prevalence and factors associated with knee osteoarthritis among middle-aged and elderly individuals in rural Tianjin: a population-based cross-sectional study. J Orthop Surg Res. 2023;18(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartley EJ, King CD, Sibille KT, Cruz-Almeida Y, Riley JL 3rd, Glover TL, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res. 2016;68(4):472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glass N, Segal NA, Sluka KA, Torner JC, Nevitt MC, Felson DT, et al. Examining sex differences in knee pain: the multicenter osteoarthritis study. Osteoarthr Cartil. 2014;22(8):1100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia SA, Vakula MN, Holmes SC, Pamukoff DN. The influence of body mass index and sex on frontal and sagittal plane knee mechanics during walking in young adults. Gait Posture. 2021;83:217–22. [DOI] [PubMed] [Google Scholar]
- 41.Ro DH, Lee DY, Moon G, Lee S, Seo SG, Kim SH, et al. Sex differences in knee joint loading: cross-sectional study in geriatric population. J Orthop Res. 2017;35(6):1283–9. [DOI] [PubMed] [Google Scholar]
- 42.Peterson JA, Lohman C, Larson RD, Bemben MG, Black CD. Lean mass is associated with, but does not mediate sex differences in pressure pain sensitivity in healthy adults. J Pain Res. 2022;16(15):3981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Zheng JJY, Li G, Yuan J, Ebert JR, Li H, et al. Pathogenesis and clinical management of obesity-related knee osteoarthritis: impact of mechanical loading. J Orthop Translat. 2020;15(24):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun AR, Udduttula A, Li J, Liu Y, Ren PG, Zhang P. Cartilage tissue engineering for obesity-induced osteoarthritis: physiology, challenges, and future prospects. J Orthop Translat. 2020;28(26):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osteoarthritis in a group of overweight subjects: a randomized, double-blind, placebo-controlled pilot study. Nutrients, 2019; 11(9):2027. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.