Abstract
Background
Low back pain is a major disorder that causes disability and is strongly associated with intervertebral disc degeneration (IDD). Because of the limitations of contemporary interventions, stem cell transplantation (SCT) has been increasingly used to regenerate degenerative discs. Nevertheless, analyses of high-impact papers in this field are rare. This study aimed to determine and analyze the 100 highest-cited documents on SCT in IDD.
Methods
The 100 highest-cited documents were retrieved from the Web of Science (WoS) database. Descriptive statistics were calculated and correlation analysis was conducted to determine the relationship between WoS citations, the Altmetric Attention Score (AAS), and Dimensions citations.
Results
The citation counts of the top 100 most cited papers ranged from 13 to 372. These studies were conducted in 17 countries and were published in 48 journals between 2003 and 2021. The top three contributing countries were the China (31), United States (22), and Japan (14). Bone marrow-derived stem cells were the most common type of stem cells (70.00%), followed by adipose-derived stem cells (13.75%), and nucleus pulposus-derived stem cells (7.50). Rabbit was the most studied species (41.25%), followed by rat (21.25%), human (13.75%), sheep (8.75%), dog (8.75%), and pig (6.25%). Tokai University School of Medicine (11) had the largest number of documents, followed by The University of Hong Kong (8), and Southeast University (4). Sakai D (10) was the most fruitful author, followed by Cheung KMC (6), Melrose J (3), Pettine K (3), Lotz JC (3), and Murphy MB (3). We observed a very high correlation between the WoS and Dimensions citations (p < 0.001, r = 0.994).
Conclusions
This study highlights the highest impact works on SCT in IDD, thereby providing a deeper understanding of the historical works related to SCT in IDD, as well as benefits for future studies in this field.
Keywords: Stem cell, Progenitor cell, Stromal cell, Cell transplantation, Intervertebral disc degeneration
Background
Low back pain (LBP) affects up to 84% of adults in their lifetime and is thought to be the most common vital musculoskeletal disorder to cause hospital visits [1–3]. LBP is also the predominant reason for sick leave and subsequent disability worldwide, representing an immense socioeconomic burden [4, 5]. The total cost of LBP in the United States is estimated to exceed $100 billion per year [6]. Intervertebral disc degeneration (IDD) is the predominant cause of LBP; however, for many patients, contemporary treatments for IDD aimed at alleviating symptoms or minimizing disability do not offer satisfactory outcomes [5–7]. Neither surgical nor non-surgical interventions are capable of hindering the progress of IDD or reversing it to regain the functional discs [3, 7, 8]. Hence, new treatment strategies focused on curing IDD are required.
Increasing attention has been paid to stem cell (SC) therapy in IDD because of the limitations of the current invention options [7–12]. With the rapid development of stem cell transplantation (SCT), numerous studies have been published on IDD [9–12]. Several important studies may have great potential to promote the growth of SCT in IDD [8–11]. The tendency of a certain field is commonly reflected in high-impact works [13–17]; thus, the evaluation of such works can help researchers and clinicians to rapidly identify the most influential papers in a specific field to deepen their research or identify novel directions in light of classic studies [16–19]. Analyses of the highest-cited papers have been conducted in various fields but have not yet been applied to SCT in IDD [13–30]. Therefore, the purpose of the present study was to determine and analyze the 100 highest-cited documents on SCT in IDD, allowing for a better understanding of the historical works as well as serving as a resource for future studies in this field.
Materials and methods
Retrieval strategy
The requirement for Institutional Review Board approval was waived because the study did not involve humans or animals. The Web of Science (WoS) database was used as the literature source, and the works were retrieved on January 10, 2023. A topic search was conducted using the following search strategy: (“stem cell” OR “progenitor cell” OR “stromal cell”) AND (“intervertebral disc” OR “intervertebral disk” OR “annulus fibrosus” OR “nucleus pulposus” OR “endplate”). The search was not limited by the publication time, article type, or language. The identified papers were listed in descending order based on WoS citations. We included all experimental in vivo studies that evaluated intervertebral disc regeneration after SCT in animal and human models, as well as review papers discussing in vivo SCT for intervertebral disc regeneration. The included studies exploring multiple cell types should have at least one group investigating SC. The studies only investigated general cell without SC were excluded. Studies only involving in vitro experiment were also excluded. Two authors independently screened the articles. In the case of disagreements regarding study selection between the two authors, a third author made the final decision. The 100 highest-cited papers on SCT in IDD were included in this study.
Data management
After the final list was determined, data extraction and analysis were performed independently by two authors. If no consensus reached, a third author was consulted to make the final decision. The extracted data included the title, year, citation count, journal, article type, country, institution, author, source species, and type of stem cells. WoS citations were collected from the citation count for each paper, which were listed in the WoS database. Citations per year (since publication) was calculated. The main authors, including the first and corresponding authors, were recorded. Each paper was further analyzed using the free Dimensions app database (www.Dimensions.ai) following previous publications [14, 15]. The number of citations in the Dimensions database and the Altmetric Attention Score (AAS) for each paper were listed in the Dimensions free app [14, 15]. Dimensions citations and AAS of the top 100 papers were collected for further analysis. To analyze the relative capability of the research output between countries, the number of papers published in each country was standardized by population size and gross domestic product (GDP). The relationships among the number of papers, population, and GDP were analyzed. For each country, the latest data of population size and GDP for 2022 were obtained from the World Bank (www.worldbank.org).
Statistical analysis
Descriptive statistics, including total counts, average counts, and percentages, were used to depict the extracted data. Correlation analysis was performed to detect the relationship between WoS citations, AAS, average AAS, and Dimensions citations. The correlation coefficient of the Pearson’s test (r) < 0.3 was defined as poor, 0.3–0.5 as low, 0.5–0.7 as moderate, 0.7–0.9 as high, and > 0.9 as very high. Statistical significance was set at p < 0.05.
Results
Top 100 list
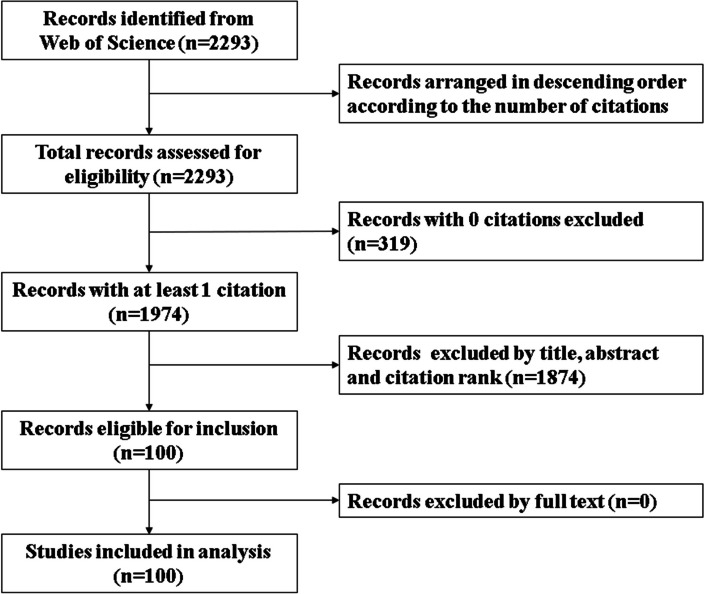
A total of 2293 records were initially identified in the WoS database. All the records were extracted and arranged in descending order based on the number of citations. After excluding 319 papers with 0 citations, 1974 papers with at least 1 citation were obtained. Based on reviewing of the title, abstract, and citation rank, 100 most cited papers were eligible of inclusion. The full texts of these papers were further reviewed. None of the papers were excluded. The 100 most cited papers on SCT in IDD were finally included (Table 1). The flowchart of study selection is depicted in Fig. 1.
Table 1.
The top 100 works on SCT in IDD according to the number of WoS citations
| Rank | First Author | Year | Title | Reference | Journal | WoS Citations | WoS Citations per Year (Since Publication) | Dimensions Citations | AAS | AAS per Year (Since Publication) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sakai D | 2003 | Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration | [31] | Biomaterials | 372 | 17.71 | 339 | 9 | 0.43 |
| 2 | Sakai D | 2006 | Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc | [32] | Biomaterials | 325 | 18.06 | 307 | 6 | 0.33 |
| 3 | Orozco L | 2011 | Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study | [33] | Transplantation | 324 | 24.92 | 343 | 32 | 2.46 |
| 4 | Sakai D | 2005 | Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration | [34] | Spine | 316 | 16.63 | 295 | 19 | 1.00 |
| 5 | Sakai D | 2012 | Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc | [35] | Nature Communications | 282 | 23.50 | 295 | 8 | 0.67 |
| 6 | Richardson SM | 2016 | Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration | [36] | Methods | 270 | 33.75 | 315 | 7 | 0.88 |
| 7 | Crevensten G | 2004 | Intervertebral disc cell therapy for regeneration: Mesenchymal stem cell implantation in rat intervertebral discs | [37] | Annals of Biomedical Engineering | 267 | 13.35 | 268 | 6 | 0.30 |
| 8 | Hiyama A | 2008 | Transplantation of mesenchymal stem cells in a canine disc degeneration model | [38] | Journal of Orthopaedic Research | 207 | 12.94 | 204 | 9 | 0.56 |
| 9 | Vadalà G | 2012 | Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation | [39] | Journal of Tissue Engineering and Regenerative Medicine | 201 | 16.75 | 214 | 10 | 0.83 |
| 10 | Yoshikawa T | 2010 | Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies | [40] | Spine | 184 | 13.14 | 190 | 0 | 0.00 |
| 11 | Sobajima S | 2008 | Feasibility of a stem cell therapy for intervertebral disc degeneration | [41] | Spine Journal | 172 | 10.75 | 166 | 5 | 0.31 |
| 12 | Henriksson HB | 2009 | Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model | [42] | Spine | 161 | 10.73 | 164 | 6 | 0.40 |
| 13 | Ganey T | 2009 | Intervertebral disc repair using adipose tissue-derived stem and regenerative cells experiments in a canine model | [43] | Spine | 150 | 10.00 | 150 | 5 | 0.33 |
| 14 | Leung VY | 2006 | Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction | [44] | European Spine Journal | 148 | 8.22 | 148 | 3 | 0.17 |
| 15 | Zhang YG | 2005 | Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans | [45] | Clinical Orthopaedics and Related Research | 134 | 7.05 | 138 | 3 | 0.16 |
| 16 | Sakai D | 2008 | Future perspectives of cell-based therapy for intervertebral disc disease | [46] | European Spine Journal | 134 | 8.38 | 129 | 0 | 0.00 |
| 17 | Serigano K | 2010 | Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model | [47] | Journal of Orthopaedic Research | 133 | 9.50 | 132 | 0 | 0.00 |
| 18 | Noriega DC | 2017 | Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial | [48] | Transplantation | 122 | 17.43 | 127 | 20 | 2.86 |
| 19 | Yang Fan | 2009 | Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells | [49] | Molecular Therapy | 118 | 7.87 | 120 | 3 | 0.20 |
| 20 | Miyamoto T | 2010 | Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits | [50] | Arthritis Research & Therapy | 105 | 7.50 | 108 | 3 | 0.21 |
| 21 | Pettine K | 2015 | Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months | [51] | Stem Cells | 102 | 11.33 | 120 | 6 | 0.67 |
| 22 | Acosta FL Jr | 2011 | Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells | [52] | Tissue Engineering Part A | 101 | 7.77 | 115 | 4 | 0.31 |
| 23 | Kumar H | 2017 | Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study | [53] | Stem Cell Research & Therapy | 95 | 13.57 | 95 | 2 | 0.29 |
| 24 | Yang H | 2010 | Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta 1 decrease rabbit intervertebral disc degeneration | [54] | Spine Journal | 85 | 6.07 | 78 | 6 | 0.43 |
| 25 | Jeong JH | 2010 | Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose-tissue-derived stromal cells | [55] | Acta Neurochirurgica | 83 | 5.93 | 74 | 0 | 0.00 |
| 26 | Hoogendoorn RJ | 2008 | Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future | [56] | Journal of Cellular and Molecular Medicine | 74 | 4.63 | 75 | 3 | 0.19 |
| 27 | Feng G | 2011 | Transplantation of mesenchymal stem cells and nucleus pulposus cells in a degenerative disc model in rabbits: a comparison of 2 cell types as potential candidates for disc regeneration Laboratory investigation | [57] | Journal of Neurosurgery: Spine | 74 | 5.69 | 76 | 0 | 0.00 |
| 28 | Yim RL | 2014 | A systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: insights and future directions for regenerative therapeutics | [58] | Stem Cells and Development | 74 | 7.40 | 74 | 6 | 0.60 |
| 29 | Sakai D | 2017 | Cell therapy for intervertebral disc repair: Clinical perspective | [59] | Journal of Orthopaedic Translation | 72 | 10.29 | 80 | 6 | 0.86 |
| 30 | Clouet J | 2019 | Intervertebral disc regeneration: From cell therapy to the development of novel bioinspired endogenous repair strategies | [60] | Advanced Drug Delivery Reviews | 71 | 14.20 | 93 | 2 | 0.40 |
| 31 | Leung VY | 2014 | Mesenchymal stem cells reduce intervertebral disc fibrosis and facilitate repair | [61] | Stem Cells | 71 | 7.10 | 76 | 8 | 0.80 |
| 32 | Haufe SM | 2006 | Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs | [62] | Stem Cells and Development | 70 | 3.89 | 77 | 3 | 0.17 |
| 33 | Ho G | 2008 | Effect of severity of intervertebral disc injury on mesenchymal stem cell-based regeneration | [63] | Connective Tissue Research | 69 | 4.31 | 64 | 3 | 0.19 |
| 34 | Wei A | 2009 | The fate of transplanted xenogeneic bone marrow-derived stem cells in rat intervertebral discs | [64] | Journal of Orthopaedic Research | 66 | 4.40 | 65 | 0 | 0.00 |
| 35 | Vadalà G | 2016 | Stem cells sources for intervertebral disc regeneration | [65] | World Journal of Stem Cells | 65 | 8.13 | 65 | 2 | 0.25 |
| 36 | Zeng Y | 2015 | Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration | [66] | Biomaterials | 65 | 7.22 | 70 | 0 | 0.00 |
| 37 | Elabd C | 2016 | Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study | [67] | Journal of Translational Medicine | 64 | 8.00 | 73 | 535 | 66.88 |
| 38 | Pettine K | 2017 | Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up | [68] | International Orthopaedics | 62 | 8.86 | 66 | 7 | 1.00 |
| 39 | Yang H | 2015 | TGF-β l suppresses inflammation in cell therapy for intervertebral disc degeneration | [69] | Scientific Reports | 59 | 6.56 | 46 | 1 | 0.11 |
| 40 | Centeno C | 2017 | Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy | [70] | Journal of Translational Medicine | 58 | 8.29 | 66 | 93 | 13.29 |
| 41 | Wang H | 2014 | Utilization of stem cells in alginate for nucleus pulposus tissue engineering | [71] | Tissue Engineering Part A | 58 | 5.80 | 56 | 1 | 0.10 |
| 42 | Chun HJ | 2012 | Tansplantation of human adipose-derived stem cells in a rabbit model of traumatic degeneration of lumbar discs | [72] | World Neurosurgery | 58 | 4.83 | 51 | 1 | 0.08 |
| 43 | Liang CZ | 2013 | Dual release of dexamethasone and TGF-β3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model | [73] | Acta Biomaterialia | 56 | 5.09 | 56 | 3 | 0.27 |
| 44 | Murrell W | 2009 | Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model | [74] | Spine Journal | 56 | 3.73 | 53 | 0 | 0.00 |
| 45 | Allon AA | 2010 | Structured coculture of stem cells and disc cells prevent disc degeneration in a rat model | [75] | Spine Journal | 54 | 3.86 | 61 | 0 | 0.00 |
| 46 | Pettine K | 2016 | Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimum two year follow-up | [76] | International Orthopaedics | 53 | 6.63 | 68 | 37 | 4.63 |
| 47 | Zhou X | 2018 | Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus | [77] | Acta Biomaterialia | 52 | 8.67 | 60 | 1 | 0.17 |
| 48 | Comella K | 2017 | Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease | [78] | Journal of Translational Medicine | 52 | 7.43 | 66 | 97 | 13.86 |
| 49 | Jeong JH | 2009 | Human mesenchymal stem cells implantation into the degenerated coccygeal disc of the rat | [79] | Cytotechnology | 49 | 3.27 | 45 | 0 | 0.00 |
| 50 | Sakai D | 2015 | Migration of bone marrow-derived cells for endogenous repair in a new tail-looping disc degeneration model in the mouse: a pilot study | [80] | Spine Journal | 49 | 5.44 | 50 | 1 | 0.11 |
| 51 | Pang X | 2014 | Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain | [81] | Pain Physician | 48 | 4.80 | 51 | 0 | 0.00 |
| 52 | Binch ALA | 2021 | Cell-based strategies for IVD repair: clinical progress and translational obstacles | [82] | Nature Reviews Rheumatology | 46 | 15.33 | 55 | 26 | 8.67 |
| 53 | Loibl M | 2019 | Controversies in regenerative medicine: Should intervertebral disc degeneration be treated with mesenchymal stem cells? | [83] | JOR Spine | 45 | 9.00 | 49 | 0 | 0.00 |
| 54 | Oehme D | 2014 | Mesenchymal progenitor cells combined with pentosan polysulfate mediating disc regeneration at the time of microdiscectomy: a preliminary study in an ovine model | [84] | Journal of Neurosurgery: Spine | 45 | 4.50 | 54 | 4 | 0.40 |
| 55 | Bendtsen M | 2011 | Autologous stem cell therapy maintains vertebral blood flow and contrast diffusion through the endplate in experimental intervertebral disc degeneration | [85] | Spine | 44 | 4.89 | 46 | 0 | 0.00 |
| 56 | Wang Z | 2015 | Efficacy of intervertebral disc regeneration with stem cells: a systematic review and meta-analysis of animal controlled trials | [86] | Gene | 44 | 3.38 | 50 | 23 | 2.56 |
| 57 | Marfia G | 2014 | Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: a preliminary study on biglycan-deficient murine model of chronic disc degeneration | [87] | Arthritis Research & Therapy | 43 | 4.30 | 43 | 6 | 0.60 |
| 58 | Hee HT | 2010 | Effects of implantation of bone marrow mesenchymal stem cells, disc distraction and combined therapy on reversing degeneration of the intervertebral disc | [88] | Journal of Bone and Joint Surgery: British Volume | 42 | 3.00 | 43 | 0 | 0.00 |
| 59 | Anderson DG | 2005 | Cell-based therapy for disc repair | [89] | Spine Journal | 41 | 2.16 | 47 | 0 | 0.00 |
| 60 | Schol J | 2019 | Cell therapy for intervertebral disc herniation and degenerative disc disease: clinical trials | [90] | International Orthopaedics | 39 | 7.80 | 49 | 3 | 0.60 |
| 61 | Omlor GW | 2010 | Methods to monitor distribution and metabolic activity of mesenchymal stem cells following in vivo injection into nucleotomized porcine intervertebral discs | [91] | European Spine Journal | 39 | 2.79 | 41 | 0 | 0.00 |
| 62 | Zeckser J | 2016 | Multipotent mesenchymal stem cell treatment for discogenic low back pain and disc degeneration | [92] | Stem Cells International | 37 | 4.63 | 38 | 5 | 0.63 |
| 63 | Cai F | 2015 | Evaluation of intervertebral disc regeneration with implantation of bone marrow mesenchymal stem cells (BMSCs) using quantitative T2 mapping: a study in rabbits | [93] | International Orthopaedics | 36 | 4.00 | 35 | 0 | 0.00 |
| 64 | Sheyn D | 2019 | Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model | [94] | Theranostics | 34 | 6.80 | 42 | 6 | 1.20 |
| 65 | Wang YT | 2010 | Regeneration potential and mechanism of bone marrow mesenchymal stem cell transplantation for treating intervertebral disc degeneration | [95] | Journal of Orthopaedic Science | 33 | 2.36 | 25 | 0 | 0.00 |
| 66 | Sun W | 2014 | Sox9 gene transfer enhanced regenerative effect of bone marrow mesenchymal stem cells on the degenerated intervertebral disc in a rabbit model | [96] | PLoS One | 31 | 3.10 | 26 | 0 | 0.00 |
| 67 | Chen X | 2016 | A comparison between nucleus pulposus-derived stem cell transplantation and nucleus pulposus cell transplantation for the treatment of intervertebral disc degeneration in a rabbit model | [97] | International Journal of Surgery | 30 | 3.75 | 29 | 0 | 0.00 |
| 68 | Gou S | 2014 | Stem cell therapy for intervertebral disk regeneration | [98] | American Journal of Physical Medicine & Rehabilitation | 30 | 3.00 | 33 | 3 | 0.30 |
| 69 | Tam V | 2014 | A comparison of intravenous and intradiscal delivery of multipotential stem cells on the healing of injured intervertebral disk | [99] | Journal of Orthopaedic Research | 30 | 3.00 | 34 | 6 | 0.60 |
| 70 | Zhou X | 2018 | Injectable decellularized nucleus pulposus-based cell delivery system for differentiation of adipose-derived stem cells and nucleus pulposus regeneration | [100] | Acta Biomaterialia | 30 | 5.00 | 37 | 0 | 0.00 |
| 71 | Hussain I | 2019 | Mesenchymal stem cell-seeded high-density collagen gel for annular repair: 6-week results from in vivo sheep models | [101] | Neurosurgery | 29 | 5.80 | 31 | 2 | 0.40 |
| 72 | Freeman BJC | 2016 | Allogeneic mesenchymal precursor cells promote healing in postero-lateral annular lesions and improve indices of lumbar intervertebral disc degeneration in an ovine model | [102] | Spine | 28 | 3.50 | 34 | 3 | 0.38 |
| 73 | Ahn J | 2015 | Transplantation of human Wharton’s jelly-derived mesenchymal stem cells highly expressing TGFβ receptors in a rabbit model of disc degeneration | [103] | Stem Cell Research & Therapy | 28 | 3.11 | 32 | 4 | 0.44 |
| 74 | Shu CC | 2017 | A histopathological scheme for the quantitative scoring of intervertebral disc degeneration and the therapeutic utility of adult mesenchymal stem cells for intervertebral disc regeneration | [104] | International Journal of Molecular Sciences | 27 | 3.86 | 31 | 4 | 0.57 |
| 75 | Melrose J | 2016 | Strategies in regenerative medicine for intervertebral disc repair using mesenchymal stem cells and bioscaffolds | [105] | Regenerative Medicine | 26 | 3.25 | 26 | 1 | 0.13 |
| 76 | Migliorini F | 2019 | Autogenic mesenchymal stem cells for intervertebral disc regeneration | [106] | International Orthopaedics | 26 | 5.20 | 31 | 2 | 0.40 |
| 77 | Ishiguro H | 2019 | Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model | [107] | Acta Biomaterialia | 25 | 5.00 | 30 | 0 | 0.00 |
| 78 | Li YY | 2014 | Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: reduced risk of osteophyte formation | [108] | Tissue Engineering Part A | 25 | 2.50 | 35 | 1 | 0.10 |
| 79 | Wang F | 2019 | Injectable hydrogel combined with nucleus pulposus-derived mesenchymal stem cells for the treatment of degenerative intervertebral disc in rats | [109] | Stem Cells International | 25 | 5.00 | 32 | 0 | 0.00 |
| 80 | Xu J | 2016 | BMP7 enhances the effect of BMSCs on extracellular matrix remodeling in a rabbit model of intervertebral disc degeneration | [110] | FEBS Journal | 22 | 2.75 | 24 | 4 | 0.50 |
| 81 | Hiraishi S | 2018 | Discogenic cell transplantation directly from a cryopreserved state in an induced intervertebral disc degeneration canine model | [111] | JOR Spine | 21 | 3.50 | 21 | 57 | 9.50 |
| 82 | Wang W | 2018 | Transplantation of hypoxic-Preconditioned bone mesenchymal stem cells retards intervertebral disc degeneration via enhancing implanted cell survival and migration in rats | [112] | Stem Cells International | 21 | 3.50 | 30 | 0 | 0.00 |
| 83 | Silverman LI | 2020 | In vitro and in vivo evaluation of discogenic cells, an investigational cell therapy for disc degeneration | [113] | Spine Journal | 19 | 4.75 | 18 | 52 | 13.00 |
| 84 | Liao JC | 2016 | Cell therapy using bone marrow-derived stem cell overexpressing BMP-7 for degenerative discs in a rat tail disc model | [114] | International Journal of Molecular Sciences | 19 | 2.38 | 20 | 1 | 0.13 |
| 85 | James G | 2016 | Mesenchymal stem cell treatment of intervertebral disc lesion prevents fatty infiltration and fibrosis of the multifidus muscle, but not cytokine and muscle fiber | [115] | Spine | 19 | 2.38 | 21 | 2 | 0.25 |
| 86 | Maidhof R | 2017 | Timing of mesenchymal stem cell delivery impacts the fate and therapeutic potential in intervertebral disc repair | [116] | Journal of Orthopaedic Research | 19 | 2.71 | 21 | 1 | 0.14 |
| 87 | Steffen F | 2017 | Bone marrow-derived mesenchymal stem cells as autologous therapy in dogs with naturally occurring intervertebral disc disease: feasibility, safety, and preliminary results | [117] | Tissue Engineering Part C: Methods | 18 | 2.57 | 20 | 70 | 10.00 |
| 88 | Wang SZ | 2016 | Intervertebral disc regeneration using platelet-rich plasma-containing bone marrow-derived mesenchymal stem cells: A preliminary investigation | [118] | Molecular Medicine Reports | 18 | 2.25 | 16 | 1 | 0.13 |
| 89 | Wei JN | 2016 | Transplantation of CXCR4 overexpressed mesenchymal stem cells augments regeneration in degenerated intervertebral discs | [119] | DNA and Cell Biology | 18 | 2.25 | 22 | 6 | 0.75 |
| 90 | Henriksson HB | 2019 | The traceability of mesenchymal stromal cells after injection into degenerated discs in patients with low back pain | [120] | Stem Cells and Development | 17 | 3.40 | 19 | 3 | 0.60 |
| 91 | Beeravolu N | 2018 | Human umbilical cord derivatives regenerate intervertebral disc | [121] | Journal of Tissue Engineering and Regenerative Medicine | 16 | 2.67 | 20 | 13 | 2.17 |
| 92 | Chiang ER | 2019 | Use of allogeneic hypoxic mesenchymal stem cells for treating disc degeneration in rabbits | [122] | Journal of Orthopaedic Research | 16 | 3.20 | 18 | 2 | 0.40 |
| 93 | Croft AS | 2021 | The application of mesenchymal stromal cells and their homing capabilities to regenerate the intervertebral disc | [123] | International Journal of Molecular Sciences | 16 | 5.33 | 19 | 2 | 0.67 |
| 94 | Sun Y | 2019 | Clinical trials of intervertebral disc regeneration: current status and future developments | [124] | International Orthopaedics | 15 | 3.00 | 20 | 1 | 0.20 |
| 95 | Hang D | 2017 | One-stage positron emission tomography and magnetic resonance imaging to assess mesenchymal stem cell survival in a canine model of intervertebral disc degeneration | [125] | Stem Cells and Development | 15 | 2.14 | 18 | 0 | 0.00 |
| 96 | Hou Y | 2016 | Study design: in vitro and in vivo assessment of bone morphogenic protein 2 combined with platelet-rich plasma on treatment of disc degeneration | [126] | International Orthopaedics | 15 | 1.88 | 14 | 0 | 0.00 |
| 97 | Daly CD | 2018 | Mesenchymal progenitor cells primed with pentosan polysulfate promote lumbar intervertebral disc regeneration in an ovine model of microdiscectomy | [127] | Spine Journal | 14 | 2.33 | 21 | 3 | 0.50 |
| 98 | Feng G | 2020 | Nanofibrous spongy microspheres to deliver rabbit mesenchymal stem cells and anti-miR-199a to regenerate nucleus pulposus and prevent calcification | [128] | Biomaterials | 14 | 3.50 | 18 | 1 | 0.25 |
| 99 | Zhou Y | 2014 | Effects of transplantation of hTIMP-1-expressing bone marrow mesenchymal stem cells on the extracellular matrix of degenerative intervertebral discs in an in vivo rabbit model | [129] | Spine | 14 | 1.40 | 20 | 1 | 0.10 |
| 100 | Shu CC | 2018 | Efficacy of administered mesenchymal stem cells in the initiation and co-ordination of repair processes by resident disc cells in an ovine (Ovis aries) large destabilizing lesion model of experimental disc degeneration | [130] | JOR Spine | 13 | 2.17 | 17 | 0 | 0.00 |
WoS Web of Science, AAS Altmetric Attention Score
Fig. 1.
Flowchart detailing the selection process for the top 100 works on SCT in IDD
The number of WoS citations per paper ranged from 13 to 372. When sorted by citations per year (since publication), the three papers with the highest total WoS citations were ranked differently: the most cited paper with 372 citations ranked fifth with 17.71 citations per year, the second most cited with 325 citations ranked fourth with 18.06 citations per year, and the third most cited with 324 citations ranked second with 24.92 citations per year (Table 2). Furthermore, according to citations per year (since publication), the top three papers with 33.75, 24.92, and 23.50 citations per year ranked 6th (270 citations), 3rd (324 citations), and 5th (282 citations) in total WoS citations respectively. The number of Dimensions citations ranged from 14 to 343. The highest AAS recorded were 535, followed by 97 and 93. The top three papers according to AAS all originated from America and also ranked as the top three when sorted by citations per year, with scores of 66.88, 13.86, and 13.29 respectively. Additionally, 27% of the studies, totaling 27, did not have an AAS. All studies were published in English.
Table 2.
The top 100 works on SCT in IDD according to WoS citations per year (since publication)
| Rank | First Author | Year | Reference | Journal | WoS Citations | Wos Citations per year (since publication) | Dimensions | AAS | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Richardson SM | 2016 | Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration | [36] | Methods | 270 | 33.75 | 315 | 7 |
| 2 | Orozco L | 2011 | Intervertebral Disc Repair by Autologous Mesenchymal Bone Marrow Cells: A Pilot Study | [33] | Transplantation | 324 | 24.92 | 343 | 32 |
| 3 | Sakai D | 2012 | Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc | [35] | Nature Communications | 282 | 23.50 | 295 | 8 |
| 4 | Sakai D | 2006 | Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc | [32] | Biomaterials | 325 | 18.06 | 307 | 6 |
| 5 | Sakai D | 2003 | Transplantation of mesenchymal stem cells embedded in Atelocollagen((R)) gel to the intervertebral disc: a potential therapeutic model for disc degeneration | [31] | Biomaterials | 372 | 17.71 | 339 | 9 |
| 6 | Noriega DC | 2017 | Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells: A Randomized Controlled Trial | [48] | Transplantation | 122 | 17.43 | 127 | 20 |
| 7 | Vadalà G | 2012 | Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation | [39] | Journal of Tissue Engineering and Regenerative Medicine | 201 | 16.75 | 214 | 10 |
| 8 | Sakai D | 2005 | Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model - Potential and limitations for stem cell therapy in disc regeneration | [34] | Spine | 316 | 16.63 | 295 | 19 |
| 9 | Binch ALA | 2021 | Cell-based strategies for IVD repair: clinical progress and translational obstacles | [82] | Nature Reviews Rheumatology | 46 | 15.33 | 55 | 26 |
| 10 | Clouet J | 2019 | Intervertebral disc regeneration: From cell therapy to the development of novel bioinspired endogenous repair strategies | [60] | Advanced Drug Delivery Reviews | 71 | 14.20 | 93 | 2 |
| 11 | Kumar H | 2017 | Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study | [53] | Stem Cell Research & Therapy | 95 | 13.57 | 95 | 2 |
| 12 | Crevensten G | 2004 | Intervertebral disc cell therapy for regeneration: Mesenchymal stem cell implantation in rat intervertebral discs | [37] | Annals of Biomedical Engineering | 267 | 13.35 | 268 | 6 |
| 13 | Yoshikawa T | 2010 | Disc Regeneration Therapy Using Marrow Mesenchymal Cell transplantation A Report of Two Case Studies | [40] | Spine | 184 | 13.14 | 190 | 0 |
| 14 | Hiyama A | 2008 | Transplantation of mesenchymal stem cells in a canine disc degeneration model | [38] | Journal of Orthopaedic Research | 207 | 12.94 | 204 | 9 |
| 15 | Pettine K | 2015 | Percutaneous injection of Autologous Bone Marrow Concentrate Cells Significantly Reduces Lumbar Discogenic Pain Through 12 Months | [51] | Stem Cells | 102 | 11.33 | 120 | 6 |
| 16 | Sobajima S | 2008 | Feasibility of a stem cell therapy for intervertebral disc degeneration | [41] | Spine Journal | 172 | 10.75 | 166 | 5 |
| 17 | Henriksson HB | 2009 | Transplantation of Human Mesenchymal Stems Cells Into Intervertebral Discs in a Xenogeneic Porcine Model | [42] | Spine | 161 | 10.73 | 164 | 6 |
| 18 | Sakai D | 2017 | Cell therapy for intervertebral disc repair: Clinical perspective | [59] | Journal of Orthopaedic Translation | 72 | 10.29 | 80 | 6 |
| 19 | Ganey T | 2009 | Intervertebral Disc Repair Using Adipose Tissue-Derived Stem and Regenerative Cells Experiments in a Canine Model | [43] | Spine | 150 | 10.00 | 150 | 5 |
| 20 | Serigano K | 2010 | Effect of Cell Number on Mesenchymal Stem Cell transplantation in a Canine Disc Degeneration Model | [47] | Journal of Orthopaedic Research | 133 | 9.50 | 132 | 0 |
| 21 | Loibl M | 2019 | Controversies in regenerative medicine: Should intervertebral disc degeneration be treated with mesenchymal stem cells? | [83] | JOR Spine | 45 | 9.00 | 49 | 0 |
| 22 | Pettine K | 2017 | Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up | [68] | International Orthopaedics | 62 | 8.86 | 66 | 7 |
| 23 | Zhou X | 2018 | Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus | [77] | Acta Biomaterialia | 52 | 8.67 | 60 | 1 |
| 24 | Sakai D | 2008 | Future perspectives of cell-based therapy for intervertebral disc disease | [46] | European Spine Journal | 134 | 8.38 | 129 | 0 |
| 25 | Centeno C | 2017 | Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy | [70] | Journal of Translational Medicine | 58 | 8.29 | 66 | 93 |
| 26 | Leung VY | 2006 | Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction | [44] | European Spine Journal | 148 | 8.22 | 148 | 3 |
| 27 | Vadalà G | 2016 | Stem cells sources for intervertebral disc regeneration | [65] | World Journal of Stem Cells | 65 | 8.13 | 65 | 2 |
| 28 | Elabd C | 2016 | Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study | [67] | Journal of Translational Medicine | 64 | 8.00 | 73 | 535 |
| 29 | Yang Fan | 2009 | Mesenchymal Stem Cells Arrest Intervertebral Disc Degeneration Through Chondrocytic Differentiation and Stimulation of Endogenous Cells | [49] | Molecular Therapy | 118 | 7.87 | 120 | 3 |
| 30 | Schol J | 2019 | Cell therapy for intervertebral disc herniation and degenerative disc disease: clinical trials | [90] | International Orthopaedics | 39 | 7.80 | 49 | 3 |
| 31 | Acosta FL Jr | 2011 | Porcine Intervertebral Disc Repair Using Allogeneic Juvenile Articular Chondrocytes or Mesenchymal Stem Cells | [52] | Tissue Engineering Part A | 101 | 7.77 | 115 | 4 |
| 32 | Miyamoto T | 2010 | Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits | [50] | Arthritis Research & Therapy | 105 | 7.50 | 108 | 3 |
| 33 | Comella K | 2017 | Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease | [78] | Journal of Translational Medicine | 52 | 7.43 | 66 | 97 |
| 34 | Yim RL | 2014 | A Systematic Review of the Safety and Efficacy of Mesenchymal Stem Cells for Disc Degeneration: Insights and Future Directions for Regenerative Therapeutics | [58] | Stem Cells and Development | 74 | 7.40 | 74 | 6 |
| 35 | Zeng Y | 2015 | Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration | [66] | Biomaterials | 65 | 7.22 | 70 | 0 |
| 36 | Leung VY | 2014 | Mesenchymal Stem Cells Reduce Intervertebral Disc Fibrosis and Facilitate Repair | [61] | Stem Cells | 71 | 7.10 | 76 | 8 |
| 37 | Zhang YG | 2005 | Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans | [45] | Clinical Orthopaedics and Related Research | 134 | 7.05 | 138 | 3 |
| 38 | Sheyn D | 2019 | Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model | [94] | Theranostics | 34 | 6.80 | 42 | 6 |
| 39 | Pettine K | 2016 | Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimum two year follow-up | [76] | International Orthopaedics | 53 | 6.63 | 68 | 37 |
| 40 | Yang H | 2015 | TGF-beta l Suppresses Inflammation in Cell Therapy for Intervertebral Disc Degeneration | [69] | Scientific Reports | 59 | 6.56 | 46 | 1 |
| 41 | Yang H | 2010 | Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta 1 decrease rabbit intervertebral disc degeneration | [54] | Spine Journal | 85 | 6.07 | 78 | 6 |
| 42 | Jeong JH | 2010 | Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose-tissue-derived stromal cells | [55] | Acta Neurochirurgica | 83 | 5.93 | 74 | 0 |
| 43 | Wang H | 2014 | Utilization of Stem Cells in Alginate for Nucleus Pulposus Tissue Engineering | [71] | Tissue Engineering Part A | 58 | 5.80 | 56 | 1 |
| 44 | Hussain I | 2019 | Mesenchymal Stem Cell-Seeded High-Density Collagen Gel for Annular Repair: 6-Week Results From In Vivo Sheep Models | [101] | Neurosurgery | 29 | 5.80 | 31 | 2 |
| 45 | Feng G | 2011 | Transplantation of mesenchymal stem cells and nucleus pulposus cells in a degenerative disc model in rabbits: a comparison of 2 cell types as potential candidates for disc regeneration Laboratory investigation | [57] | Journal of Neurosurgery: Spine | 74 | 5.69 | 76 | 0 |
| 46 | Sakai D | 2015 | Migration of bone marrow-derived cells for endogenous repair in a new tail-looping disc degeneration model in the mouse: a pilot study | [80] | Spine Journal | 49 | 5.44 | 50 | 1 |
| 47 | Croft AS | 2021 | The Application of Mesenchymal Stromal Cells and Their Homing Capabilities to Regenerate the Intervertebral Disc | [123] | International Journal of Molecular Sciences | 16 | 5.33 | 19 | 2 |
| 48 | Migliorini F | 2019 | Autogenic mesenchymal stem cells for intervertebral disc regeneration | [106] | International Orthopaedics | 26 | 5.20 | 31 | 2 |
| 49 | Liang CZ | 2013 | Dual release of dexamethasone and TGF-beta 3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model | [73] | Acta Biomaterialia | 56 | 5.09 | 56 | 3 |
| 50 | Zhou X | 2018 | Injectable decellularized nucleus pulposus-based cell delivery system for differentiation of adipose-derived stem cells and nucleus pulposus regeneration | [100] | Acta Biomaterialia | 30 | 5.00 | 37 | 0 |
| 51 | Wang F | 2019 | Injectable Hydrogel Combined with Nucleus Pulposus-Derived Mesenchymal Stem Cells for the Treatment of Degenerative Intervertebral Disc in Rats | [109] | Stem Cells International | 25 | 5.00 | 32 | 0 |
| 52 | Ishiguro H | 2019 | Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model | [107] | Acta Biomaterialia | 25 | 5.00 | 30 | 0 |
| 53 | Wang Z | 2015 | Efficacy of intervertebral disc regeneration with stem cells - A systematic review and meta-analysis of animal controlled trials | [86] | Gene | 44 | 4.89 | 50 | 23 |
| 54 | Chun HJ | 2012 | Transplantation of Human Adipose-Derived Stem Cells in a Rabbit Model of Traumatic Degeneration of Lumbar Discs | [72] | World Neurosurgery | 58 | 4.83 | 51 | 1 |
| 55 | Pang X | 2014 | Human Umbilical Cord Mesenchymal Stem Cell transplantation for the Treatment of Chronic Discogenic Low Back Pain | [81] | Pain Physician | 48 | 4.80 | 51 | 0 |
| 56 | Silverman LI | 2020 | In vitro and in vivo evaluation of discogenic cells, an investigational cell therapy for disc degeneration | [113] | Spine Journal | 19 | 4.75 | 18 | 52 |
| 57 | Hoogendoorn RJ | 2008 | Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future | [56] | Journal of Cellular and Molecular Medicine | 74 | 4.63 | 75 | 3 |
| 58 | Zeckser J | 2016 | Multipotent Mesenchymal Stem Cell Treatment for Discogenic Low Back Pain and Disc Degeneration | [92] | Stem Cells International | 37 | 4.63 | 38 | 5 |
| 59 | Oehme D | 2014 | Mesenchymal progenitor cells combined with pentosan polysulfate mediating disc regeneration at the time of microdiscectomy: a preliminary study in an ovine model | [84] | Journal of Neurosurgery: Spine | 45 | 4.50 | 54 | 4 |
| 60 | Wei A | 2009 | The Fate of transplanted Xenogeneic Bone Marrow-Derived Stem Cells in Rat Intervertebral Discs | [64] | Journal of Orthopaedic Research | 66 | 4.40 | 65 | 0 |
| 61 | Ho G | 2008 | Effect of severity of intervertebral disc injury on mesenchymal stem cell-based regeneration | [63] | Connective Tissue Research | 69 | 4.31 | 64 | 3 |
| 62 | Marfia G | 2014 | Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: a preliminary study on biglycan-deficient murine model of chronic disc degeneration | [87] | Arthritis Research & Therapy | 43 | 4.30 | 43 | 6 |
| 63 | Cai F | 2015 | Evaluation of intervertebral disc regeneration with implantation of bone marrow mesenchymal stem cells (BMSCs) using quantitative T2 mapping: a study in rabbits | [93] | International Orthopaedics | 36 | 4.00 | 35 | 0 |
| 64 | Haufe SM | 2006 | Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs | [62] | Stem Cells and Development | 70 | 3.89 | 77 | 3 |
| 65 | Allon AA | 2010 | Structured coculture of stem cells and disc cells prevent disc degeneration in a rat model | [75] | Spine Journal | 54 | 3.86 | 61 | 0 |
| 66 | Shu CC | 2017 | A Histopathological Scheme for the Quantitative Scoring of Intervertebral Disc Degeneration and the Therapeutic Utility of Adult Mesenchymal Stem Cells for Intervertebral Disc Regeneration | [104] | International Journal of Molecular Sciences | 27 | 3.86 | 31 | 4 |
| 67 | Chen X | 2016 | A comparison between nucleus pulposus-derived stem cell transplantation and nucleus pulposus cell transplantation for the treatment of intervertebral disc degeneration in a rabbit model | [97] | International Journal of Surgery | 30 | 3.75 | 29 | 0 |
| 68 | Murrell W | 2009 | Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model | [74] | Spine Journal | 56 | 3.73 | 53 | 0 |
| 69 | Freeman BJC | 2016 | Allogeneic Mesenchymal Precursor Cells Promote Healing in Postero-lateral Annular Lesions and Improve Indices of Lumbar Intervertebral Disc Degeneration in an Ovine Model | [102] | Spine | 28 | 3.50 | 34 | 3 |
| 70 | Wang W | 2018 | Transplantation of Hypoxic-Preconditioned Bone Mesenchymal Stem Cells Retards Intervertebral Disc Degeneration via Enhancing Implanted Cell Survival and Migration in Rats | [112] | Stem Cells International | 21 | 3.50 | 30 | 0 |
| 71 | Hiraishi S | 2018 | Discogenic cell transplantation directly from a cryopreserved state in an induced intervertebral disc degeneration canine model | [111] | JOR Spine | 21 | 3.50 | 21 | 57 |
| 72 | Feng G | 2020 | Nanofibrous spongy microspheres to deliver rabbit mesenchymal stem cells and anti-miR-199a to regenerate nucleus pulposus and prevent calcification | [128] | Biomaterials | 14 | 3.50 | 18 | 1 |
| 73 | Henriksson HB | 2019 | The Traceability of Mesenchymal Stromal Cells After Injection Into Degenerated Discs in Patients with Low Back Pain | [120] | Stem Cells and Development | 17 | 3.40 | 19 | 3 |
| 74 | Bendtsen M | 2011 | Autologous Stem Cell Therapy Maintains Vertebral Blood Flow and Contrast Diffusion Through the Endplate in Experimental Intervertebral Disc Degeneration | [85] | Spine | 44 | 3.38 | 46 | 0 |
| 75 | Jeong JH | 2009 | Human mesenchymal stem cells implantation into the degenerated coccygeal disc of the rat | [79] | Cytotechnology | 49 | 3.27 | 45 | 0 |
| 76 | Migliorini F | 2016 | Strategies in regenerative medicine for intervertebral disc repair using mesenchymal stem cells and bioscaffolds | [105] | Regenerative Medicine | 26 | 3.25 | 26 | 1 |
| 77 | Chiang ER | 2019 | Use of Allogeneic Hypoxic Mesenchymal Stem Cells For Treating Disc Degeneration in Rabbits | [122] | Journal of Orthopaedic Research | 16 | 3.20 | 18 | 2 |
| 78 | Ahn J | 2015 | Transplantation of human Wharton’s jelly-derived mesenchymal stem cells highly expressing TGFβ receptors in a rabbit model of disc degeneration | [103] | Stem Cell Research & Therapy | 28 | 3.11 | 32 | 4 |
| 79 | Sun W | 2014 | Sox9 Gene Transfer Enhanced Regenerative Effect of Bone Marrow Mesenchymal Stem Cells on the Degenerated Intervertebral Disc in a Rabbit Model | [96] | PLoS One | 31 | 3.10 | 26 | 0 |
| 80 | Hee HT | 2010 | Effects of implantation of bone marrow mesenchymal stem cells, disc distraction and combined therapy on reversing degeneration of the intervertebral disc | [88] | Journal of Bone and Joint Surgery: British Volume | 42 | 3.00 | 43 | 0 |
| 81 | Gou S | 2014 | Stem Cell Therapy for Intervertebral Disk Regeneration | [98] | American Journal of Physical Medicine & Rehabilitation | 30 | 3.00 | 33 | 3 |
| 82 | Tam V | 2014 | A Comparison of Intravenous and Intradiscal Delivery of Multipotential Stem Cells on the Healing of Injured Intervertebral Disk | [99] | Journal of Orthopaedic Research | 30 | 3.00 | 34 | 6 |
| 83 | Sun Y | 2019 | Clinical trials of intervertebral disc regeneration: current status and future developments | [124] | International Orthopaedics | 15 | 3.00 | 20 | 1 |
| 84 | Omlor GW | 2010 | Methods to monitor distribution and metabolic activity of mesenchymal stem cells following in vivo injection into nucleotomized porcine intervertebral discs | [91] | European Spine Journal | 39 | 2.79 | 41 | 0 |
| 85 | Xu J | 2016 | BMP7 enhances the effect of BMSCs on extracellular matrix remodeling in a rabbit model of intervertebral disc degeneration | [110] | FEBS Journal | 22 | 2.75 | 24 | 4 |
| 86 | Maidhof R | 2017 | Timing of Mesenchymal Stem Cell Delivery Impacts the Fate and Therapeutic Potential in Intervertebral Disc Repair | [116] | Journal of Orthopaedic Research | 19 | 2.71 | 21 | 1 |
| 87 | Beeravolu N | 2018 | Human umbilical cord derivatives regenerate intervertebral disc | [121] | Journal of Tissue Engineering and Regenerative Medicine | 16 | 2.67 | 20 | 13 |
| 88 | Steffen F | 2017 | Bone Marrow-Derived Mesenchymal Stem Cells as Autologous Therapy in Dogs with Naturally Occurring Intervertebral Disc Disease: Feasibility, Safety, and Preliminary Results | [117] | Tissue Engineering Part C: Methods | 18 | 2.57 | 20 | 70 |
| 89 | Li YY | 2014 | Delivering Mesenchymal Stem Cells in Collagen Microsphere Carriers to Rabbit Degenerative Disc: Reduced Risk of Osteophyte Formation | [108] | Tissue Engineering Part A | 25 | 2.50 | 35 | 1 |
| 90 | James G | 2016 | Mesenchymal Stem Cell Treatment of Intervertebral Disc Lesion Prevents Fatty Infiltration and Fibrosis of the Multifidus Muscle, but not Cytokine and Muscle Fiber Changes | [115] | Spine | 19 | 2.38 | 21 | 2 |
| 91 | Liao JC | 2016 | Cell Therapy Using Bone Marrow-Derived Stem Cell Overexpressing BMP-7 for Degenerative Discs in a Rat Tail Disc Model | [114] | International Journal of Molecular Sciences | 19 | 2.38 | 20 | 1 |
| 92 | Wang YT | 2010 | Regeneration potential and mechanism of bone marrow mesenchymal stem cell transplantation for treating intervertebral disc degeneration | [95] | Journal of Orthopaedic Science | 33 | 2.36 | 25 | 0 |
| 93 | Daly CD | 2018 | Mesenchymal progenitor cells primed with pentosan polysulfate promote lumbar intervertebral disc regeneration in an ovine model of microdiscectomy | [127] | Spine Journal | 14 | 2.33 | 21 | 3 |
| 94 | Wei JN | 2016 | Transplantation of CXCR4 Overexpressed Mesenchymal Stem Cells Augments Regeneration in Degenerated Intervertebral Discs | [119] | DNA and Cell Biology | 18 | 2.25 | 22 | 6 |
| 95 | Wang SZ | 2016 | Intervertebral disc regeneration using platelet-rich plasma-containing bone marrow-derived mesenchymal stem cells: A preliminary investigation | [118] | Molecular Medicine Reports | 18 | 2.25 | 16 | 1 |
| 96 | Shu CC | 2018 | Efficacy of administered mesenchymal stem cells in the initiation and co-ordination of repair processes by resident disc cells in an ovine (Ovis aries) large destabilizing lesion model of experimental disc degeneration | [130] | JOR Spine | 13 | 2.17 | 17 | 0 |
| 97 | Anderson DG | 2005 | Cell-based therapy for disc repair | [89] | Spine Journal | 41 | 2.16 | 47 | 0 |
| 98 | Hang D | 2017 | One-Stage Positron Emission Tomography and Magnetic Resonance Imaging to Assess Mesenchymal Stem Cell Survival in a Canine Model of Intervertebral Disc Degeneration | [125] | Stem Cells and Development | 15 | 2.14 | 18 | 0 |
| 99 | Hou Y | 2016 | Study design: in vitro and in vivo assessment of bone morphogenic protein 2 combined with platelet-rich plasma on treatment of disc degeneration | [126] | International Orthopaedics | 15 | 1.88 | 14 | 0 |
| 100 | Zhou Y | 2014 | Effects of transplantation of hTIMP-1-Expressing Bone Marrow Mesenchymal Stem Cells on the Extracellular Matrix of Degenerative Intervertebral Discs in an In Vivo Rabbit Model | [129] | Spine | 14 | 1.40 | 20 | 1 |
WoS Web of Science, AAS Altmetric Attention Score
Year of publication
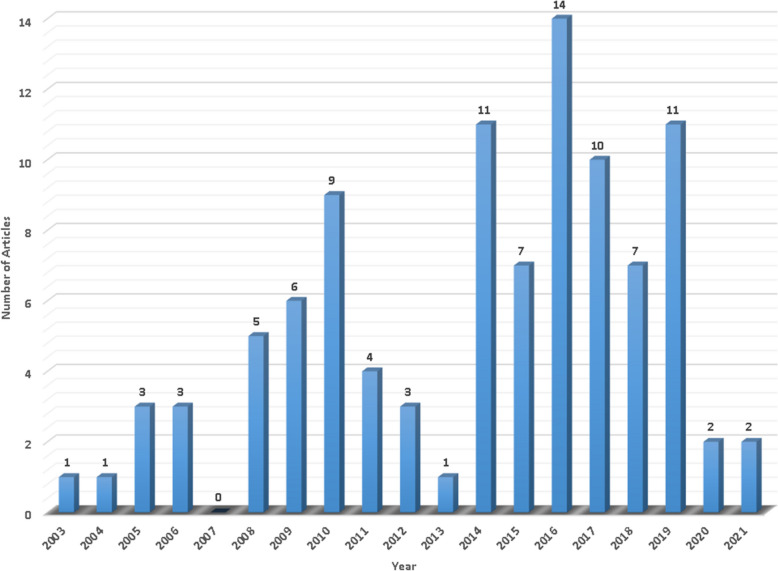
Figure 2 presents the annual distribution of the top 100 studies, which were published between 2003 and 2021. The highest number of works were published in 2016, with 14 papers, followed by 2014 and 2019, each with 11 papers.
Fig. 2.
Annual distribution of the top 100 works on SCT in IDD, showing that the peak publication year was 2016, followed by 2014 and 2019
Article type
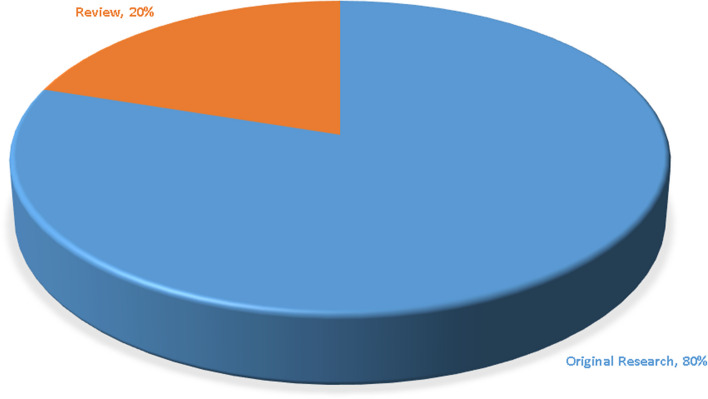
The article types of the top 100 works are shown in Fig. 3. A total of 80 original studies were included. The average number of citations of these works was 77.51. The remaining 20 papers were reviews, with an average of 65.30 citations.
Fig. 3.
Article types among the top 100 works, comprising 80 original research articles and 20 review articles
Source journal
A total of 48 journals contributed to the 100 studies, with 19 journals publishing at least two of these studies (Table 3). The most frequently represented journals were Spine and The Spine Journal, each with 8 publications, followed by International Orthopaedics with 7, and the Journal of Orthopaedic Research with 6. Spine also had the highest total citations at 916, followed by Biomaterials with 776, and The Spine Journal with 490. Among the 48 journals, Nature Reviews Rheumatology had the highest impact factor (33.7), followed by Nature Communications (16.6) and Advanced Drug Delivery Reviews (16.1). No significant correlation was found between the average number of WoS citations and the average AAS (r = −0.036, p = 0.882).
Table 3.
Journals with two or more papers among the top 100 works
| Journal Title | No. of Papers | Total WoS Citations | Average WoS Citations | Average AAS | Impact Factor |
|---|---|---|---|---|---|
| Spine | 8 | 916 | 114.50 | 4.50 | 3.0 |
| Spine Journal | 8 | 490 | 61.25 | 8.38 | 4.5 |
| International Orthopaedics | 7 | 246 | 35.14 | 7.14 | 2.7 |
| Journal of Orthopaedic Research | 6 | 471 | 78.50 | 3.00 | 2.8 |
| Biomaterials | 4 | 776 | 194.00 | 4.00 | 14.0 |
| Stem Cells and Development | 4 | 176 | 44.00 | 3.00 | 4.0 |
| Acta Biomaterialia | 4 | 163 | 40.75 | 1.00 | 9.7 |
| European Spine Journal | 3 | 321 | 107.00 | 1.00 | 2.8 |
| Tissue Engineering Part A | 3 | 184 | 61.33 | 2.00 | 4.1 |
| Journal of Translational Medicine | 3 | 174 | 58.00 | 241.67 | 7.4 |
| Stem Cells International | 3 | 83 | 27.67 | 1.67 | 4.3 |
| JOR Spine | 3 | 79 | 26.33 | 19.00 | 3.7 |
| International Journal of Molecular Sciences | 3 | 62 | 20.67 | 2.33 | 5.6 |
| Transplantation | 2 | 446 | 223.00 | 26.00 | 6.2 |
| Journal of Tissue Engineering and Regenerative Medicine | 2 | 217 | 108.50 | 11.50 | 3.3 |
| Stem Cells | 2 | 173 | 86.50 | 7.00 | 5.2 |
| Arthritis Research & Therapy | 2 | 148 | 74.00 | 4.50 | 4.9 |
| Stem Cell Research & Therapy | 2 | 123 | 61.50 | 3.00 | 7.5 |
| Journal of Neurosurgery: Spine | 2 | 119 | 59.50 | 2.00 | 2.8 |
WoS Web of Science, AAS Altmetric Attention Score
Country distribution
Table 4 presents the country affiliations of the top 100 most-cited studies. Seventeen countries contributed to these influential papers. China led with 31 publications, followed by the United States with 22 and Japan with 14. In terms of total citations, Japan ranked first with 2,264 citations, followed by the United States with 1,539, and China with 1,439. Ten countries published at least two papers each. No significant correlation was observed between the average number of WoS citations and the average AAS (r = 0.099, p = 0.705). When research output was normalized by population, Australia ranked first (34.97), followed by Switzerland (34.49) and Ireland (19.89). Similarly, when normalized by GDP, Australia again ranked first (58.34), with Switzerland (36.91) and Sweden (31.88) following.
Table 4.
Countries of the top 100 works
| Countrya | No. of Papers | Total WoS Citations |
Average WoS Citations | Average AAS | No. per Billion Populations | No. per $ 10,000 Billion GDP |
|---|---|---|---|---|---|---|
| China | 31 | 1439 | 46.42 | 1.77 | 2.19 | 17.48 |
| United States | 22 | 1539 | 69.95 | 41.09 | 6.63 | 9.57 |
| Japan | 14 | 2264 | 161.71 | 8.64 | 11.14 | 28.35 |
| Australia | 9 | 294 | 32.67 | 1.89 | 34.97 | 58.34 |
| South Korea | 5 | 313 | 62.60 | 1.40 | 19.31 | 27.80 |
| Italy | 3 | 309 | 103.00 | 6.00 | 5.08 | 14.29 |
| Switzerland | 3 | 79 | 26.33 | 36.00 | 34.49 | 36.91 |
| Spain | 2 | 446 | 223.00 | 26.00 | 4.23 | 14.03 |
| Sweden | 2 | 178 | 89.00 | 4.50 | 19.20 | 31.88 |
| Germany | 2 | 65 | 32.50 | 1.00 | 2.41 | 4.74 |
| Netherlands | 1 | 74 | 74.00 | 3.00 | 5.70 | 9.82 |
| Denmark | 1 | 44 | 44.00 | 0.00 | 17.07 | 25.18 |
| Singapore | 1 | 42 | 42.00 | 0.00 | 18.34 | 25.19 |
| Canada | 1 | 34 | 34.00 | 6.00 | 2.61 | 5.02 |
| Ireland | 1 | 46 | 46.00 | 26.00 | 19.89 | 20.06 |
| United Kingdom | 1 | 270 | 270.00 | 7.00 | 1.49 | 3.14 |
| France | 1 | 71 | 71.00 | 2.00 | 1.48 | 3.40 |
WoS Web of Science, AAS Altmetric Attention Score, GDP Gross Domestic Product
aPopulation and GDP of each country for the year 2022 were obtained from the World Bank (www.worldbank.org)
Institution of origin
The affiliated institutions that contributed two or more papers are listed in Table 5, with fifteen institutions included in the list. Tokai University School of Medicine, with 11 papers, had the leading publication record, followed by The University of Hong Kong (8), and Southeast University (4). Tokai University School of Medicine had the highest total citations (1950), followed by the University of Hong Kong (550), and the University of Valladolid and CSIC (446). Regarding the average number of citations, the University of Valladolid and CSIC ranked first (233.00), followed by Tokai University School of Medicine (177.27), and the University of California (140.67).
Table 5.
Institutions with two or more papers among the top 100 works
| Institutions | No. of Papers | Total WoS Citations | Average WoS Citations |
|---|---|---|---|
| Tokai University School of Medicine | 11 | 1950 | 177.27 |
| The University of Hong Kong | 8 | 550 | 68.75 |
| Southeast University | 4 | 105 | 26.25 |
| Zhejiang University | 3 | 138 | 46.00 |
| University of California | 3 | 422 | 140.67 |
| Celling Biosciences | 3 | 217 | 72.33 |
| Kolling Institute | 3 | 66 | 22.00 |
| University of Valladolid and CSIC | 2 | 446 | 223.00 |
| Campus Bio-Medico University of Rome | 2 | 266 | 133.00 |
| Sahlgrenska Hospital | 2 | 178 | 89.00 |
| University of Ulsan | 2 | 132 | 66.00 |
| CHA University | 2 | 123 | 61.50 |
| Mayo Clinic | 2 | 74 | 37.00 |
| Monash University | 2 | 59 | 29.50 |
| Second Military Medical University | 2 | 36 | 18.00 |
WoS Web of Science
Main author
Table 6 lists the first and corresponding authors who contributed two or more papers. Sakai D led the list with 10 papers, followed by Cheung KMC with 6, and Melrose J, Pettine K, Lotz JC, and Murphy MB, each with 3 papers. Sakai D also achieved the highest total number of citations (1,911), followed by Cheung KMC (451) and García-Sancho J (446). In terms of average citations per paper, García-Sancho J had the highest average (233.00), followed by Sakai D (191.10) and Lotz JC (140.67). When considering average citations per year (since publication), García-Sancho J again ranked highest (21.18), with Sakai D (12.59) and Vadalà G (12.44) following.
Table 6.
First/corresponding authors with two or more papers of the top 100 works
| Authors | As First Author | As Corresponding Author | No. of Papers | Total WoS Citations | Average WoS Citations | Average WoS Citations per Year (Since Publication) |
|---|---|---|---|---|---|---|
| Sakai D | 7 | 9 | 10 | 1911 | 191.10 | 12.59 |
| Cheung KMC | 6 | 6 | 451 | 75.17 | 5.58 | |
| Melrose J | 1 | 3 | 3 | 66 | 22.00 | 3.62 |
| Pettine K | 3 | 3 | 217 | 72.33 | 8.94 | |
| Lotz JC | 3 | 3 | 422 | 140.67 | 8.33 | |
| Murphy MB | 3 | 3 | 217 | 72.33 | 8.94 | |
| Schol J | 1 | 2 | 2 | 111 | 55.50 | 9.04 |
| Vadalà G | 2 | 2 | 266 | 133.00 | 12.44 | |
| Leung VY | 2 | 2 | 219 | 109.50 | 7.66 | |
| Henriksson HB | 2 | 2 | 178 | 89.00 | 7.07 | |
| Yang H | 2 | 2 | 144 | 72.00 | 6.31 | |
| Jeong JH | 2 | 2 | 132 | 66.00 | 4.60 | |
| Feng G | 2 | 2 | 88 | 44.00 | 4.60 | |
| Zhou X | 2 | 2 | 82 | 41.00 | 6.83 | |
| Shu CC | 2 | 2 | 40 | 20.00 | 3.01 | |
| García-Sancho J | 2 | 2 | 446 | 223.00 | 21.18 | |
| Choi KH | 2 | 2 | 132 | 66.00 | 4.60 | |
| Lee SH | 2 | 2 | 123 | 61.50 | 8.34 | |
| Chen Q | 2 | 2 | 108 | 54.00 | 6.88 | |
| Qu W | 2 | 2 | 74 | 37.00 | 3.94 | |
| Wu XT | 2 | 2 | 54 | 27.00 | 3.13 |
WoS Web of Science
Classification of species
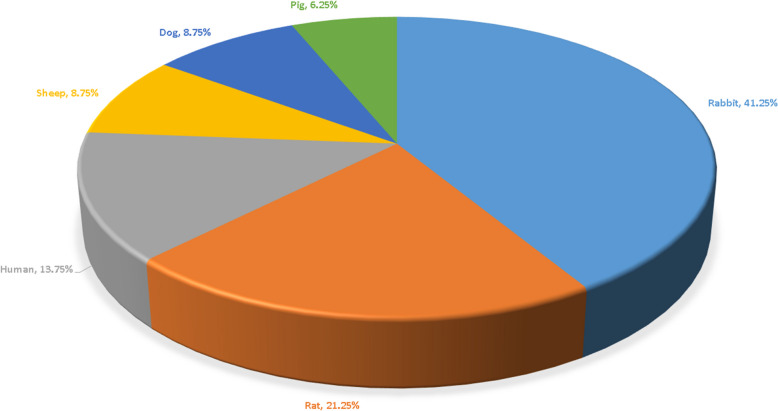
Figure 4 presents the classification of the species used in the 80 original studies on SCT. A total of six species were represented across these studies. Rabbits were the most commonly studied species, appearing in 33 papers (41.25%), followed by rats in 17 studies (21.25%), humans in 11 studies (13.75%), and sheep and dogs in 7 studies each (8.75%). Pigs were the least represented, featured in 5 studies (6.25%).
Fig. 4.
Species examined in the original research studies. Six species were included, with rabbit being the most frequently studied (41.25%), followed by rat (21.25%), human (13.75%), sheep (8.75%), dog (8.75%), and pig (6.25%)
In the 11 clinical trials, sample sizes varied from 2 to 33 participants. These trials explored various spinal conditions, including low back pain, low back pain with radicular symptoms, lumbar discogenic pain, and lumbar spinal stenosis. The follow-up periods ranged from 1 to 6 years. Bone marrow-derived stem cells (BMSCs) were the most frequently employed in 7 trials, while adipose-derived stem cells (ADSCs) were used in 2 trials. Umbilical cord-derived stem cells (UCSCs) and hematopoietic stem cells (HSCs) were each utilized in 1 trial.
Category of stem cells
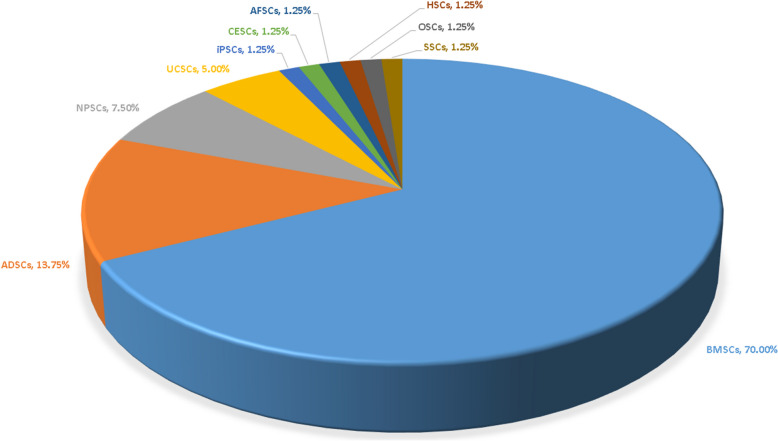
Figure 5 illustrates the types of stem cells investigated in the 80 original studies. Among these, most studies (79) focused on a single type of stem cell, while one study explored four different types. In total, ten types of stem cells were examined, including BMSCs, ADSCs, nucleus pulposus-derived stem cells (NPSCs), UCSCs, induced pluripotent stem cells (iPSCs), cartilage endplate-derived stem cells (CESCs), annulus fibrosus-derived stem cells (AFSCs), HSCs, olfactory stem cells (OSCs), and synovial stem cells (SSCs). BMSCs were the most frequently discussed, featured in 56 studies (70.00%), followed by ADSCs in 11 studies (13.75%), and NPSCs in 6 studies (7.50%).
Fig. 5.
Stem cell types discussed in the original research. Ten different types of stem cells were included, with BMSCs being the most frequently discussed (70.00%), followed by ADSCs (13.75%) and NPSCs (7.50%)
Correlation analysis
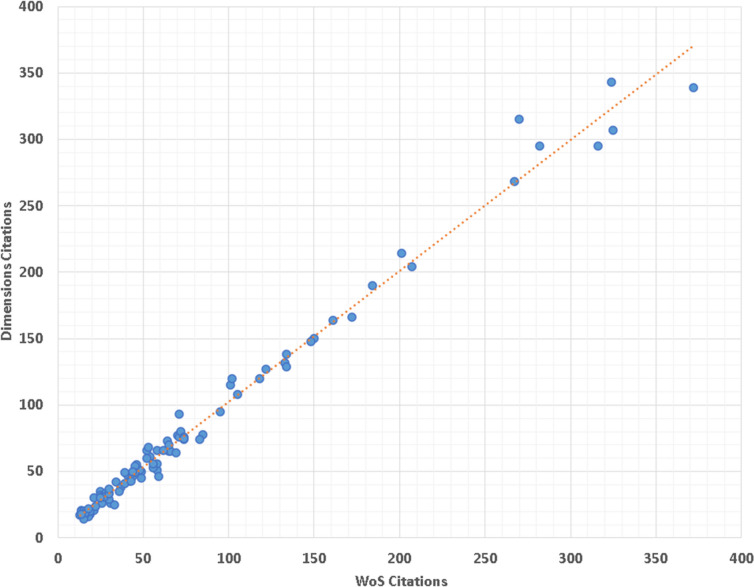
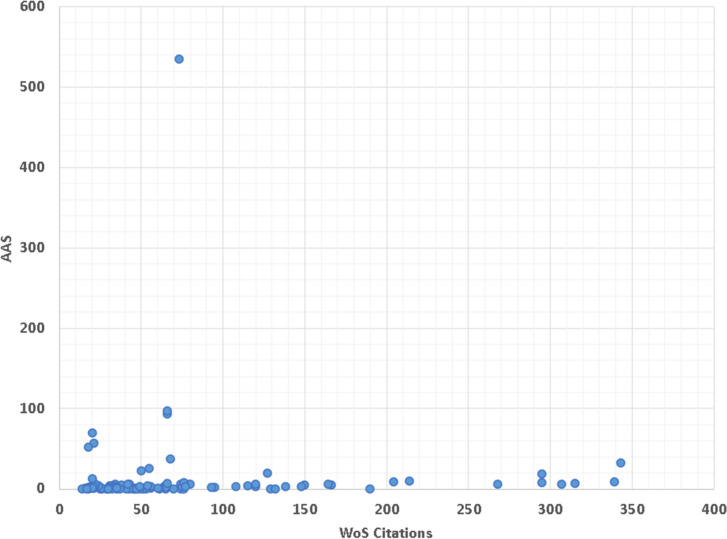
The citation counts of the top 100 WoS works were very high correlated with their citations in Dimensions (r = 0.994, p < 0.001). Figure 6 shows a clear linear correlation between WoS citations and Dimensions citations. In addition, a poor correlation was found between the number of citations of WoS and AAS (r = 0.000, p = 0.996) (Fig. 7).
Fig. 6.
Scatter plot illustrating the correlation between WoS and Dimensions citations, demonstrating a very high linear correlation (r = 0.994, p < 0.001)
Fig. 7.
Scatter plot assessing the correlation between WoS citations and AAS, showing no significant correlation between the two metrics (r = 0.000, p = 0.996)
Discussion
LBP has become the leading cause of disability worldwide, severely influencing the quality of life of patients and placing a huge burden on both society and the economy [2, 3, 5, 6]. LBP is associated with IDD [9–11, 98], for which the current strategies are limited and cannot solve the root cause [11, 98]. Therefore, there is a crucial need to develop new treatment options to retard IDD and restore disc functions [7, 8, 11, 12, 98]. The progress in SCT may provide potent strategies for treating IDD, while some of the most influential work may change clinical practice and motivate discussions, disputes, and further studies [8–10, 12, 98]. Although numerous publications analyzing the highest-cited works have been reported in many fields [13–30], there have been no such papers on SCT in IDD. Thus, this is the first study to determine and analyze the highest impact works on SCT in IDD.
The top 100 papers were published in English, demonstrating that English is the most important and influential language in the scientific community [16, 20–22, 24, 25]. Spine and Spine Journal published the largest number of works, followed by International Orthopaedics, and Journal of Orthopaedic Research, demonstrating that these journals have a strong influence on SCT in IDD. One reason for this observation may be that investigators tend to submit vital work to high-impact journals in their fields [20–25]. Another possibility is that investigators tend to cite papers published in important journals [13–19]. Moreover, journals with high impact factors, such as Nature Reviews Rheumatology, published at least one paper on stem cells in IDD. This finding suggests that some high-quality works on this topic could be accepted in high-impact journals [14, 16, 18, 19].
The 100 highest impact works were published by authors from 17 countries. The top three countries, including China, the United States, and Japan, produced 67 papers, accounting for about 70% of the top 100 works. This indicates that high-impact work is still concentrated in a few countries, most of which are developed, with the exception of China. This finding demonstrates that the economic status of a country is associated with the research output of high-impact works [13, 20, 22, 24]. Therefore, there is a need to improve the quality of work in non-developed countries.
It is surprising that China is the most fruitful country for the publication of research relating to SCT in IDD. Indeed, the high scientific productivity of the United States has been proven in many fields [13, 17, 18, 21–25]. This may be attributed to the advantages of the United States in terms of a large number of researchers and sufficient funds [13–22, 24, 25]. Our findings may indicate that China have played an increasingly important role in the field of SCT in IDD. However, when the research output was normalized by population or GDP, Australia, Switzerland, Sweden, and Ireland were more prolific. For each country, it may make more sense to adjust by the number of researchers and financial resources used on SCT in IDD, not the total population and GDP. However, it should be recognized that it is rather difficult to obtain these data from each country in the field of SCT in IDD. Nevertheless, when considering the population and economic status of these countries, the findings may reflect their relatively high research output on SCT in IDD.
Some institutions and authors have excellent records in the top 100 list. Sakai D from Tokai University School of Medicine ranks first with the highest total citations, indicating his significant influence in the field of SCT in IDD. Other notable authors such as Cheung KMC, Melrose J, Pettine K, Lotz JC, Murphy MB, Schol J, Vadalà G, and García-Sancho J also have impressive publication records. Our findings underscore the high quality of their work on SCT in IDD.
Citations per year (since publication) is calculated as an indicator that evaluates the relative influence of a paper, irrespective of the time elapsed since its publication [24, 25, 28, 30, 131]. We found that the top study based on citation per year (since publication) was a review on SCT in IDD. The second study, which investigated the transplantation of mesenchymal stromal cells, is notable for its high citation per year (since publication), likely because it represents the first human clinical study investigating SCT for the treatment of disc degeneration disease [3]. The 3rd, 4th, and 5th highest cited papers are also from Sakai D, further evidencing his significant impact in SCT on IDD. Given their high citation per year (since publication), these studies are expected to maintain strong citation records in the future [23, 132].
In our comprehensive analysis of the top 100 papers on SCT in IDD, a detailed comparison of total WoS citations and citations per year since publication reveals a dynamic landscape of research impact [24, 25, 28, 30, 131]. Notably, while papers with the highest total WoS citations indicate long-standing contributions to the field, their annual citations suggest varying levels of ongoing influence [18, 22, 28, 132]. For instance, the most cited paper, with 372 total citations, ranks only fifth when evaluated by its annual citation rate of 17.71, highlighting strong historical impact but a relatively moderated contemporary influence. Conversely, papers with fewer total citations but higher citations per year, such as those with 33.75, 24.92, and 23.50 citations per year, are emerging as significant recent contributors, emphasizing the importance of considering both longevity and immediacy in assessing research impact. Additionally, the integration of AAS alongside traditional citation metrics provides a layered understanding of research visibility and engagement [14, 15]. The highest recorded AAS was 535, indicative of substantial immediate attention and public engagement. Analyzing average AAS per year since publication offers further insight into the sustained impact of these studies over time. For instance, the top-ranked paper by AAS also shows a high annual engagement rate, suggesting ongoing discussions and relevance within the community long after publication. This dual perspective highlights not only the peak interest at the time of publication but also the enduring resonance of research in broader discussions, thereby enriching our understanding of both immediate and lasting scholarly influence [14, 15, 133]. Such analysis is crucial for discerning which topics capture transient attention versus those that foster prolonged academic and public discourse, thus providing a more nuanced view of the dynamics within scientific communication [14, 15].
Animal models play a pivotal role in advancing research on IDD, particularly in evaluating the safety and efficacy of SCT [134, 135]. These models provide researchers with valuable insights into the mechanisms of SCT and its potential therapeutic applications. Rabbits emerged as the most commonly used species in SCT research, appearing in 41.25% of the studies. Their relatively larger disc dimensions, compared to smaller models like rats, make them ideal for facilitating SCT [136]. Additionally, the cost-effectiveness and availability of rabbits, compared to larger animals like sheep or pigs, contribute to their popularity in this area of research [136, 137]. Despite the advantages of animal models, translating these findings to human clinical applications remains challenging. Humans constituted only 13.75% of the studies, reflecting the complexity of advancing preclinical data into clinical trials. Ethical concerns, safety considerations, and the inherent complexity of human spinal conditions create barriers to conducting large-scale human trials [136, 137]. While larger animals such as sheep and pigs could provide closer physiological similarities to humans, their high maintenance costs and limited availability reduce their use in SCT research [137].
In clinical trials, sample sizes were small, ranging from 2 to 33 participants, reflecting the early stages of SCT research [136, 138]. The trials addressed various spinal conditions, including low back pain and lumbar spinal stenosis, demonstrating the potential versatility of SCT. Follow-up durations from 1 to 6 years allowed researchers to assess both short-term and long-term outcomes, providing valuable insights into the sustained efficacy of these therapies. BMSCs were the most frequently used, employed in 7 of the 11 trials, likely due to their established regenerative potential and safety profile [11, 60]. Although alternative stem cell types are being explored, BMSCs remain the dominant choice, indicating a need for further research into the effectiveness of other cell sources. The results of the studies were largely positive, showing improvements in radiologic outcomes such as increased water content in the discs, improved Pfirrmann grading, and reduced disc bulge size as observed in MRI scans. Additionally, SCT was associated with improvements in lumbar function, reduced pain, and enhanced quality of life for patients. Importantly, most studies reported minor or no adverse events, further supporting SCT’s potential as a safe and effective treatment for IDD [9, 60, 138]. However, despite these promising findings, limitations remain. The small sample sizes and lack of large-scale randomized controlled trials limit the generalizability of the results [60, 138]. To confirm the safety and efficacy of SCT as a treatment for IDD, larger, well-designed RCTs are needed. These future studies should focus on expanding the sample sizes and exploring the use of different stem cell sources to ensure the broader applicability of SCT in clinical settings.
Some of the studies included in our analysis investigated multiple cell types, not just SC. These studies highlight that certain cell types, such as articular chondrocytes [52], may offer advantages in treating IDD compared to SC, as they are better suited to survive in the ischemic disc microenvironment [8, 11, 98]. We consider these findings highly significant for the field of SCT in IDD and have therefore included these studies in our top 100 list. These insights could encourage researchers to modify SC to enhance their adaptation to the challenging disc environment or to explore new stem cell types with superior regenerative potential. In addition, BMSCs are the most widely used stem cell type, likely due to their excellent biological properties and ease of harvesting with minimal harm [3, 98]. However, as our understanding of IDD deepens, increasing evidence suggests that the harsh microenvironment of degenerated discs limits the efficacy of BMSCs [10, 98]. Consequently, there is growing interest in utilizing resident stem cells, such as (NPSCs, AFSCs, and CESCs, which demonstrate better tolerance to disc conditions [9, 10]. We anticipate that research on resident stem cells in IDD will expand rapidly in the near future.
Traditional indicators of academic influence, largely comprising impact factors and citations, provide an important view of the quality of research [20, 21, 139]. Nevertheless, social media substantially alters the way in which knowledge is shared [14, 15, 133, 139]. Worldwide platforms such as X (formerly known as Twitter) and Facebook allow investigators to share their work with many more readers, many of whom will not be familiar with the academic field and may not be reflected by traditional indicators [133, 139]. AAS is an indicator of the online sharing activities a paper has received [14, 15]. In this study, the AAS of 27% of the included studies was zero, meaning that these studies had no online activities. Moreover, the citation counts of the top 100 works in WoS were very high correlated with their citations in Dimensions (r = 0.994, p < 0.001). This result is similar to the findings for previous publications in other fields [14, 15]. This suggests that this new database could be an alternative to WoS and could compensate for the bias of Altmetric because of the rapid change in social media. Altmetrics can be used as a useful index to investigate the impact of work on society, but not as a reliable index of work quality [14, 15, 133, 139]. A poor correlation was demonstrated between the number of citations in WoS and AAS (r = 0.000, p = 0.996), which is similar to the findings of similar publications [14, 15]. Therefore, the number of WoS citations is not directly comparable to the AAS values; this may be attributed to the fact that different databases cover different journals, which may affect the citations of articles [139]. However, different databases can be used to assess different aspects of the work.
In our analysis of SCT research in IDD, the AAS may indicate geographic discrepancies that potentially challenge its universality and fairness [133, 140]. Altmetric’s system, primarily monitoring Western-oriented platforms like X (formerly known as Twitter) and Facebook, biases engagement metrics towards American content, while popular platforms in Europe and Asia such as LinkedIn and Sina Weibo are underrepresented due to recent policy changes and data stream closures [140]. This skew may distort perceptions of the impact and relevance of research across different regions, potentially affecting academic recognition and funding decisions [131, 140]. To address these issues, it is essential for Altmetric to broaden its scope to include a more diverse array of data sources, ensuring that altmetrics provide a balanced view of global research impact [140]. As the academic and publishing communities become increasingly aware of these biases, there is a pressing need for altmetrics to evolve to reflect the diverse nature of scientific discourse more accurately, thereby enhancing the fairness and accuracy of research impact assessments and ensuring equitable recognition of scholarly contributions worldwide [131, 140].
This study has several limitations. First, although a comprehensive literature search has been performed, some papers may not be identified due to the intrinsic limitations of the search strategy. Second, the citation count is used as an indicator of the impact of a work, which may not be absolutely reliable. Indeed, as older works have more time to receive citations, influential papers published in recent years may have fewer citations, resulting in their exclusion from the top 100 list. Third, the number of citations is usually influenced by multiple factors, such as self-citation, and may not reflect the objective impact of the studies. Fourth, only the WoS database was retrieved to identify the top-cited publications. The high-impact works in other sources, such as books, websites, and other databases could not be included in this study. Fifth, our analysis was limited to first authors and corresponding authors, which is not without its shortcomings. This method may miss recognizing the important contributions of co-authors in collaborative studies, including those with shared first authorships. Sixth, reviewing the most highly cited papers in this field does not necessarily reveal how best stem cells might be used to affect disc repair. Seventh, our study employed a cross-sectional design with data collected at a single point in time. Consequently, it may not capture temporal changes in specific cell types, species, journals, countries, citations, or AAS. It is important to note that paper rankings could shift if the search is conducted at another time. Eight, our analysis incorporates AAS, which introduce another layer of temporal bias. These scores tend to favor recent publications due to their immediate visibility on social media platforms, potentially underrepresenting the historical impact of older research that predated the widespread use of these digital tools. Therefore, while AAS provide valuable insights into current public and scholarly engagement, they should be interpreted with caution. Despite these constraints, our study establishes a foundational baseline for future analyses, enabling subsequent studies to track longitudinal changes in the field’s dynamics and the evolving impact of specific research outputs. This will allow for a more comprehensive understanding of how different factors influence research recognition over time.
Conclusions
For the first time, we provide an analytic study of the 100 highest impact works on SCT in IDD, thereby providing a list of the most influential publications in this field. The current study should disseminate beneficial knowledge to researchers and clinicians, expand the understanding of the historical works regarding SCT in IDD, and be helpful in guiding further research on this topic.
Acknowledgements
None.
Statement of human and animal rights
Not applicable.
Abbreviations
- SCT
Stem cell transplantation
- IDD
Intervertebral disc degeneration
- WoS
Web of Science
- AAS
Altmetric Attention Score
- LBP
Low back pain
- SC
Stem cell
- GDP
Gross domestic product
- BMSCs
Bone marrow-derived stem cells
- ADSCs
Adipose-derived stem cells
- NPSCs
Nucleus pulposus-derived stem cells
- UCSCs
Umbilical cord-derived stem cells
- iPSCs
Induced pluripotent stem cells
- CESCs
Cartilage endplate-derived stem cells
- AFSCs
Annulus fibrosus-derived stem cells
- HSCs
Hematopoietic stem cells
- OSCs
Olfactory stem cells
- SSCs
Synovial stem cells
Authors’ contributions
Study conception and design was performed by XL and QL. Acquisition of data was conducted by XC, HL, and BH, Analysis and interpretation of data was done by HL, BH, and JR. Drafting the manuscript was performed by XC, HL, and BH. Critical revision of manuscript was conducted by JR, XL, QL. All authors read and approved the final manuscript.
Funding
None.
Data availability
All data in this paper are available upon reasonable requests from corresponding author.
Declarations
Ethics approval and consent to participate
No approval of Institutional Reviewed Board was needed due to not involving human and animals in this study.
Consent for publication
There are no human subjects in this article, and informed consent is not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaofeng Chen and Hao Li contributed equally to this work.
Contributor Information
Xi Li, Email: panyulixi@163.com.
Qian Li, Email: liqianpanyu@outlook.com.
References
- 1.Walker BF. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord. 2000;13(3):205–17. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei A, Shen B, Williams L, Diwan A. Mesenchymal stem cells: potential application in intervertebral disc regeneration. Transl Pediatr. 2014;3(2):71–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–5. [DOI] [PubMed] [Google Scholar]
- 5.Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(6):968–74. [DOI] [PubMed] [Google Scholar]
- 6.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. [DOI] [PubMed] [Google Scholar]
- 7.Krut Z, Pelled G, Gazit D, Gazit Z. Stem cells and exosomes: new therapies for intervertebral disc degeneration. Cells. 2021;10(9): 2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S, Tam V, Cheung KM, Long D, Lv M, Wang T, et al. Stem cell-based approaches for intervertebral disc regeneration. Curr Stem Cell Res Ther. 2011;6(4):317–26. [DOI] [PubMed] [Google Scholar]
- 9.Du Y, Wang Z, Wu Y, Liu C, Zhang L. Intervertebral disc stem/progenitor cells: a promising seed for intervertebral disc regeneration. Stem Cells Int. 2021;2021:2130727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyu FJ, Cheung KM, Zheng Z, Wang H, Sakai D, Leung VY. IVD progenitor cells: a new horizon for understanding disc homeostasis and repair. Nat Rev Rheumatol. 2019;15(2):102–12. [DOI] [PubMed] [Google Scholar]
- 11.Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11(4):243–56. [DOI] [PubMed] [Google Scholar]
- 12.Vadala G, Ambrosio L, Russo F, Papalia R, Denaro V. Stem cells and intervertebral disc regeneration overview-what they can and can’t do. Int J Spine Surg. 2021;15(s1):40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung AWK. The 100 most cited papers concerning the Insular cortex of the brain: a bibliometric analysis. Front Hum Neurosci. 2018;12: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martelli AJ, Machado RA, Martelli DRB, Neves LTD, Martelli Junior H. The 100 most-cited papers in oral medicine and pathology. Braz Oral Res. 2020;35:e020. [DOI] [PubMed] [Google Scholar]
- 15.Garcovich D, Marques Martinez L, Adobes Martin M. Citation classics in paediatric dentistry: a bibliometric study on the 100 most-cited articles. Eur Arch Paediatr Dent. 2020;21(2):249–61. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, Ibrahim AM, Koolen PG, Markarian MK, Lee BT, Lin SJ. Highest impact articles in microsurgery: a citation analysis. J Reconstr Microsurg. 2015;31(7):527–40. [DOI] [PubMed] [Google Scholar]
- 17.Eberlin KR, Labow BI, Upton J 3, Taghinia AH. High-impact articles in hand surgery. Hand (N Y). 2012;7(2):157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagni M, Khan NR, Cohen HL, Choudhri AF. Highly cited works in radiology: the top 100 cited articles in radiologic journals. Acad Radiol. 2014;21(8):1056–66. [DOI] [PubMed] [Google Scholar]
- 19.Badhiwala JH, Nassiri F, Witiw CD, Mansouri A, Alotaibi N, Eagles M, et al. Highly cited works in spinal disorders: the top 100 most cited papers published in spine journals. Spine (Phila Pa 1976). 2018;43(24):1746–55. [DOI] [PubMed] [Google Scholar]
- 20.Holzer LA, Holzer G. The 50 highest cited papers in hip and knee arthroplasty. J Arthroplasty. 2014;29(3):453–7. [DOI] [PubMed] [Google Scholar]
- 21.Familiari F, Castricini R, Galasso O, Gasparini G, Ianno B, Ranuccio F. The 50 highest cited papers on rotator cuff tear. Arthroscopy. 2021;37(1):61–8. [DOI] [PubMed] [Google Scholar]
- 22.Vielgut I, Dauwe J, Leithner A, Holzer LA. The fifty highest cited papers in anterior cruciate ligament injury. Int Orthop. 2017;41(7):1405–12. [DOI] [PubMed] [Google Scholar]
- 23.Namdari S, Baldwin K, Kovatch K, Huffman GR, Glaser D. Fifty most cited articles in orthopedic shoulder surgery. J Shoulder Elb Surg. 2012;21(12):1796–802. [DOI] [PubMed] [Google Scholar]
- 24.Virk SS, Yu E. The top 50 articles on minimally invasive spine surgery. Spine (Phila Pa 1976). 2017;42(7):513–9. [DOI] [PubMed] [Google Scholar]
- 25.Malik AT, Jain N, Yu E, Khan SN. The top 50 most-cited articles on cervical spondylotic myelopathy. World Neurosurg. 2018;116:e1168-80. [DOI] [PubMed] [Google Scholar]
- 26.Yin M, Xu C, Mo W. The 100 most cited articles on lumbar spinal stenosis: a bibliometric analysis. Global Spine J. 2022;12(3):381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue JH, Hu ZP, Lai P, Cai DQ, Wen ES. The 100 most-cited articles in Parkinson’s disease. Neurol Sci. 2018;39(9):1537–45. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Zhao T, Reidler JS, Chen X, Zhang H, Shao H, et al. The top 100 most-cited articles on kyphoplasty and vertebroplasty. World Neurosurg. 2020;135:e435-446. [DOI] [PubMed] [Google Scholar]
- 29.Ruegsegger N, Ahmad SS, Benneker LM, Berlemann U, Keel MJ, Hoppe S. The 100 most influential publications in cervical spine research. Spine (Phila Pa 1976). 2016;41(6):538–48. [DOI] [PubMed] [Google Scholar]
- 30.Ding Z, Ren Y, Cao H, Li J. Top 100 most cited articles on anterior cervical discectomy and fusion. Front Surg. 2022;9: 1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24(20):3531–41. [DOI] [PubMed] [Google Scholar]
- 32.Sakai D, Mochida J, Iwashina T, Hiyama A, Omi H, Imai M, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27(3):335–45. [DOI] [PubMed] [Google Scholar]
- 33.Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92(7):822–8. [DOI] [PubMed] [Google Scholar]
- 34.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976). 2005;30(21):2379–87. [DOI] [PubMed] [Google Scholar]
- 35.Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson SM, Kalamegam G, Pushparaj PN, Matta C, Memic A, Khademhosseini A, et al. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods. 2016;99:69–80. [DOI] [PubMed] [Google Scholar]
- 37.Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004;32(3):430–4. [DOI] [PubMed] [Google Scholar]
- 38.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26(5):589–600. [DOI] [PubMed] [Google Scholar]
- 39.Vadalà G, Sowa G, Hubert M, Gilbertson LG, Denaro V, Kang JD. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6(5):348–55. [DOI] [PubMed] [Google Scholar]
- 40.Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine (Phila Pa 1976). 2010;35(11):E475-80. [DOI] [PubMed] [Google Scholar]
- 41.Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8(6):888–96. [DOI] [PubMed] [Google Scholar]
- 42.Henriksson HB, Svanvik T, Jonsson M, Hagman M, Horn M, Lindahl A, et al. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976). 2009;34(2):141–8. [DOI] [PubMed] [Google Scholar]
- 43.Ganey T, Hutton WC, Moseley T, Hedrick M, Meisel HJ. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: experiments in a canine model. Spine (Phila Pa 1976). 2009;34(21):2297–304. [DOI] [PubMed] [Google Scholar]
- 44.Leung VY, Chan D, Cheung KM. Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J. 2006;15(Suppl 3):S406-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang YG, Guo X, Xu P, Kang LL, Li J. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res. 2005;430:219–26. [DOI] [PubMed] [Google Scholar]
- 46.Sakai D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J. 2008;17(Suppl 4):452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28(10):1267–75. [DOI] [PubMed] [Google Scholar]
- 48.Noriega DC, Ardura F, Hernández-Ramajo R, Martín-Ferrero M, Sánchez-Lite I, Toribio B, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101(8):1945–51. [DOI] [PubMed] [Google Scholar]
- 49.Yang F, Leung VY, Luk KD, Chan D, Cheung KM. Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol Ther. 2009;17(11):1959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyamoto T, Muneta T, Tabuchi T, Matsumoto K, Saito H, Tsuji K, et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12(6):R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pettine KA, Murphy MB, Suzuki RK, Sand TT. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015;33(1):146–56. [DOI] [PubMed] [Google Scholar]
- 52.Acosta FL Jr, Metz L, Adkisson HD, Liu J, Carruthers-Liebenberg E, Milliman C, et al. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A. 2011;17(23–24):3045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar H, Ha DH, Lee EJ, Park JH, Shim JH, Ahn TK, et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res Ther. 2017;8(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang H, Wu J, Liu J, Ebraheim M, Castillo S, Liu X, et al. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J. 2010;10(9):802–10. [DOI] [PubMed] [Google Scholar]
- 55.Jeong JH, Lee JH, Jin ES, Min JK, Jeon SR, Choi KH. Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose-tissue-derived stromal cells. Acta Neurochir (Wien). 2010;152(10):1771–7. [DOI] [PubMed] [Google Scholar]
- 56.Hoogendoorn RJ, Lu ZF, Kroeze RJ, Bank RA, Wuisman PI, Helder MN. Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future. J Cell Mol Med. 2008;12(6a):2205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng G, Zhao X, Liu H, Zhang H, Chen X, Shi R, et al. Transplantation of mesenchymal stem cells and nucleus pulposus cells in a degenerative disc model in rabbits: a comparison of 2 cell types as potential candidates for disc regeneration. J Neurosurg Spine. 2011;14(3):322–9. [DOI] [PubMed] [Google Scholar]
- 58.Yim RL, Lee JT, Bow CH, Meij B, Leung V, Cheung KM, et al. A systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: insights and future directions for regenerative therapeutics. Stem Cells Dev. 2014;23(21):2553–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakai D, Schol J. Cell therapy for intervertebral disc repair: clinical perspective. J Orthop Translat. 2017;9:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clouet J, Fusellier M, Camus A, Le Visage C, Guicheux J. Intervertebral disc regeneration: from cell therapy to the development of novel bioinspired endogenous repair strategies. Adv Drug Deliv Rev. 2019;146:306–24. [DOI] [PubMed] [Google Scholar]
- 61.Leung VY, Aladin DM, Lv F, Tam V, Sun Y, Lau RY, et al. Mesenchymal stem cells reduce intervertebral disc fibrosis and facilitate repair. Stem Cells. 2014;32(8):2164–77. [DOI] [PubMed] [Google Scholar]
- 62.Haufe SM, Mork AR. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006;15(1):136–7. [DOI] [PubMed] [Google Scholar]
- 63.Ho G, Leung VY, Cheung KM, Chan D. Effect of severity of intervertebral disc injury on mesenchymal stem cell-based regeneration. Connect Tissue Res. 2008;49(1):15–21. [DOI] [PubMed] [Google Scholar]
- 64.Wei A, Tao H, Chung SA, Brisby H, Ma DD, Diwan AD. The fate of transplanted xenogeneic bone marrow-derived stem cells in rat intervertebral discs. J Orthop Res. 2009;27(3):374–9. [DOI] [PubMed] [Google Scholar]
- 65.Vadalà G, Russo F, Ambrosio L, Loppini M, Denaro V. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016;8(5):185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng Y, Chen C, Liu W, Fu Q, Han Z, Li Y, et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration. Biomaterials. 2015;59:53–65. [DOI] [PubMed] [Google Scholar]
- 67.Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med. 2016;14(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pettine KA, Suzuki RK, Sand TT, Murphy MB. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int Orthop. 2017;41(10):2097–103. [DOI] [PubMed] [Google Scholar]
- 69.Yang H, Cao C, Wu C, Yuan C, Gu Q, Shi Q, et al. TGF-βl suppresses inflammation in cell therapy for intervertebral disc degeneration. Sci Rep. 2015;5: 13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Centeno C, Markle J, Dodson E, Stemper I, Williams CJ, Hyzy M, et al. Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med. 2017;15(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H, Zhou Y, Huang B, Liu LT, Liu MH, Wang J, et al. Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng Part A. 2014;20(5–6):908–20. [DOI] [PubMed] [Google Scholar]
- 72.Chun HJ, Kim YS, Kim BK, Kim EH, Kim JH, Do BR, et al. Transplantation of human adipose-derived stem cells in a rabbit model of traumatic degeneration of lumbar discs. World Neurosurg. 2012;78(3–4):364–71. [DOI] [PubMed] [Google Scholar]
- 73.Liang CZ, Li H, Tao YQ, Peng LH, Gao JQ, Wu JJ, et al. Dual release of dexamethasone and TGF-β3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model. Acta Biomater. 2013;9(12):9423–33. [DOI] [PubMed] [Google Scholar]
- 74.Murrell W, Sanford E, Anderberg L, Cavanagh B, Mackay-Sim A. Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model. Spine J. 2009;9(7):585–94. [DOI] [PubMed] [Google Scholar]
- 75.Allon AA, Aurouer N, Yoo BB, Liebenberg EC, Buser Z, Lotz JC. Structured coculture of stem cells and disc cells prevent disc degeneration in a rat model. Spine J. 2010;10(12):1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pettine K, Suzuki R, Sand T, Murphy M. Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimum two year follow-up. Int Orthop. 2016;40(1):135–40. [DOI] [PubMed] [Google Scholar]
- 77.Zhou X, Wang J, Fang W, Tao Y, Zhao T, Xia K, et al. Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018;71:496–509. [DOI] [PubMed] [Google Scholar]
- 78.Comella K, Silbert R, Parlo M. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J Transl Med. 2017;15(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeong JH, Jin ES, Min JK, Jeon SR, Park CS, Kim HS, et al. Human mesenchymal stem cells implantation into the degenerated coccygeal disc of the rat. Cytotechnology. 2009;59(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakai D, Nishimura K, Tanaka M, Nakajima D, Grad S, Alini M, et al. Migration of bone marrow-derived cells for endogenous repair in a new tail-looping disc degeneration model in the mouse: a pilot study. Spine J. 2015;15(6):1356–65. [DOI] [PubMed] [Google Scholar]
- 81.Pang X, Yang H, Peng B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician. 2014;17(4):E525-530. [PubMed] [Google Scholar]
- 82.Binch ALA, Fitzgerald JC, Growney EA, Barry F. Cell-based strategies for IVD repair: clinical progress and translational obstacles. Nat Rev Rheumatol. 2021;17(3):158–75. [DOI] [PubMed] [Google Scholar]
- 83.Loibl M, Wuertz-Kozak K, Vadala G, Lang S, Fairbank J, Urban JP. Controversies in regenerative medicine: should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine. 2019;2(1):e1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oehme D, Ghosh P, Shimmon S, Wu J, McDonald C, Troupis JM, et al. Mesenchymal progenitor cells combined with pentosan polysulfate mediating disc regeneration at the time of microdiscectomy: a preliminary study in an ovine model. J Neurosurg Spine. 2014;20(6):657–69. [DOI] [PubMed] [Google Scholar]
- 85.Bendtsen M, Bünger CE, Zou X, Foldager C, Jørgensen HS. Autologous stem cell therapy maintains vertebral blood flow and contrast diffusion through the endplate in experimental intervertebral disc degeneration. Spine (Phila Pa 1976). 2011;36(6):E373-9. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z, Perez-Terzic CM, Smith J, Mauck WD, Shelerud RA, Maus TP, et al. Efficacy of intervertebral disc regeneration with stem cells - a systematic review and meta-analysis of animal controlled trials. Gene. 2015;564(1):1–8. [DOI] [PubMed] [Google Scholar]
- 87.Marfia G, Campanella R, Navone SE, Zucca I, Scotti A, Figini M, et al. Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: a preliminary study on biglycan-deficient murine model of chronic disc degeneration. Arthritis Res Ther. 2014;16(5):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hee HT, Ismail HD, Lim CT, Goh JC, Wong HK. Effects of implantation of bone marrow mesenchymal stem cells, disc distraction and combined therapy on reversing degeneration of the intervertebral disc. J Bone Joint Surg Br. 2010;92(5):726–36. [DOI] [PubMed] [Google Scholar]
- 89.Anderson DG, Risbud MV, Shapiro IM, Vaccaro AR, Albert TJ. Cell-based therapy for disc repair. Spine J. 2005;5(6 Suppl):s297-303. [DOI] [PubMed] [Google Scholar]
- 90.Schol J, Sakai D. Cell therapy for intervertebral disc herniation and degenerative disc disease: clinical trials. Int Orthop. 2019;43(4):1011–25. [DOI] [PubMed] [Google Scholar]
- 91.Omlor GW, Bertram H, Kleinschmidt K, Fischer J, Brohm K, Guehring T, et al. Methods to monitor distribution and metabolic activity of mesenchymal stem cells following in vivo injection into nucleotomized porcine intervertebral discs. Eur Spine J. 2010;19(4):601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeckser J, Wolff M, Tucker J, Goodwin J. Multipotent mesenchymal stem cell treatment for discogenic low back pain and disc degeneration. Stem Cells Int. 2016;2016: 3908389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai F, Wu XT, Xie XH, Wang F, Hong X, Zhuang SY, et al. Evaluation of intervertebral disc regeneration with implantation of bone marrow mesenchymal stem cells (BMSCs) using quantitative T2 mapping: a study in rabbits. Int Orthop. 2015;39(1):149–59. [DOI] [PubMed] [Google Scholar]
- 94.Sheyn D, Ben-David S, Tawackoli W, Zhou Z, Salehi K, Bez M, et al. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics. 2019;9(25):7506–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang YT, Wu XT, Wang F. Regeneration potential and mechanism of bone marrow mesenchymal stem cell transplantation for treating intervertebral disc degeneration. J Orthop Sci. 2010;15(6):707–19. [DOI] [PubMed] [Google Scholar]
- 96.Sun W, Zhang K, Liu G, Ding W, Zhao C, Xie Y, et al. Sox9 gene transfer enhanced regenerative effect of bone marrow mesenchymal stem cells on the degenerated intervertebral disc in a rabbit model. PLoS One. 2014;9(4): e93570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen X, Zhu L, Wu G, Liang Z, Yang L, Du Z. A comparison between nucleus pulposus-derived stem cell transplantation and nucleus pulposus cell transplantation for the treatment of intervertebral disc degeneration in a rabbit model. Int J Surg. 2016;28:77–82. [DOI] [PubMed] [Google Scholar]
- 98.Gou S, Oxentenko SC, Eldrige JS, Xiao L, Pingree MJ, Wang Z, et al. Stem cell therapy for intervertebral disk regeneration. Am J Phys Med Rehabil. 2014;93(11 Suppl 3):S122-31. [DOI] [PubMed] [Google Scholar]
- 99.Tam V, Rogers I, Chan D, Leung VY, Cheung KM. A comparison of intravenous and intradiscal delivery of multipotential stem cells on the healing of injured intervertebral disk. J Orthop Res. 2014;32(6):819–25. [DOI] [PubMed] [Google Scholar]
- 100.Zhou X, Wang J, Huang X, Fang W, Tao Y, Zhao T, et al. Injectable decellularized nucleus pulposus-based cell delivery system for differentiation of adipose-derived stem cells and nucleus pulposus regeneration. Acta Biomater. 2018;81:115–28. [DOI] [PubMed] [Google Scholar]
- 101.Hussain I, Sloan SR, Wipplinger C, Navarro-Ramirez R, Zubkov M, Kim E, et al. Mesenchymal stem cell-seeded high-density collagen gel for annular repair: 6-week results from in vivo sheep models. Neurosurgery. 2019;85(2):E350-9. [DOI] [PubMed] [Google Scholar]
- 102.Freeman BJC, Kuliwaba JS, Jones CF, Shu CC, Colloca CJ, Zarrinkalam MR, et al. Allogeneic mesenchymal precursor cells promote healing in postero-lateral annular lesions and improve indices of lumbar intervertebral disc degeneration in an ovine model. Spine (Phila Pa 1976). 2016;41(17):1331–9. [DOI] [PubMed] [Google Scholar]
- 103.Ahn J, Park EM, Kim BJ, Kim JS, Choi B, Lee SH, et al. Transplantation of human Wharton’s jelly-derived mesenchymal stem cells highly expressing TGFβ receptors in a rabbit model of disc degeneration. Stem Cell Res Ther. 2015;6:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shu CC, Smith MM, Smith SM, Dart AJ, Little CB, Melrose J. A histopathological scheme for the quantitative scoring of intervertebral disc degeneration and the therapeutic utility of adult mesenchymal stem cells for intervertebral disc regeneration. Int J Mol Sci. 2017;18(5):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Melrose J. Strategies in regenerative medicine for intervertebral disc repair using mesenchymal stem cells and bioscaffolds. Regen Med. 2016;11(7):705–24. [DOI] [PubMed] [Google Scholar]
- 106.Migliorini F, Rath B, Tingart M, Baroncini A, Quack V, Eschweiler J. Autogenic mesenchymal stem cells for intervertebral disc regeneration. Int Orthop. 2019;43(4):1027–36. [DOI] [PubMed] [Google Scholar]
- 107.Ishiguro H, Kaito T, Yarimitsu S, Hashimoto K, Okada R, Kushioka J, et al. Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater. 2019;87:118–29. [DOI] [PubMed] [Google Scholar]
- 108.Li YY, Diao HJ, Chik TK, Chow CT, An XM, Leung V, et al. Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: reduced risk of osteophyte formation. Tissue Eng Part A. 2014;20(9–10):1379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang F, Nan LP, Zhou SF, Liu Y, Wang ZY, Wang JC, et al. Injectable hydrogel combined with nucleus pulposus-derived mesenchymal stem cells for the treatment of degenerative intervertebral disc in rats. Stem Cells Int. 2019;2019:8496025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu J, E XQ, Wang NX, Wang MN, Xie HX, Cao YH, et al. BMP7 enhances the effect of BMSCs on extracellular matrix remodeling in a rabbit model of intervertebral disc degeneration. FEBS J. 2016;283(9):1689–700. [DOI] [PubMed] [Google Scholar]
- 111.Hiraishi S, Schol J, Sakai D, Nukaga T, Erickson I, Silverman L, et al. Discogenic cell transplantation directly from a cryopreserved state in an induced intervertebral disc degeneration canine model. JOR Spine. 2018;1(2): e1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang W, Wang Y, Deng G, Ma J, Huang X, Yu J, et al. Transplantation of hypoxic-preconditioned bone mesenchymal stem cells retards intervertebral disc degeneration via enhancing implanted cell survival and migration in rats. Stem Cells Int. 2018;2018:7564159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Silverman LI, Dulatova G, Tandeski T, Erickson IE, Lundell B, Toplon D, et al. In vitro and in vivo evaluation of discogenic cells, an investigational cell therapy for disc degeneration. Spine J. 2020;20(1):138–49. [DOI] [PubMed] [Google Scholar]
- 114.Liao JC. Cell therapy using bone marrow-derived stem cell overexpressing BMP-7 for degenerative discs in a rat tail disc model. Int J Mol Sci. 2016;17(2): 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.James G, Blomster L, Hall L, Schmid AB, Shu CC, Little CB, et al. Mesenchymal stem cell treatment of intervertebral disc lesion prevents fatty infiltration and fibrosis of the Multifidus muscle, but not cytokine and muscle fiber changes. Spine (Phila Pa 1976). 2016;41(15):1208–17. [DOI] [PubMed] [Google Scholar]
- 116.Maidhof R, Rafiuddin A, Chowdhury F, Jacobsen T, Chahine NO. Timing of mesenchymal stem cell delivery impacts the fate and therapeutic potential in intervertebral disc repair. J Orthop Res. 2017;35(1):32–40. [DOI] [PubMed] [Google Scholar]
- 117.Steffen F, Smolders LA, Roentgen AM, Bertolo A, Stoyanov J. Bone marrow-derived mesenchymal stem cells as autologous therapy in dogs with naturally occurring intervertebral disc disease: feasibility, safety, and preliminary results. Tissue Eng Part C Methods. 2017;23(11):643–51. [DOI] [PubMed] [Google Scholar]
- 118.Wang SZ, Jin JY, Guo YD, Ma LY, Chang Q, Peng XG, et al. Intervertebral disc regeneration using platelet–rich plasma–containing bone marrow–derived mesenchymal stem cells: a preliminary investigation. Mol Med Rep. 2016;13(4):3475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wei JN, Cai F, Wang F, Wu XT, Liu L, Hong X, et al. Transplantation of CXCR4 overexpressed mesenchymal stem cells augments regeneration in degenerated intervertebral discs. Dna Cell Biol. 2016;35(5):241–8. [DOI] [PubMed] [Google Scholar]
- 120.Henriksson HB, Papadimitriou N, Hingert D, Baranto A, Lindahl A, Brisby H. The traceability of mesenchymal stromal cells after injection into degenerated discs in patients with low back Pain. Stem Cells Dev. 2019;28(17):1203–11. [DOI] [PubMed] [Google Scholar]
- 121.Beeravolu N, Brougham J, Khan I, McKee C, Perez-Cruet M, Chaudhry GR. Human umbilical cord derivatives regenerate intervertebral disc. J Tissue Eng Regen Med. 2018;12(1):e579-91. [DOI] [PubMed] [Google Scholar]
- 122.Chiang ER, Ma HL, Wang JP, Chang MC, Liu CL, Chen TH, et al. Use of allogeneic hypoxic mesenchymal stem cells for treating disc degeneration in rabbits. J Orthop Res. 2019;37(6):1440–50. [DOI] [PubMed] [Google Scholar]
- 123.Croft AS, Illien-Jünger S, Grad S, Guerrero J, Wangler S, Gantenbein B. The application of mesenchymal stromal cells and their homing capabilities to regenerate the intervertebral disc. Int J Mol Sci. 2021;22(7): 3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun Y, Leung VY, Cheung KM. Clinical trials of intervertebral disc regeneration: current status and future developments. Int Orthop. 2019;43(4):1003–10. [DOI] [PubMed] [Google Scholar]
- 125.Hang D, Li F, Che W, Wu X, Wan Y, Wang J, et al. One-stage positron emission tomography and magnetic resonance imaging to assess mesenchymal stem cell survival in a canine model of intervertebral disc degeneration. Stem Cells Dev. 2017;26(18):1334–43. [DOI] [PubMed] [Google Scholar]
- 126.Hou Y, Shi G, Shi J, Xu G, Guo Y, Xu P. Study design: in vitro and in vivo assessment of bone morphogenic protein 2 combined with platelet-rich plasma on treatment of disc degeneration. Int Orthop. 2016;40(6):1143–55. [DOI] [PubMed] [Google Scholar]
- 127.Daly CD, Ghosh P, Zannettino ACW, Badal T, Shimmon R, Jenkin G, et al. Mesenchymal progenitor cells primed with pentosan polysulfate promote lumbar intervertebral disc regeneration in an ovine model of microdiscectomy. Spine J. 2018;18(3):491–506. [DOI] [PubMed] [Google Scholar]
- 128.Feng G, Zhang Z, Dang M, Rambhia KJ, Ma PX. Nanofibrous spongy microspheres to deliver rabbit mesenchymal stem cells and anti-miR-199a to regenerate nucleus pulposus and prevent calcification. Biomaterials. 2020;256: 120213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yi Z, Guanjun T, Lin C, Zifeng P. Effects of transplantation of hTIMP-1-Expressing bone marrow mesenchymal stem cells on the extracellular matrix of degenerative intervertebral discs in an in vivo rabbit model. Spine (Phila Pa 1976). 2014;39(11):E669-75. [DOI] [PubMed] [Google Scholar]
- 130.Shu CC, Dart A, Bell R, Dart C, Clarke E, Smith MM, et al. Efficacy of administered mesenchymal stem cells in the initiation and co-ordination of repair processes by resident disc cells in an ovine (Ovis aries) large destabilizing lesion model of experimental disc degeneration. JOR Spine. 2018;1(4):e1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bano JM, Rate WRt, Civilette MD, Wyand TJ, Samuelson ER, Bodendorfer BM, et al. The top 100 most impactful articles on the achilles tendon according to altmetric attention score and number of citations. Orthop J Sports Med. 2024;12(3):23259671241232710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huo YQ, Pan XH, Li QB, Wang XQ, Jiao XJ, Jia ZW, et al. Fifty top-cited classic papers in orthopedic elbow surgery: a bibliometric analysis. Int J Surg. 2015;18:28–33. [DOI] [PubMed] [Google Scholar]
- 133.Richardson MA, Park W, Echternacht SR, Bell DE. Altmetric attention score: evaluating the social media impact of burn research. J Burn Care Res. 2021;42(6):1181–5. [DOI] [PubMed] [Google Scholar]
- 134.Alini M, Diwan AD, Erwin WM, Little CB, Melrose J. An update on animal models of intervertebral disc degeneration and low back pain: exploring the potential of artificial intelligence to improve research analysis and development of prospective therapeutics. JOR Spine. 2023;6(1): e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oehme D, Goldschlager T, Ghosh P, Rosenfeld JV, Jenkin G. Cell-based therapies used to treat lumbar degenerative disc disease: a systematic review of animal studies and human clinical trials. Stem Cells Int. 2015;2015:946031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sheng SR, Wang XY, Xu HZ, Zhu GQ, Zhou YF. Anatomy of large animal spines and its comparison to the human spine: a systematic review. Eur Spine J. 2010;19(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Munda M, Velnar T. Stem cell therapy for degenerative disc disease: bridging the gap between preclinical promise and clinical potential. Biomol Biomed. 2024;24(2):210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martin-Martin A, Thelwall M, Orduna-Malea E, Delgado Lopez-Cozar E. Google Scholar, microsoft academic, scopus, dimensions, web of science, and OpenCitations’ COCI: a multidisciplinary comparison of coverage via citations. Scientometrics. 2021;126(1):871–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Altmetric. Attention sources tracked by Altmetric. https://help.altmetric.com/support/solutions/articles/6000235983-attention-sources-tracked-by-altmetric. Accessed 10 Aug 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in this paper are available upon reasonable requests from corresponding author.