Abstract
Objective
This study retrospectively analyzed the relationship between serum-free triiodothyronine (FT3) levels and the prognosis of coronary atherosclerotic cardiopathy (CHD) in patients from alpine regions treated with drug-coated balloons (DCB).
Methods
Data from 201 CHD patients with DCB at Hulunbuir People’s Hospital between September 2019 and August 2023 were included. Patients were divided into two groups based on the occurrence of major adverse cardiovascular events (MACE) after surgery. Univariate and multivariate logistic regression analyses were conducted to identify risk factors. The predictive efficiency of these risk factors for MACE was evaluated using the ROC curve.
Results
The poor prognosis group had significantly higher ages, a greater proportion of patients with a history of previous coronary interventions, and elevated levels of N-terminal pro-B-type natriuretic peptide compared to the good prognosis group. In contrast, FT3 levels were significantly lower (P < 0.05). No significant differences were observed in surgical parameters such as DCB target lesion site, lesion length, or puncture approach between the groups (P > 0.05). Multivariate binary logistic regression analysis identified FT3 level as an independent predictor factor of MACE in CHD patients treated with DCB. The optimal cut-off value for FT3 in predicting adverse prognosis following DCB surgery was 3.30 pmol/L, with a sensitivity of 72.5%, specificity of 62.8%, and an area under the curve (AUC) of 0.741 (P < 0.05).
Conclusion
Decreased FT3 levels serve as a biomarker for predicting the occurrence of MACE in patients from alpine regions undergoing DCB treatment for CHD. There is a significant correlation between reduced FT3 levels and the incidence of MACE in these patients.
Keywords: Coronary atherosclerotic cardiopathy, Drug-coated balloons, Free triiodothyronine, Prognosis
Introduction
Coronary atherosclerotic cardiopathy (CHD) currently affects an estimated 11 million individuals in China, with the prevalence to rise [1]. Established risk factors for CHD include advanced age, male gender, hypertension, diabetes, dyslipidemia, smoking, and a family history of early-onset CHD [2, 3]. Recent studies have highlighted the influence of environmental temperature on cardiovascular health, with fluctuation linked to CHD incidence and mortality rates, indirectly heightening health risks through various mechanisms [4]. During cold winters, cardiac death rates peak, particularly among elderly and male patients [5]. Hulunbuir City, located in Inner Mongolia in the northeastern China, experiences harsh winter with significant annual temperature fluctuations and frequent cold waves, representing a regional meteorological challenge [6]. As a result, investigating CHD prevention and treatment in this region provides valuable insights for managing CHD in the alpine areas of China.
Current CHD management includes both pharmacological and revascularization strategies, with percutaneous coronary intervention (PCI) being widely used in clinical settings [2]. The introduction of drug-eluting stents (DES) has greatly improved the symptoms and prognosis in CHD patients. However, challenges like small vessel disease (SVD), side branch lesions (SB), and in-stent restenosis (ISR) remain sub-optimally addressed by stent deployment [7]. Recently, drug-coated balloons (DCB) have emerged as a pivotal treatment modality in interventional cardiology, offering enhanced management for SVD, SB, and ISR, thereby benefiting a broader patient population [8]. Therefore, identifying high-risk groups for DCB treatment through laboratory testing and preemptively assessing postoperative complications in CHD patients treated with DCB is crucial.
Thyroid hormones (TH) play a crucial role in regulating growth, development, and metabolism, significantly impacting the circulatory system. Free triiodothyronine (FT3), the most potent hormone in the TH group, regulates cardiac contractions, heart rate, and blood pressure [9, 10]. Reduced FT3 levels have been associated with arteriosclerosis and an increased incidence of CHD, negatively affecting its prognosis [11]. Additionally, FT3 has been identified as a significant predictor of cardiac mortality [12]. Despite this knowledge, no domestic studies have yet assessed how variations in FT3 levels in alpine regions may impact the prognosis of patients undergoing DCB treatment for CHD.
Materials and methods
Study population
A retrospective analysis was conducted on 206 patients who underwent DCB treatment for CHD in the cardiology department of Hulunbuir People’s Hospital from September 2019 to August 2023. Data were collected from 201 patients who met the inclusion and exclusion criteria (5 patients were excluded), with effective follow-up conducted in 191 cases (127 males and 64 females), while 10 cases were lost to follow-up.
Prior to the study, we conducted a small observational analysis, which suggested a possible correlation between decreased FT3 levels and MACE. Based on this observation, we calculated the required sample size using SPSS 21 software for comparison of means between two groups. The preliminary investigation showed that the mean FT3 levels in the two groups were 4.3 and 3.8, with standard deviations of approximately 0.9. Setting the type I error (α) at 0.05, the type II error (β) at 0.2, and an expected adverse event rate of 20%, the minimum required sample size was calculated to be 163 participants. Accounting for a potential 20% data loss, the final required sample size was determined to be 205 participants.
Inclusion and exclusion criteria
Inclusion criteria
Patients with a history of CHD or those diagnosed with CHD via coronary angiography who underwent DCB expansion surgery.
Intraoperative lesion treatment was deemed satisfactory, meeting the standards outlined in the Chinese Expert Consensus on Clinical Application of Drug-Coated Balloons [13].
All participants provided informed consent and signed consent form to participate in the study.
Participants were aged 18 years or older.
Exclusion criteria
Patients requiring remedial stent placement due to intraoperative dissection.
Patients diagnosed with subclinical hypothyroidism, subclinical hyperthyroidism, hyperthyroidism, or those receiving thyroid hormone medication and other iodine-containing preparations upon admission.
Patients with severe infections, significant cardiac, liver, or kidney dysfunction, hematological disorders, malignant tumors, or sepsis.
Patients with a life expectancy of less than one year.
Data collection
General information for the 191 patients was documented, including name, age, gender, contact details, hospital ID, diagnosis, medical history (including hypertension, diabetes, smoking, previous coronary interventions, and prior myocardial infarction), as well as surgical dates. Examination data include thyroid function tests (TSH, FT3, and FT4), complete blood count, coagulation profile, comprehensive biochemistry, homocysteine levels, glycated hemoglobin (for patients with abnormal blood sugar or diabetes), cardiac biomarkers, N-terminal pro-B-type natriuretic peptide levels, and preoperative echocardiographic ejection fraction assessment. Surgical details comprised target vessel location, lesion length, percentage of lesion stenosis, puncture approach, DCB dimensions (length and diameter), and calculation of the Gensini score (GS score) for each patient [14].
Treatment standards
Preoperative examination and oral medication regimen
Before enrollment, the aforementioned pre-operative examinations were conducted. Patients were informed about their condition, surgical objectives, and associated risks before signing the informed consent form for surgery. All patients underwent a standardized dual antiplatelet therapy (DAPT) regimen pre-operatively, which included oral administration of enteric-coated aspirin 100 mg with a loading dose of 300 mg once daily before surgery, alongside clopidogrel bisulfate 75 mg once daily with a loading dose of 300 mg before surgery, or ticagrelor 90 mg twice daily with a loading dose of 180 mg before surgery.
Surgical procedure
During coronary angiography, patients suitable for DCB intervention are carefully selected. The initial step involves dilation using a traditional or semi-compliant balloon, maintaining a balloon-to-artery diameter ratio of 0.8 to 1.0 and applying moderate pressure (typically 8 to 14 atmospheres, where 1 atmosphere equals 101.325 kPa) to minimize the risk of dissection. If initial dilation is inadequate, a non-compliant or cutting balloon may be used for further pre-dilation. After achieving sufficient pre-dilation, the outcome is assessed to determine eligibility for DCB treatment. DCB intervention proceeds under the following conditions: absence of dissection or presence of type A or B dissection; achievement of TIMI flow grade 3; residual stenosis ≤ 30%. The selected DCB diameter should match the vessel diameter (recommended diameter ratio of 0.8-1.0), and dilation maintained for 60 s at 10 atmospheres of pressure. To ensure adequate coverage between the treated area or stent and the DCB, the DCB must extend 2 to 3 mm beyond each end of the pre-treated area. Additionally, the DCB must reach the lesion site within 2 min of entering the body to prevent inadequate treatment.
Postoperative management and oral medication regimen
Following surgery, patients receive pressure bandaging at the radial artery puncture site for 4 to 6 h, with close monitoring for hematoma formation and any improvements in preoperative CHD symptoms, as well as monitoring for bleeding or melena. All patients are required to continue DAPT for at least one month post-surgery, as recommended by expert consensus (oral intake is recommended for 12 months) to prevent thrombosis formation [13]. Postoperative electrocardiograms and urinalysis are also performed to ensure comprehensive post-surgical care.
Follow-up records
Follow-up for patients undergoing DCB treatment for CHD is conducted through three methods: phone calls, outpatient clinic visits, and readmission records. The follow-up period ranges from six months to two years post-surgery. Patients are categorized based on prognosis: those readmitted for recurrent angina requiring further coronary imaging assessments (including coronary angiography or coronary artery enhanced CT) to evaluate restenosis at the target lesion site post-DCB are classified into the poor prognosis group; if no restenosis requiring intervention is observed during follow-up imaging assessments, they are categorized into the good prognosis group. Patients experiencing other major adverse cardiovascular events (MACE)-such as recurrent acute myocardial infarction (AMI), post-DCB target lesion revascularization (TLR), cardiac death, malignant arrhythmia, and heart failure-are also categorized into the poor prognosis group (n = 40). The remaining patients are defined as the control group or the good prognosis group (n = 151). Statistical analysis is conducted using R software version 4.2.0 to compare the correlation between FT3 levels and prognosis in the poor and good prognosis groups.
Statistical methods
Data analysis and graph plotting are conducted using R software version 4.2.0. For categorical data such as age, TSH, FT3, FT4, and platelet count, c2 tests are used. For measurement data that follows a normal distribution, such as gender, history of hypertension, diabetes, and previous coronary interventions, t-tests are applied. For data that does not follow a normal distribution, such as the Gensini score, the Kruskal-Wallis test (a non-parametric test) is employed. Both univariate and multivariate logistic regression analyses are conducted to assess the observed indicators, and ROC curves are plotted to predict the potential impact of these indicators on patient prognosis. A P-value of < 0.05 is considered statistically significant.
Results
Comparison of general clinical data between groups
Patients were classified into a good prognosis group (151 cases) and a poor prognosis group (40 cases) based on the occurrence of MACE following DCB treatment for CHD. Statistically significant differences between the groups were observed for age, history of coronary intervention, FT3 levels, N-terminal pro-B-type natriuretic peptide measurements, and GS scores (P < 0.05). However, no significant differences were found between the groups regarding gender, presence of hypertension, diabetes, history of myocardial infarction, smoking history, diagnostic results, platelet count, TSH and FT4 levels, fibrinogen, high-density lipoprotein, low-density lipoprotein, uric acid, triglycerides, total cholesterol, cardiac injury markers, homocysteine, glycated hemoglobin, and echocardiographic ejection fraction (Table 1).
Table 1.
A comparison of general clinical data and biochemical markers between groups with good and poor prognosis
| Observational indicator | Good prognosis group (n = 151) | Poor prognosis group (n = 40) | t/X2 | P |
|---|---|---|---|---|
| Age (years, mean ± s) | 59 ± 9.7 | 65 ± 14 | -2.483 | 0.017 |
| Gender [n (%)] | 0.624 | 0.430 | ||
| Male | 103(68.2%) | 24(60.0%) | ||
| Female | 48(31.8%) | 16 (40.0%) | ||
| History of high blood pressure [n (%)] | 111(73.5%) | 34(85.0%) | 1.698 | 0.193 |
| History of diabetes [n (%)] | 55(36.4%) | 21(52.5%) | 2.773 | 0.096 |
| Previous coronary intervention [n (%)] | 45(29.8%) | 21(52.5%) | 6.236 | 0.013 |
| Previous myocardial infarction [n (%)] | 30(19.9%) | 10(25.0%) | 0.241 | 0.624 |
| Smoking history [n (%)] | 98(64.9%) | 23(57.5%) | 0.461 | 0.497 |
| Diagnosis [n (%)] | 7.063 | 0.070 | ||
| Acute non-ST elevation myocardial infarction | 22(14.6%) | 7(17.5%) | ||
| Acute ST elevation myocardial infarction | 30(19.9%) | 1(2.5%) | ||
| Stable angina | 20(13.2%) | 7(17.5%) | ||
| Unstable angina | 79(52.3%) | 25(62.5%) | ||
| TSH (uIU/ml, mean ± s) | 2.8 ± 2.6 | 5.3 ± 15 | − 1.053 | 0.299 |
| FT3(pmol/L, mean ± s) | 4.4 ± 0.72 | 4. 1 ± 0.90 | 2.013 | 0.049 |
| FT4(pmol/L, mean ± s) | 16 ± 2.5 | 15 ± 3.0 | 0.540 | 0.592 |
| Platelet count (× 109/L, mean ± s) | 210 ± 52 | 220 ± 70 | -0.646 | 0.521 |
| Fibrinogen (g/L, mean ± s) | 3. 1 ± 0.90 | 3.2 ± 0.70 | -0.721 | 0.473 |
| Uric acid (µmol/L, mean ± s) | 340 ± 85 | 330 ± 91 | 0.290 | 0.773 |
| Triglycerides (mmol/L, mean ± s) | 1.9 ± 1.2 | 1.8 ± 0.78 | 1.007 | 0.317 |
| Total cholesterol (mmol/L, mean ± s) | 4. 1 ± 1. 1 | 4. 1 ± 1.3 | 0.040 | 0.969 |
| High-density lipoprotein (mmol/L, mean ± s) | 1.0 ± 0.29 | 1.0 ± 0.20 | -0.458 | 0.648 |
| Low-density lipoprotein (mmol/L, mean ± s) | 2.5 ± 0.95 | 2.5 ± 0.97 | 0.329 | 0.743 |
| Cardiac injury markers (ng/L, mean ± s) | 320 ± 810 | 200 ± 360 | 1.314 | 0.191 |
| N-terminal pro b-type natriuretic peptide (pg/ml, mean ± s) | 330 ± 570 | 900 ± 1400 | -2.476 | 0.017 |
| Homocysteine (µmol/L, mean ± s) | 16 ± 10 | 16 ± 8.0 | 0.067 | 0.947 |
| Glycated hemoglobin (%, mean ± s) | 2.9 ± 4.0 | 3.9 ± 3.7 | − 1.468 | 0.147 |
| Ejection fraction (%, mean ± s) | 62 ± 7.4 | 61 ± 7.8 | 0.743 | 0.460 |
| Gensini score (mean ± s) | 40 ± 25 | 44 ± 22 | -0.986 | 0.032 |
Comparison of surgery-related data between the two groups
A univariate analysis comparing the surgical data of the two groups revealed no significant differences in lesion length, lesion stenosis percentage, puncture approach, DCB length, and DCB diameter (Table 2).
Table 2.
Comparison of surgery-related data between the good and poor prognosis groups
| Observational indicator | Good prognosis group (n = 151) | Poor prognosis group (n = 40) | t/X2 | P |
|---|---|---|---|---|
| Target vessel [n (%)] | 3.9367 | 0.268 | ||
| Left anterior descending | 34(22.5%) | 15(37.5%) | ||
| Left circumflex | 1(0.7%) | 0(0%) | ||
| Right coronary artery | 17(11.3%) | 4(10.0%) | ||
| Other | 99(65.6%) | 21(52.5%) | ||
| Lesion length (mm, mean ± s) | 24 ± 4.6 | 25 ± 4.3 | -0.953 | 0.344 |
| Lesion stenosis percentage (%, mean ± s) | 96 ± 5.8 | 96 ± 5.0 | -0.312 | 0.756 |
| Puncture approach [n (%)] | 0.000 | 1 | ||
| Right radial artery | 150(99.3%) | 40(100%) | ||
| Left radial artery | 1(0.7%) | 0(0%) | ||
| DCB length (mm, mean ± s) | 24 ± 4.6 | 24 ± 5.5 | -0.049 | 0.961 |
| DCB diameter (mm, mean ± s) | 2.4 ± 0.39 | 2.4 ± 0.36 | − 1.134 | 0.261 |
Logistic regression analysis of risk factors in the poor prognosis group
A univariate binary logistic regression analysis was conducted, with the occurrence of MACE post-DCB as the dependent variable. The analysis showed significant differences in age, history of coronary intervention, FT3 level, N-terminal pro-B-type natriuretic peptide measurement, diagnosis, and GS scores (P < 0.05) (Table 3). Subsequent multivariate logistic regression analysis indicated that the FT3 level and history of coronary intervention of a patient independently predict the risk of MACE following DCB treatment for CHD (Table 4).
Table 3.
Univariate binary logistic regression analysis of observational indicators
| Observational indicator | Reference indicator | B | Wald | OR value 95% confidence interval | P |
|---|---|---|---|---|---|
| Gender (female) | 0.358 | 0.952 | 1.431(0.688 ~ 2.923) | 0.329 | |
| Age (years) | 0.054 | 8.698 | 1.056(1.02 ~ 1.097) | 0.003 | |
| History of high blood pressure | No | 0.714 | 2.215 | 2.042(0.847 ~ 5.724) | 0.137 |
| History of diabetes | No | 0.657 | 3.351 | 1.929(0.955 ~ 3.927) | 0.067 |
| Previous intervention history | No | 0.957 | 6.941 | 2.604(1.279 ~ 5.347) | 0.008 |
| History of myocardial infarction | No | 0.296 | 0.501 | 1.344(0.571 ~ 2.985) | 0.479 |
| Smoking history | No | 0.312 | 0.743 | 0.732(0.361 ~ 1.504) | 0.389 |
| 0.045 | 1.284 | 1.046(0.995 ~ 1. 171) | 0.257 | ||
| -0.83 | 11.886 | 0.436(0.265 ~ 0.685) | < 0.001 | ||
| -0.041 | 0.356 | 0.96(0.838 ~ 1.098) | 0.550 | ||
| Platelets (× 109/L) | 0.002 | 0.587 | 1.002(0.996 ~ 1.008) | 0.443 | |
| Fibrinogen (g/L) | 0.126 | 0.392 | 1. 135(0.754 ~ 1.673) | 0.531 | |
| Uric acid (µmol/L) | -0.001 | 0.091 | 0.999(0.995 ~ 1.003) | 0.762 | |
| Triglycerides (mmol/L) | -0. 144 | 0.645 | 0.866(0.589 ~ 1.201) | 0.422 | |
| Total cholesterol (mmol/L) | -0.007 | 0.002 | 0.993(0.728 ~ 1.339) | 0.966 | |
| High-density lipoprotein (mmol/L) | 0.23 | 0.139 | 1.259(0.335 ~ 4.04) | 0.710 | |
| Low-density lipoprotein (mmol/L) | -0.063 | 0. 112 | 0.939(0.642 ~ 1.353) | 0.738 | |
| Cardiac injury markers (ng/L) | 0 | 0.733 | 1(0.999 ~ 1) | 0.392 | |
| N-terminal pro B-type natriuretic peptide (pg/mL) | 0.001 | 10.436 | 1.001(1 ~ 1.001) | 0.001 | |
| Homocysteine (µmol/L) | -0.001 | 0.003 | 0.999(0.957 ~ 1.033) | 0.953 | |
| Glycated hemoglobin (%) | 0.061 | 1.918 | 1.063(0.974 ~ 1. 158) | 0.166 | |
| Ejection fraction (%) | -0.018 | 0.589 | 0.983(0.94 ~ 1.03) | 0.443 | |
| Gensini score | 0.006 | 0.842 | 1.006(0.992 ~ 1.02) | 0.035 | |
| Stable angina | Acute non-ST elevation myocardial infarction | -2.256 | 4.166 | 0. 105(0.005 ~ 0.648) | 0.041 |
| Acute ST elevation myocardial infarction | 0.095 | 0.024 | 1. 1(0.323 ~ 3.753) | 0.877 | |
| Unstable angina | -0.005 | 0 | 0.995(0.393 ~ 2.76) | 0.991 |
Table 4.
Multifactorial binary logistic regression analysis of observational indicators
| Observational indicator | Reference indicator | B | Wald | OR value 95% confidence interval | P |
|---|---|---|---|---|---|
| Age (years) | 0.034 | 3.116 | 1.034(0.997 ~ 1.076) | 0.078 | |
| Previous coronary intervention history | No | 0.956 | 5.307 | 2.602(1.153 ~ 5.924) | 0.021 |
| FT3(pmol/L) | -0.582 | 4.176 | 0.559(0.311 ~ 0.967) | 0.041 | |
| N-terminal pro B-type natriuretic peptide (pg/mL) | 0 | 2.994 | 1(1 ~ 1.001) | 0.084 | |
| Stable angina | Acute non-ST elevation myocardial infarction | − 1.633 | 1.965 | 0. 195(0.009 ~ 1.429) | 0.161 |
| Acute ST elevation myocardial infarction | -0.261 | 0. 124 | 0.77(0.169 ~ 3.239) | 0.724 | |
| Unstable angina | 0.387 | 0.457 | 1.473(0.5 ~ 4.863) | 0.499 |
Comparison of adverse prognosis events between high FT3 level group and low FT3 level group
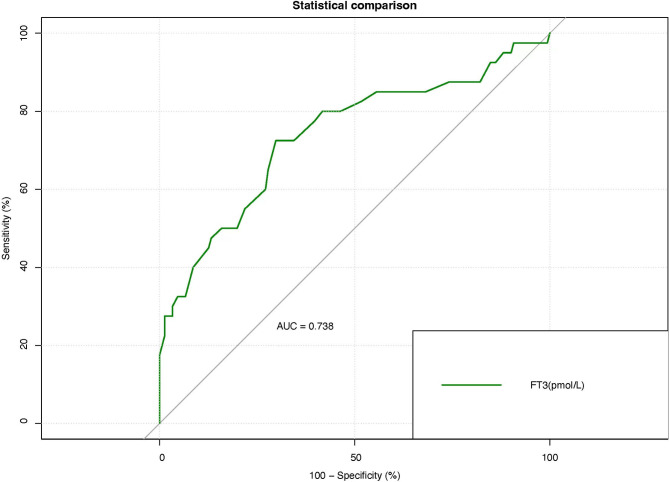
An ROC model was constructed using FT3 as a key biomarker (Fig. 1). The results indicated that the cut-off value for FT3 in predicting the occurrence of MACE post-DCB treatment for CHD is 3.30 pmol/L, with a sensitivity of 72.5%, specificity of 62.8%, and an AUC of 0.741. Patients with FT3 levels above 3.30 pmol/L were classified into the high-level group, while those with levels below this threshold were placed in the low-level group. A statistically significant difference in MACE occurrence post-DCB was observed between the high and low-level groups (P < 0.05) (Table 5).
Fig. 1.
The ROC curve for FT3 predicting adverse prognosis events following DCB treatment
Table 5.
Comparison of adverse prognosis events in high and low FT3 level groups
| High FT3 level group (n = 111) | Low FT3 level group (n = 90) | X2 | P | |
|---|---|---|---|---|
| Poor prognosis group/n (%) | 21(18.9) | 19(21. 1) | 4.086 | 0.043 |
Discussion
FT3 is the primary active hormone among TH. In many non-thyroidal illnesses, a decrease in FT3 while TSH and FT4 levels remain normal is known as low triiodothyronine syndrome (LT3S), with its incidence increasing with age [15]. Over the past decades, numerous studies have explored the relationship between LT3S and the cardiovascular disease prognosis; however, research on the impact of reduced FT3 on the outcomes of DCB treatment for patients with CHD is still relatively limited. Studies have indicated that decreased FT3 may elevate the risks of restenosis and thrombosis following DCB treatment and is closely related to the severity of coronary stenosis [16–21]. These findings suggest that a reduction in FT3 may be a risk factor for complications after arterial balloon dilation treatment for CHD. Additionally, a decrease in FT3 can promote arteriosclerosis through various mechanisms, including effects on lipid metabolism, alterations in arterial smooth muscle structure, vascular wall thickening, decreased compliance, endothelial dysfunction, a hypercoagulable state, impaired fibrinolytic function, hyperhomocysteinemia, systemic inflammation, and platelet dysfunction [2–27]. Furthermore, lower FT3 levels are associated with an increased risk of cardiac death and serves as an important predictive marker [28, 29]. To validate the impact of decreased FT3 on the efficacy of DCB treatment, this study established a predictive model for FT3 levels and the risk of MACE post-DCB treatment for CHD. The study confirmed that the risk of MACE significantly increases in a patient when FT3 levels fall below 3.30 pmol/L. This finding aligns with previous studies and provides strong support for predicting major adverse events postoperatively in CHD patients within clinical settings.
Severe fluctuations in environmental temperature can affect FT3 levels and contribute to the development of various diseases, including cardiovascular disease. Some studies suggest that short-term exposure to cold can elevate TH and FT3 levels, viewed as a compensatory response to increase heat production [30, 31]. However, observations of long-term changes in endocrine levels in individuals living in polar environments have found a decrease in FT3, with no significant changes detected in FT4 and TSH levels. Pathogenic analysis indicates that prolonged exposure to cold significantly raises both the internal production and clearance rates of FT3 [32–34]. The study area, Hulunbuir City, situated near the inland arctic region of China, frequently experiences polar cyclones, suggesting that residents exposed to cold conditions may similarly experience fluctuations in FT3 levels.
The impact of temperature changes on the circulatory system primarily manifests through alterations in vasomotor status, leading to fluctuations in blood pressure and contributing to cardiovascular diseases. Research reveals that while cardiovascular events can occur at any time, they are more likely to be triggered by extreme weather conditions like cold or hot temperatures [35]. Studies from various regions show that the highest incidence rates of acute coronary syndrome occur during the cold winter season [36, 37]. However, cold temperatures are not the only significant factor affecting cardiovascular health, and their impact remains controversial. Some studies report varying degrees of cardiovascular effects due to cold, with more pronounced impact observed in the incidence of cardiac arrest and acute myocardial infarction (AMI), suggesting the influence of additional related variables [38–43]. Further research indicates that human blood pressure, along with HDL, LDL, and glucose, tends to be slightly elevated in winter compared to summer [44, 45]. Additionally, research shows that individuals with dyslipidemia treated with statin medications achieve target LDL levels more readily in summer than in winter, suggesting that seasonal changes may affect lipoprotein metabolism [46]. Beyond hemodynamic changes caused by temperature shifts, cold weather can also alter arteriosclerotic risk factors, potentially destabilizing vulnerable plaques in individuals at high risk for cardiovascular disease. This increases the likelihood of plaque rupture and occlusive thrombosis, thus precipitating acute cardiovascular events.
Residents of alpine regions not only experiences endocrine changes triggered by temperature fluctuations but also increased cardiovascular risks due to vasoconstriction directly caused by cold exposure. Furthermore, the tendency to consume higher-fat diets during winter to combat severe colds, combined with reduced physical activity due to limited outdoor engagement, further heightens the risk of CHD. Therefore, comprehensive strategies for CHD prevention and control in alpine regions should address climate influences, lifestyle habits, and social adaptation. Implementing measures such as intensified health education, promoting healthier dietary choices, encouraging lifestyle modifications, and advocating for moderate indoor exercise during winter months are crucial. The results from this study on patients residing in Hulunbuir City demonstrate that decreased FT3 levels are associated with an increased incidence of MACE following DCB treatment in patients living in alpine regions. In future clinical practice, ongoing monitoring of FT3 levels in similar patients could serve as a valuable prognostic indicator, complementing the aforementioned measures aimed at mitigating the progression of stenotic lesions. Such efforts are essential for enhancing survival rates and improving the quality of life for patients with CHD residing in alpine regions.
The objective of this study was to retrospectively assess the relationship between FT3 levels in the alpine region and prognosis after DCB treatment for CHD. However, several limitations should be acknowledged. First, the sample size is relatively small due to regional restrictions, and the follow-up observation period for patients is limited. Second, the study involved only a single measurement of thyroid function at the time of patient admission, lacking dynamic observation of FT3 levels over time. The research team plans to address these limitations in future studies by continuously monitoring the prognosis of enrolled patients, particularly through coronary angiography to assess progression at the DCB target lesion site and corresponding changes in FT3 levels. Third, the study cannot conclusively determine a causal relationship between elevated FT3 levels and the occurrence of MACE following DCB treatment for CHD. To investigate this further, the team intends to conduct mechanistic animal model studies using mouse models lacking the FT3 factor. Finally, the current clinical study primarily analyzed the correlation between FT3 and various factors post-DCB treatment for CHD, without comparing results to a healthy (negative control) group or further stratifying patient risk factors for comparison. Future research will aim to fill these gaps by including a larger group of clinical patients and incorporating comprehensive comparative analyses. Despite these limitations, our study is one of the first to focus on FT3 levels as a predictor of adverse outcomes in patients from alpine regions undergoing DCB treatment, offering valuable insights into better identifying and managing high-risk patients in this unique setting.
Conclusion
Reduced FT3 levels are identified as a biomarker for predicting adverse prognostic events in patients from alpine regions undergoing DCB treatment for CHD. There is a significant correlation between decreased FT3 levels and the incidence of MACE following DCB treatment for CHD in patients from alpine regions.
Abbreviations
- DCB
Drug-coated balloons
- FT3
Free triiodothyronine
- CHD
Coronary heart disease
- AMI
Acute myocardial infarction
- TLR
Target lesion revascularization
- MACE
Major adverse cardiovascular events
- ROC
Receiver operating characteristic
- AUC
Area under curve
- PCI
Percutaneous coronary intervention
- PTCA
Percutaneous transluminal coronary angioplasty
- BMS
Bare-metal stents
- DES
Drug-eluting stents
- SVD
Small vessel disease
- SB
Side branch
- ISR
In-stent restenosis
- TH
Thyroid hormones
- DAPT
Dual antiplatelet therapy
- GS
Gensini score
- LT3S
Low triiodothyronine syndrome
Author contributions
Qin-Bao Zhang: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. Gang Wu: Conceptualization, Validation, Writing – original draft, Writing – review & editing. Ze-Ying Wang: Data curation, Formal Analysis, Software. Zhi-Liang Cui: Formal Analysis, Software, Visualization. Hong-Xia Zhang: Data curation, Formal Analysis, Investigation. All authors read and approved the final draft.
Funding
No external funding was received.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted with approval from the Ethics Committee of Hulunbuir People’s Hospital (Approval Number: 2023SYY-11). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.China Cardiovascular Health and Disease Report Writing Group, Hu SS, Wang ZW. Summary of the China Cardiovascular Health and Disease Report 2022. Chin J Interv Cardiol. 2023;31(07):485–508. [Google Scholar]
- 2.Yang LX, Guo RW. The Chinese guidelines for percutaneous coronary intervention (2016) guide clinical practice in acute coronary syndromes. Chin J Interv Cardiol. 2016;24(12):714–7. 10.3969/j.issn.1004-8812.2016.12.013 [Google Scholar]
- 3.Xia YM, Gao H, Wang QS, Feng X, Wang YQ, Xu ZX. Characteristics of traditional Chinese medicine syndrome in patients with coronary heart disease at different disease stages. World J Tradit Chin Med. 2022;8:218–24. 10.4103/wjtcm.wjtcm_65_21 [Google Scholar]
- 4.Wang LS. A study of the relationship between regression of acute coronary syndromes and climate change in the elderly. Tianjin Med Uni. 2014. 10.7666/d.Y2396426 [Google Scholar]
- 5.McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, Lockery JE, Kirpach B, Storey E, Shah RC, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Johnston CI, Ryan J, Radziszewska B, Jelinek M, Malik M, Eaton CB, Brauer D, Cloud G, Wood EM, Mahady SE, Satterfield S, Grimm R, Murray AM, ASPREE Investigator Group. Effect of aspirin on Cardiovascular events and bleeding in the healthy Elderly. N Engl J Med. 2018;379(16):1509–18. 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang HQ. Characterization of spatial and temporal variations of winter temperatures in the Hulunbuir region. Nanjing Uni Inf Eng. 2014.
- 7.Fei MF, Luo LJ. Progress in the Prevention of Restenosis in Coronary arrays. J Changjiang Uni. 2011;8(12):218–20. doi: CNKI:SUN:CJDL.0.2011-12-094. [Google Scholar]
- 8.Chen YD. Current status of drug-coated balloons in the interventional treatment of coronary heart disease. Chin Cardiovasc J. 2020;25(02):101–3. 10.3969/j.issn.1007-5410.2020.02.001 [Google Scholar]
- 9.Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation. 1993;87(5):1435–41. 10.1161/01.cir.87.5.1435 [DOI] [PubMed] [Google Scholar]
- 10.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344(7):501–9. 10.1056/NEJM200102153440707 [DOI] [PubMed] [Google Scholar]
- 11.Grais IM, Sowers JR. Thyroid and the heart. Am J Med. 2014;127(8):691–8. 10.1016/j.amjmed.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, L’Abbate A, Donato L. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107(5):708–13. 10.1161/01.cir.0000048124.64204.3f [DOI] [PubMed] [Google Scholar]
- 13.Expert Group on the Chinese Expert Consensus on the Clinical Use of Drug-Coated Balloon. Chinese expert consensus on the clinical application of drug-coated balloons. Chin J Interv Cardiol. 2016;24(2). 10.3969/j.issn.1004-8812.2016.02.001
- 14.Zhang XY, Wang SX. Correlation between LDL-C/HDL-C and Gensini score in patients with acute coronary syndromes. Chin J Emerg Resusc Disaster Med. 2023;18(01):4–7. [Google Scholar]
- 15.Boelaert K. Thyroid dysfunction in the elderly. Nat Rev Endocrinol. 2013;9(4):194–204. 10.1038/nrendo.2013.30 [DOI] [PubMed] [Google Scholar]
- 16.Pereg D, Tirosh A, Elis A, Neuman Y, Mosseri M, Segev D, Lishner M, Hermoni D. Mortality and coronary heart disease in euthyroid patients. Am J Med. 2012;125(8):826. .e7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YH, Park DW, Kim WJ, Lee JY, Yun SC, Kang SJ, Lee SW, Lee CW, Hong MK, Park SW, Park SJ. Impact of the extent of coronary artery disease on outcomes after revascularization for unprotected left main coronary artery stenosis. J Am Coll Cardiol. 2010;55(23):2544–52. 10.1016/j.jacc.2009.11.094 [DOI] [PubMed] [Google Scholar]
- 18.He CJ, Zhu CY, Fan HY, Qian YZ, Zhai CL, Hu HL. Low T3 syndrome predicts more adverse events in patients with hypertrophic cardiomyopathy. Clin Cardiol. 2023;46(12):1569–77. 10.1002/clc.24156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraler S, Wenzl FA, Georgiopoulos G, Obeid S, Liberale L, von Eckardstein A, Muller O, Mach F, Räber L, Losdat S, Schmiady MO, Stellos K, Stamatelopoulos K, Camici GG, Srdic A, Paneni F, Akhmedov A, Lüscher TF. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts premature death in acute coronary syndromes. Eur Heart J. 2022;43(19):1849–60. 10.1093/eurheartj/ehac143 [DOI] [PubMed] [Google Scholar]
- 20.Karabağ Y, Çağdaş M, Rencuzogullari I, Karakoyun S, Artaç İ, İliş D, Atalay E, Yesin M, Gürsoy MO. Halil Tanboğa I. Relationship between C-reactive protein/albumin ratio and coronary artery disease severity in patients with stable angina pectoris. J Clin Lab Anal. 2018;32(7):e22457. 10.1002/jcla.22457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daswani R, Jayaprakash B, Shetty R, Rau NR. Association of thyroid function with severity of coronary artery disease in Euthyroid patients. J Clin Diagn Res. 2015;9(6):OC10–3. 10.7860/JCDR/2015/10908.6059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakker O, Hudig F, Meijssen S, Wiersinga WM. Effects of triiodothyronine and amiodarone on the promoter of the human LDL receptor gene. Biochem Biophys Res Commun. 1998;249(2):517–21. 10.1006/bbrc.1998.9174 [DOI] [PubMed] [Google Scholar]
- 23.Waterhouse DF, McLaughlin AM, Walsh CD, Sheehan F, O’Shea D. An examination of the relationship between normal range thyrotropin and cardiovascular risk parameters: a study in healthy women. Thyroid. 2007;17(3):243–8. 10.1089/thy.2006.0208 [DOI] [PubMed] [Google Scholar]
- 24.Coceani M, Iervasi G, Pingitore A, Carpeggiani C, L’Abbate A. Thyroid hormone and coronary artery disease: from clinical correlations to prognostic implications. Clin Cardiol. 2009;32(7):380–5. 10.1002/clc.20574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88(6):2438–44. 10.1210/jc.2003-030398 [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Sara JD, Matsuzawa Y, Gharib H, Bell MR, Gulati R, Lerman LO, Lerman A. Clinical outcomes of patients with hypothyroidism undergoing percutaneous coronary intervention. Eur Heart J. 2016;37(26):2055–65. 10.1093/eurheartj/ehv737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin LQ, Wu BX, Lin MY, Chen QX, Xu DP. Interim analysis report of kuanxiong aerosol in improving angina and quality of life after percutaneous coronary intervention. World J Tradit Chin Med. 2022;8:87–91. 10.4103/wjtcm.wjtcm_26_21 [Google Scholar]
- 28.Flynn RW, Macdonald TM, Jung RT, Morris AD, Leese GP. Mortality and vascular outcomes in patients treated for thyroid dysfunction. J Clin Endocrinol Metab. 2006;91(6):2159–64. 10.1210/jc.2005-1833 [DOI] [PubMed] [Google Scholar]
- 29.Marraccini P, Bianchi M, Bottoni A, Mazzarisi A, Coceani M, Molinaro S, Lorenzoni V, Landi P, Iervasi G. Prevalence of thyroid dysfunction and effect of contrast medium on thyroid metabolism in cardiac patients undergoing coronary angiography. Acta Radiol. 2013;54(1):42–7. 10.1258/ar.2012.120326 [DOI] [PubMed] [Google Scholar]
- 30.Mahwi TO, Abdulateef DS. Relation of different components of climate with human pituitary-thyroid Axis and FT3/FT4 ratio: a study on euthyroid and SCH subjects in two different Seasons. Int J Endocrinol. 2019;2019:2762978. 10.1155/2019/2762978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed HL, Silverman ED, Shakir KM, Dons R, Burman KD, O’Brian JT. Changes in serum triiodothyronine (T3) kinetics after prolonged Antarctic residence: the polar T3 syndrome. J Clin Endocrinol Metab. 1990;70(4):965–74. 10.1210/jcem-70-4-965 [DOI] [PubMed] [Google Scholar]
- 32.Reed HL, Brice D, Shakir KM, Burman KD, D’Alesandro MM, O’Brian JT. Decreased free fraction of thyroid hormones after prolonged Antarctic residence. J Appl Physiol (1985). 1990;69(4):1467–72. 10.1152/jappl.1990.69.4.1467 [DOI] [PubMed] [Google Scholar]
- 33.Hassi J, Sikkilä K, Ruokonen A, Leppäluoto J. The pituitary-thyroid axis in healthy men living under subarctic climatological conditions. J Endocrinol. 2001;169(1):195–203. 10.1677/joe.0.1690195 [DOI] [PubMed] [Google Scholar]
- 34.Leonard WR, Levy SB, Tarskaia LA, Klimova TM, Fedorova VI, Baltakhinova ME, Krivoshapkin VG, Snodgrass JJ. Seasonal variation in basal metabolic rates among the Yakut (Sakha) of Northeastern Siberia. Am J Hum Biol. 2014 Jul-Aug;26(4):437–45. 10.1002/ajhb.22524 [DOI] [PubMed]
- 35.Imai C, Barnett AG, Hashizume M, Honda Y. The role of Influenza in the Delay between Low Temperature and Ischemic Heart Disease: evidence from Simulation and Mortality Data from Japan. Int J Environ Res Public Health. 2016;13(5):454. 10.3390/ijerph13050454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodzic E, Perla S, Iglica A, Vucijak M. Seasonal incidence of Acute Coronary Syndrome and its features. Mater Sociomed. 2018;30(1):10–4. 10.5455/msm.2018.30.10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou WR, Wang Y, Sun YM, Niu KJ. Analysis of prehospital emergency care for acute coronary syndromes and the relationship with meteorological factors. Chin J Chron Dis Prev Control. 2020;28(08):591–5. 10.16386/j.cjpccd.issn.1004-6194.2020.08.009 [Google Scholar]
- 38.Brook RD, Weder AB, Rajagopalan S. Environmental hypertensionology the effects of environmental factors on blood pressure in clinical practice and research. J Clin Hypertens (Greenwich). 2011;13(11):836–42. 10.1111/j.1751-7176.2011.00543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang M, Yoo JK, Stickford ASL, Moore JP, Hendrix JM, Crandall CG, Fu Q. Early sympathetic neural responses during a cold pressor test linked to pain perception. Clin Auton Res. 2021;31(2):215–24. 10.1007/s10286-019-00635-7 [DOI] [PubMed] [Google Scholar]
- 40.Zha YP, Wang YK, Deng Y, Zhang RW, Tan X, Yuan WJ, Deng XM, Wang WZ. Exercise training lowers the enhanced tonically active glutamatergic input to the rostral ventrolateral medulla in hypertensive rats. CNS Neurosci Ther. 2013;19(4):244–51. 10.1111/cns.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Cao Y, Hong D, Zheng D, Richtering S, Sandset EC, Leong TH, Arima H, Islam S, Salam A, Anderson C, Robinson T, Hackett ML. Ambient temperature and stroke occurrence: a systematic review and Meta-analysis. Int J Environ Res Public Health. 2016;13(7):698. 10.3390/ijerph13070698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keatinge WR, Coleshaw SR, Cotter F, Mattock M, Murphy M, Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br Med J (Clin Res Ed). 1984;289(6456):1405–8. 10.1136/bmj.289.6456.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karakayali M, Omar T, Artac I, Ilis D, Arslan A, Altunova M, Cagin Z, Karabag Y, Karakoyun S, Rencuzogullari I. The prognostic value of HALP score in predicting in-hospital mortality in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis. 2023;34(7):483–8. 10.1097/MCA.0000000000001271 [DOI] [PubMed] [Google Scholar]
- 44.Marti-Soler H, Gubelmann C, Aeschbacher S, Alves L, Bobak M, Bongard V, Clays E, de Gaetano G, Di Castelnuovo A, Elosua R, Ferrieres J, Guessous I, Igland J, Jørgensen T, Nikitin Y, O’Doherty MG, Palmieri L, Ramos R, Simons J, Sulo G, Vanuzzo D, Vila J, Barros H, Borglykke A, Conen D, De Bacquer D, Donfrancesco C, Gaspoz JM, Giampaoli S, Giles GG, Iacoviello L, Kee F, Kubinova R, Malyutina S, Marrugat J, Prescott E, Ruidavets JB, Scragg R, Simons LA, Tamosiunas A, Tell GS, Vollenweider P, Marques-Vidal P. Seasonality of cardiovascular risk factors: an analysis including over 230 000 participants in 15 countries. Heart. 2014;100(19):1517–23. 10.1136/heartjnl-2014-305623 [DOI] [PubMed] [Google Scholar]
- 45.Rencuzogullari I, Çağdaş M, Karabağ Y, Karakoyun S, Yesin M, Çinar T, Tanik VO, Burak C, Tanboğa İH. Value of syntax score II for predicting in-hospital and long-term survival in octogenarians with ST-segment elevation myocardial infarction: a comparison of six different risk scores. Arch Gerontol Geriatr. 2019 Jul-Aug;83:37–43. 10.1016/j.archger.2019.03.016 [DOI] [PubMed]
- 46.Tung P, Wiviott SD, Cannon CP, Mruphy SA, Mccabe CH, Gibson CM. Seasonal variation in lipids in patients following acute coronary syndrome on fixed doses of pravastatin(40 mg) or atorvastatin(80 mg). Am J Cardiol. 2009;103:1056–60. 10.1016/j.amjcard.2008.12.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.