Abstract
Background
Air pollution (AP) has become a substantial environmental issue affecting human cardiorespiratory health. Physical exercise (PE) is widely accepted to promote cardiorespiratory health. There is a paucity of research on the point at which the level of polluted environment engaged in PE could be used as a preventive approach to compensate for the damages of AP.
Objectives
To assess the effects of acute moderate-intensity PE on the cardio-respiratory and inflammatory responses of young adults in varying levels of AP, and to determine the pollution level at which engaging in short-term PE is considered safe.
Methods
We constructed a real-world crossover study of 30 healthy young adults with repeated measures. Participants participated in 90 min of moderate-intensity PE in different (low, medium, high) AP exposure scenarios. Cardiorespiratory health was measured by assessing systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), peak expiratory flow (PEF), mean forced expiratory flow between 25% and 75% of FVC (FEF25–75%), and fractional exhaled nitric oxide (FeNO) before and after the intervention. Blood samples were also collected simultaneously. The percentage changes in cardiorespiratory health markers after exercise in the three AP levels environments were compared using linear mixed-effects models.
Results
Compared to the changes observed post-exercise in the low-level AP environment, only PEF (-9.36, P = 0.018) showed a significant decrease, and eosinophils showed a significant increase in the medium-level environment (25.64, P = 0.022), with no significant differences in other indicators. Conversely, post-exercise in the high-level AP environment resulted in a significant increase in DBP (6.5, P = 0.05), lung inflammation (FeNO: 13.3, p < 0.001), inflammatory cell counts (WBC: 27.0, p < 0.001; neutrophils: 26.8, p < 0.001; lymphocytes: 32.2, p < 0.001; monocytes: 28.2, p < 0.001; and eosinophils: 48.9, p < 0.001), and inflammatory factors (IL-1β: 0.76, P = 0.003; IL-10: 0.17, P = 0.02; IL-6: 0.1, P = 0.17; TNF-α: 0.97, P = 0.011; CRP: 0.17, P = 0.003). Additionally, there were significant declines in lung function parameters, including FVC (-6.84, P = 0.04), FEV1 (-8.97, P = 0.009), and PEF (-9.50, P = 0.013).
Conclusions
Acute PE in low and medium-level AP environments is generally secure concerning short-term effects on cardiorespiratory health among healthy young adults. However, acute PE in high-level AP environments can be detrimental to cardiorespiratory health, significantly increasing the body’s inflammatory response.
Trial registration
ChiCTR2000031851; Registered 12.04.2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-21045-z.
Keywords: Air pollution, PM2.5, Physical exercise, Cardiorespiratory health, Inflammatory response
Highlights
This study can generate more impactful information on the combined short-term effects of acute physical exercise and different levels of air pollution on cardio-respiratory responses among young adults.
Acute physical exercise in medium- and low-level pollution environments is relatively safe for cardiorespiratory health.
Acute physical exercise in high levels of air pollution significantly increases the body’s inflammatory response.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-21045-z.
Introduction
Billions of people worldwide are exposed to environments with air pollution (AP) [1]. AP poses significant health threats to humanity [2, 3], particularly impacting cardiorespiratory health [4, 5]. The pollutants in the air, especially particulate matter (PM), may induce systemic chronic inflammatory responses and oxidative stress in the body, which could be mechanistic pathways leading to adverse health outcomes in cardiorespiratory health [6].
As is well known, regular physical exercise (PE) brings numerous benefits to physical health, such as enhancing cardiorespiratory function, reducing inflammatory responses, and improving immunity [7–9]. However, an increase in PE also entails an increased risk of exposure to AP. Engaging in PE in environments with AP may lead to higher inhalation doses of pollutants due to deeper and faster breathing and increased ventilation [6, 10], which could potentially exacerbate the adverse effects of AP on cardiorespiratory health. Thus, the effect of PE in AP environments on cardiorespiratory health has become a hot topic of investigation in recent years.
Reviewing prior research reveals a lack of consensus due to differences in study populations, levels of pollution, research designs, and exercise protocols. Most studies have focused on middle-aged and elderly individuals or those with cardiorespiratory diseases, with fewer studies conducted on healthy young adults [11, 12]. There are also significant variations in the levels of pollution exposure across different studies. For instance, in the survey by Matt et al. [13], the difference between low and high levels of PM10 exposure was 58 µg/m³, whereas in the study by Kocot et al. [12], the difference between low and high levels of PM10 exposure was 156 µg/m³. Although both studies dealt with low and high levels of exposure, there was a significant difference between the two in terms of the magnitude of exposure levels. Additionally, due to ethical reasons, there is limited research on the cardiorespiratory health effects of exercising in environments with high AP concentrations. Variations in study designs and exercise regimens (such as type, duration, and intensity of exercise) can lead to significant differences in the amount of pollutants inhaled. For example, Matt et al. [13]conducted 2 h of moderate-intensity intermittent physical exercise (such as 15-minute intermittent cycle ergometry), while Kocot et al. [12]only performed 15-minute submaximal exercise trials on a cycle ergometer. Therefore, there is a significant difference in the amount of pollutants inhaled by both, which can result in changes in measurement outcomes. Some studies suggest that compared to rest, acute PE can counteract the adverse effects of AP on cardiorespiratory health [13–15], while others argue that continuing exercise as AP levels rise may decrease cardiorespiratory function [12, 16] and increase inflammatory responses [17, 18]in the body. Therefore, determining the safety threshold for PE in AP environments is a goal that warrants continuous exploration.
Consequently, in this study, we have decided to investigate the effects of moderate-intensity PE on the cardio-respiratory and inflammatory responses of young adults in varying levels of AP, and to determine the pollution level at which engaging in PE is considered safe.
Methods
Study design and participants
This study employed a self-controlled crossover design, wherein participants engaged in 90 min of moderate-intensity PE under three different AP concentrations (low, medium, and high). Inclusion criteria for participants were as follows: (1) aged 18–30 and enrolled university students; (2) physically healthy (the physical examination report from the past 3 months is normal); (3) no medication intake within the past 3 weeks; (4) no history of pulmonary or cardiovascular diseases; (5) absence of symptoms such as cold or fever; (6) non-smokers; (7) voluntary participation and cooperation with researchers throughout the study. Exclusion criteria included: (1) nasal allergy sufferers; (2) participants experiencing discomfort such as cold or fever during the trial period ; (3) participants who consumed alcohol the day before the trial; (4) inability to tolerate moderate-to-high-intensity exercise; (5) female participants menstruating during the exercise period. All participants provided voluntary informed consent after agreeing to participate in the study. The study received approval from the Ethics Committee of Shanghai University of Sport (Approval No.: 102772019RT001).
The experiments were conducted between September 2023 and December 2023. Participants were instructed to abstain from alcohol consumption and vigorous physical exercise 24 h before the experiment and avoid exposure to high pollution levels in the air. Additionally, coffee and soy milk intake were prohibited within 3 h before testing. To minimize the influence of different measurement times on the results, all experiments and measurements were conducted at the same time of day, and participants were required to consume a standardized meal to reduce interference from the diet. On the day of the experiment, participants arrived at the laboratory at 7:30 a.m., having already consumed their meal prior to arrival. After a brief rest of at least 30 min, they underwent baseline health indicator measurements. Subsequently, they walked to the 100-meter playground for a 90-minute moderate-intensity exercise session, which took place from 9:30 a.m. to 11:00 a.m. The exercise regimen included warm-up running (5 min), warm-up exercises (5 min), aerobic exercises (40 min), games (30 min), and stretching and relaxation (10 min). The entire exercise intervention was led by experienced coaches.
After the 90-minute exercise intervention, participants returned to the laboratory immediately for post-intervention health indicator measurements. Blood samples were collected first, with venous blood collection completed within 15 min after exercise, followed by the measurement of cardiorespiratory health indicators within 30 min after the exercise intervention. Each participant completed PE under three different AP environments, and between each experiment, participants underwent a washout period of at least 2 weeks.
Physical exercise monitoring
Before the exercise, participants uniformly wore Polar heart rate monitors to objectively monitor their exercise intensity. The exercise intensity was controlled moderately corresponding to 70% of each participant’s maximum heart rate. The maximum heart rate was calculated based on the latest international standard algorithms considering age and gender, where for males, the maximum heart rate = 220 - age; for females, the maximum heart rate = 206 − 0.88 × age [19]. During the exercise session, researchers and coaches could adjust the exercise pace in real-time based on the monitoring results to ensure that the predetermined exercise intensity was achieved.
Environmental exposure monitoring
The research design involved conducting moderate-intensity exercise experiments under three different AP levels (low: PM2.5 ≤ 75 µg/m3, medium: 75 µg/m3 < PM2.5 ≤115 µg/m3, and high: PM2.5 > 115 µg/m3), based on the latest air quality guidelines from the World Health Organization (WHO) [20]and China’s Ambient Air Quality Standards (GB 3095 − 2012). The experimental environment was conducted on an outdoor sports field, exposed to natural environmental pollution, with no noise pollution. Before the experiment, participants rested indoors and went outside to conduct the trial at a designated time. The testing period was during the autumn and winter seasons, and each experiment was conducted at the same time of day, specifically on sunny days. If the conditions were not met, the exercise intervention experiment was not conducted.
During the 90-minute exercise intervention period, the air temperature, relative humidity, and PM2.5 concentration at the monitoring site were monitored. For PM2.5 measurement, the SidePak™ AM520i Personal Aerosol Monitor was used to detect changes in ambient PM2.5 concentration, with the instrument sampling every 10 s. Data on other air pollutants were obtained from environmental monitoring stations located 3 km from the experimental center, with the average values during the intervention period taken as the pollution levels for the experiment.
Cardiorespiratory health measurements
The equipment used for heart rate and blood pressure testing was the Omron J710 Blood Pressure Monitor from Japan, which measured systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse. Three consecutive measurements were taken each time, and the average was recorded.
For pulmonary function testing, in accordance with the standards of the European Respiratory Society (ERS) and the American Thoracic Society (ATS) [21], the Italian New Spirolab® Pulmonary Function Testing Instrument was employed. The measured indices included forced vital capacity (FVC), forced expiratory volume in one second (FEV1), peak expiratory flow (PEF), and maximal mid-expiratory flow (FEF25− 75%).
Fractional exhaled nitric oxide (FeNO) was measured using the portable NIOX MINO Analyzer (Aerocrine AB, Solna, Sweden).
Blood sample collection
Thirty minutes before the start of the exercise and within 15 min after its completion, trained medical personnel collected venous blood samples from the study participants. Blood samples were drawn using Ethylenediaminetetraacetic Acid (EDTA) anticoagulant tubes for routine blood tests. Serum samples for the measurement of IL-1β, IL-10, IL-6, TNF-α, and CRP were centrifuged and aliquoted within 4 h after blood collection and stored at -80 °C.
Ethical considerations
Ethics approval for the study was obtained from the Ethics Committee of Shanghai University of Sport (Ethics approval no: 102772019RT001) and registered in the Chinese Clinical Trial Registry (Registered No: ChiCTR2000031851). Written informed consent was obtained from participants before they participated in the study.
Statistical analysis
Statistical analysis of the data was conducted using R 4.3.2 software. Descriptive analysis was primarily focused on the basic information of the study participants, pollutant concentrations, and fundamental characteristics of health indicators. Continuous variables were described using arithmetic means and standard deviations, while categorical variables were described using proportions. Changes in health indicators before and after exercise were expressed as relative differences ((post-exercise - baseline) / baseline), where a relative difference < 0% indicated a decrease after exercise. The Shapiro-Wilk test was employed to assess the distribution of data. Paired t-tests were used to evaluate the statistical significance of differences between pre- and post-exercise measurements in each experiment. Due to the non-normal distribution of pollutant concentrations, Wilcoxon rank-sum tests were utilized to assess differences between the three different AP levels. Considering the repeated study design, linear mixed-effects models (LME) were constructed using the ‘lme4’ package in R. These models analyzed changes relative to baseline values after exercise across the three experiments. The study participants’ ID was included as a random effect in the model to account for individual variability in all health outcomes. Gender, age, and body mass index (BMI) were included as fixed effects. This study considered a two-tailed p-value < 0.05 to indicate statistical significance.
Results
Subject characteristics
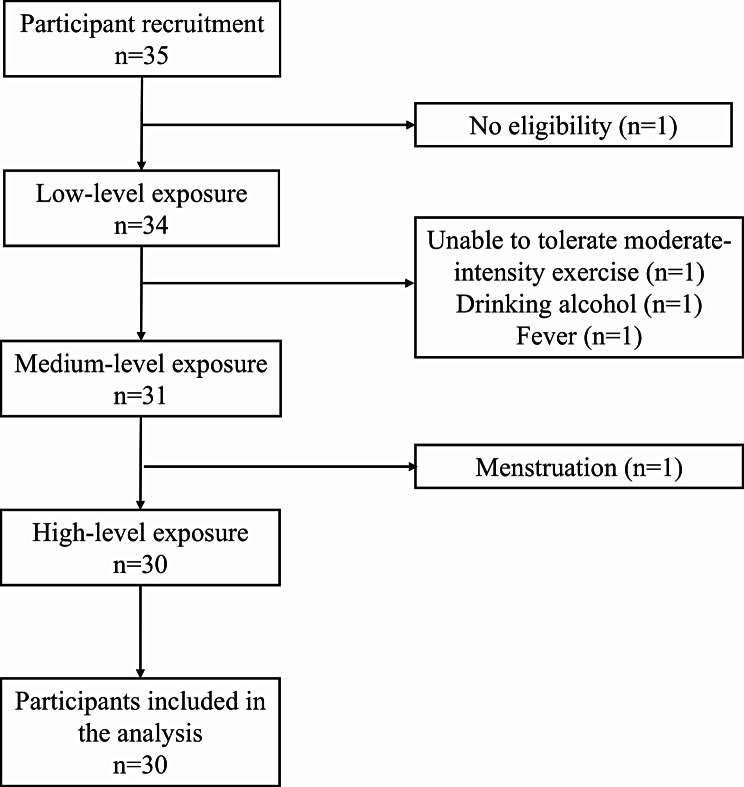
A total of 35 participants were recruited, and ultimately, 30 participants completed exposures in all three scenarios (Fig. 1). Among them, there were 16 males (53.3%) and 14 females (46.7%). The mean age of the 30 participants was 20.1 ± 0.9 years, with an average BMI of 23.0 ± 1.9 kg/m². Specific characteristics of the participants are presented in Table 1.
Fig. 1.
Participant inclusion and exclusion flowchart
Table 1.
Characteristics of the study group(Mean ± SD)
| Characteristics | Male | Female | All |
|---|---|---|---|
| N(%) | 16(53.3%) | 14(46.7%) | 30 |
| Age(years) | 20.4 ± 0.8 | 19.7 ± 0.9 | 20.1 ± 0.9 |
| Height(cm) | 175.9 ± 3.2 | 165.6 ± 5.4 | 171.1 ± 6.7 |
| Body mass(kg) | 66.4 ± 8.9 | 56.0 ± 8.0 | 61.6 ± 9.9 |
| BMI(kg/m2) | 21.7 ± 0.8 | 24.5 ± 1.6 | 23.0 ± 1.9 |
SD: standard deviation; BMI: body mass index
Pollution levels
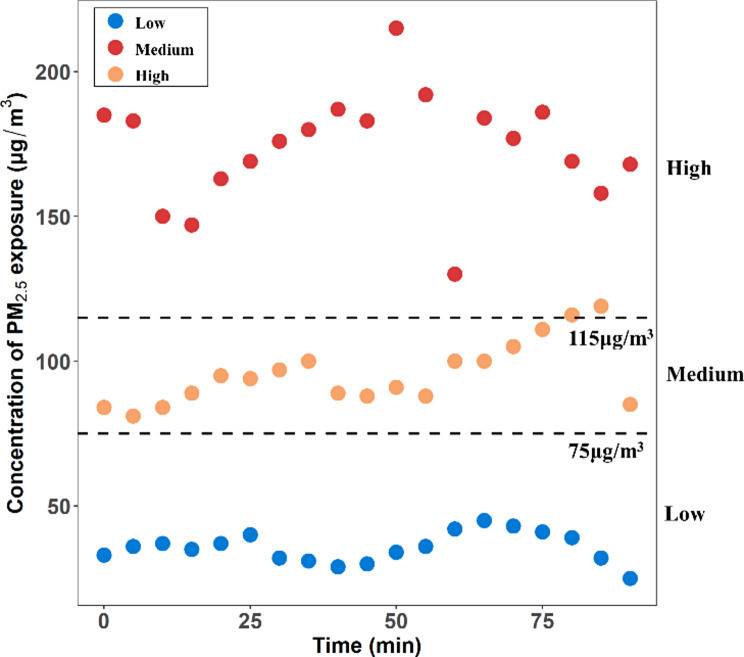
During the 90-minute exercise intervention experiments, the environmental levels of PM2.5 were monitored using the SidePak™ AM520i Individual Aerosol Monitor, showing variations over time (Fig. 2). The average concentrations of PM2.5 were 35.63 ± 5.23 µg/m3, 95.58 ± 10.98 µg/m3, and 174.26 ± 17.86 µg/m3, respectively. Detailed characteristics of the environmental conditions recorded during the three experiments are provided in Table 2. Significant differences were observed in the levels of PM2.5, inhalable particulate matter PM10, SO2, and NO2 during the three intervention periods. Specifically, the AP levels at the medium level were significantly higher than those at the low level, while the AP levels at the high level were significantly higher than those at the medium level. The pollution concentration levels in the experiment align with expectations.
Fig. 2.
Changes in PM2.5 concentration during the exposure period
Table 2.
Distribution of environmental conditions recorded during exercise trials
| Variable | Low | Medium | High | p Value* |
|---|---|---|---|---|
| SO2(µg/m3) | 14.2 ± 3.3 | 33.6 ± 19.2 | 44.0 ± 24.4 | <0.0142 |
| NO2(µg/m3) | 24.0 ± 5.6 | 65.2 ± 9.9 | 65.4 ± 18.7 | <0.0003 |
| PM10(µg/m3) | 46.4 ± 6.1 | 147.8 ± 16.7 | 187.0 ± 12.1 | <0.0019 |
| PM2.5(µg/m3) | 35.63 ± 5.23 | 95.58 ± 10.98 | 174.26 ± 17.86 | <0.0001 |
| Temperature(℃) | 16.61 ± 0.94 | 9.22 ± 0.76 | 1.3 ± 1.4 | <0.0001 |
| Relative humidity(%) | 58.84 ± 0.91 | 35.54 ± 0.84 | 36.22 ± 2.5 | <0.0001 |
All data are presented as mean ± standard deviation. *: significant differences between the three experimental conditions (Wilcoxon test results)
Cardiorespiratory health
In this study, measurements of cardiorespiratory function and airway inflammation indicators were taken before and after each of the three exercise interventions. We calculated the percentage change of cardiorespiratory-related health indicators relative to the baseline to adjust for individual differences at the baseline level and analyzed the changes in cardiorespiratory health indicators among three different levels of AP. In addition, we analyzed the mean differences in cardiorespiratory health measurements before and after PE in each level of AP environment. The specific results are presented in Supplementary Table 1. Figure 3 shows the percentage changes before and after PE in three different AP level environments.
Fig. 3.
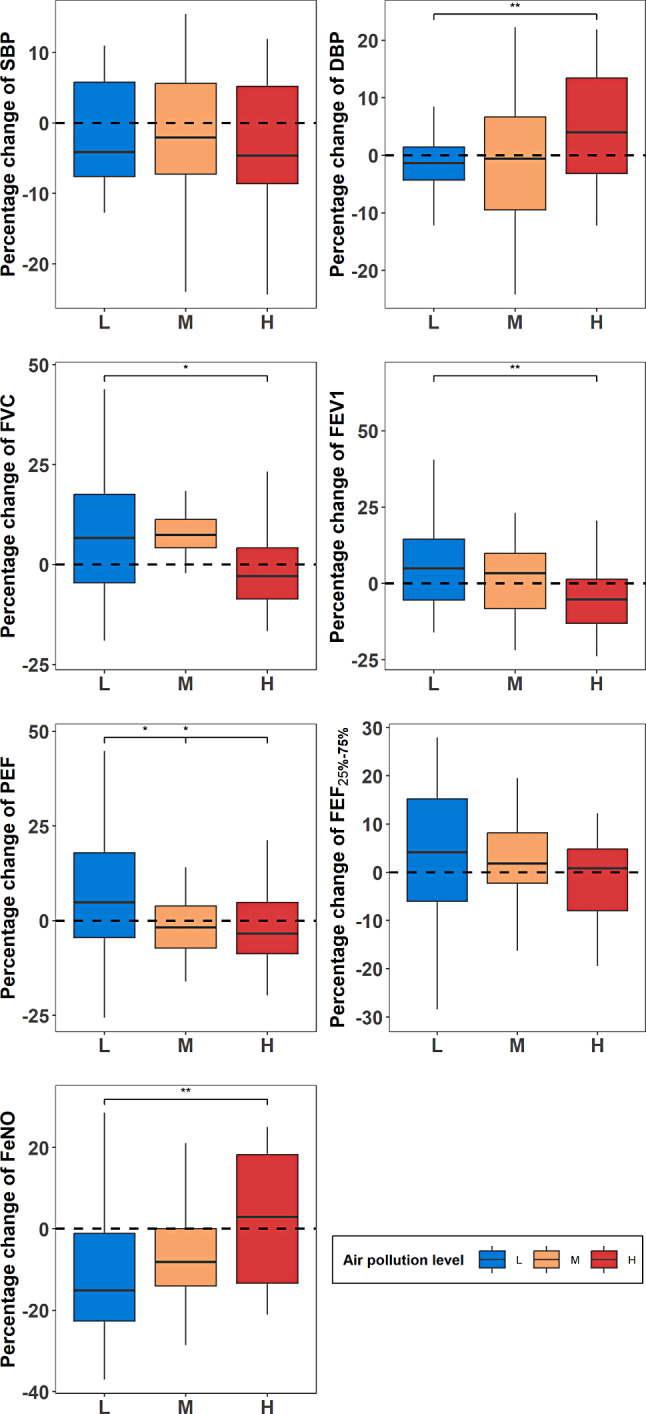
Percentage change in cardiorespiratory health measurements relative to baseline
We observed that after exercise in environments with three different AP concentrations, blood pressure decreased in all three concentration environments, with SBP decreasing below 0, indicating a decrease after exercise. DBP tends to be decreased after exercise in environments with medium to low pollution concentrations, while it increased in environments with high pollution concentrations (3.5 mm/Hg, p = 0.03).
For lung function, post-exercise mean values of FVC and FEV1 significantly increased in the low pollution level environment (0.2 L, P = 0.041) (0.2 L, P = 0.049), while mean values of PEF and FEF25 − 75% also increased, albeit without significant differences. Additionally, post-exercise FVC still showed a weak significant increase in the medium pollution level environment (0.3 ± 0.5, P = 0.05). However, post-exercise mean values of FEV1 significantly decreased in the high-level AP environment (-0.2 ± 0.4, P = 0.031), with other lung function indicators also showing a tendency of decreases in the high pollution level environment.
For airway inflammation indicators, we found that FeNO significantly decreased after exercise in both the low pollution level (-3 ppb, p < 0.001) and medium pollution level (-1.7 ppb, p = 0.038) environments, while it tend to be increased in the high pollution level environment, although without significant differences. Moreover, after exposure to environments with three different concentrations of pollution, the percentage change in FeNO values showed an increasing trend.
Based on the observed changes in cardiorespiratory function indicators pre- and post-exercise, it is evident that exercising in medium-level AP environments may not lead to as beneficial significant changes in cardiorespiratory function indicators as seen with exercise in low pollution environments. Conversely, exercising in high pollution environments yields adverse effects. This suggests that AP may diminish the benefits of PE on cardiorespiratory function.
Subsequently, we utilized LME to adjust for participants’ gender, age, and BMI. Using the percentage change in cardiorespiratory health indicators after exercising in the low pollution level environment as the reference, we further confirmed the aforementioned results. The specific results were consistent with the description provided in Fig. 3 above, and the analytical outcomes are detailed in Table 3.
Table 3.
Differences in cardiorespiratory health among three different AP levels
| SBP | DBP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p Value* | Coefficient | 95% CI | p Value* | |||||
| M | -0.62 | -4.76 | 3.41 | 0.770 | -3.44 | 5.21 | 7.66 | 0.685 | ||
| H | -1.44 | -5.58 | 2.59 | 0.496 | 6.45 | 2.11 | 10.75 | 0.005 | ||
| FVC | FEV1 | |||||||||
| Coefficient | 95% CI | p Value* | Coefficient | 95% CI | p Value* | |||||
| M | -0.43 | -7.24 | 6.0 | 0.9 | -6.57 | -13.44 | 0.01 | 0.062 | ||
| H | -6.84 | -13.41 | -0.68 | 0.04 | -8.97 | -15.62 | -2.63 | 0.009 | ||
| PEF | FEF 25 − 75% | |||||||||
| Coefficient | 95% CI | p Value* | Coefficient | 95% CI | p Value* | |||||
| M | -9.36 | -16.71 | -2.00 | 0.018 | -0.87 | -13.29 | 2.93 | 0.828 | ||
| H | -9.50 | -16.60 | -2.41 | 0.013 | -4.35 | -15.88 | 0.63 | 0.248 | ||
| FeNO | ||||||||||
| Coefficient | 95% CI | p Value* | ||||||||
| M | 5.2 | -2.20 | 12.56 | 0.183 | ||||||
| H | 13.3 | 5.93 | 20.69 | 0.001 | ||||||
Mixed effect models adjusted for gender, age, and BMI. Exposure scenario with reference to ‘Low level AP and PE exposure’. *: Statistical significance in the results of the linear mixed-effects model. M: Medium level AP and PE exposure; H: High level AP and PE exposure. Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; PEF, Peak expiratory flow; FEF25–75%, mean forced expiratory flow between 25% and 75% of FVC; FeNO, fractionated exhaled nitric oxide
The LME analysis revealed that the changes in SBP at medium and high levels of AP environment both showed no significant difference compared to the low concentration. However, DBP showed a significant increase in the high concentration of AP compared to the low level (6.45, P = 0.05), with no significant difference observed in relative changes between medium and low levels.
Regarding lung function, compared to the changes after exercise in environments with low pollution concentrations, PEF significantly decreased after exercise in environments with medium (-9.36, P = 0.018) and high (-9.50, P = 0.013) pollution concentrations. FVC and FEV1 showed no significant differences after exercise in medium concentrations but significantly decreased after exercise in high concentrations (-6.84, P = 0.04) (-8.97, P = 0.009).
Regarding the airway inflammation indicator FeNO, compared to the changes after exercise in environments with low pollution concentrations, there was no significant difference after exercise in environments with medium concentrations, but FeNO significantly increased after exercise in environments with high concentrations (13.3, P = 0. 001).
The results further demonstrate that changes in cardiorespiratory health indicators post-exercise in high-level AP environments are significantly decreased compared to low-level AP environments. Conversely, in low and medium-level AP environments, differences in cardiorespiratory health indicator changes are not statistically significant.
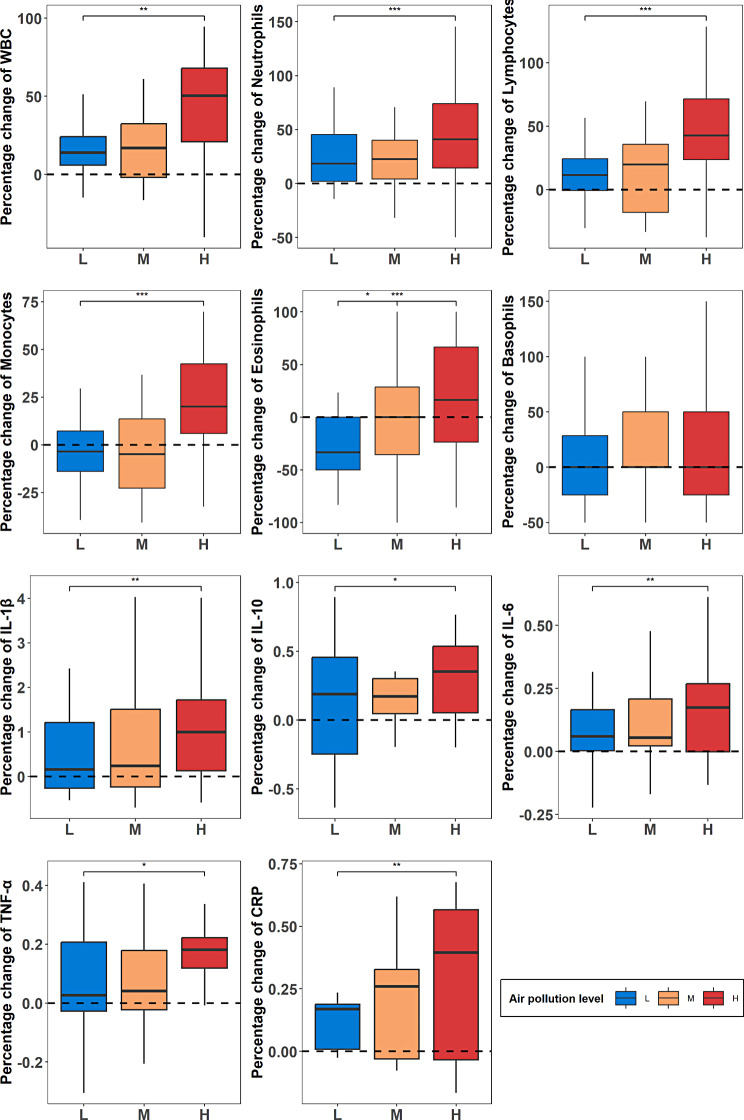
Effects on circulating inflammation markers
Figure 4 illustrates the percentage change relative to baseline in inflammatory markers following exercise across three different levels of AP. Overall, the change in inflammatory markers after exercise in environments with high pollution concentrations was notably higher than those in medium and low concentrations, while the changes between medium and low concentrations were small. Moreover, the majority of inflammatory markers showed an increase after exercise regardless of the AP concentration, except monocytes and eosinophils, which exhibited a slight decrease in change after exercise in environments with medium and low concentrations.
Fig. 4.
Percentage change in inflammatory markers relative to baseline
Table 4 presents the results of LME analysis comparing the differences in percentage change of inflammatory markers between medium and high levels of AP with reference to the percentage change at the low pollution level. The results indicate that compared to the changes observed after exercise in the low pollution environment, there was a significant increase in the levels of white blood cells (27.0, p < 0.001), neutrophils (26.8, p < 0.001), lymphocytes (32.2, p < 0.001), monocytes (28.2, p < 0.001), eosinophils (48.9, p < 0.001), IL-1β (0.76, P = 0.003), IL-10 (0.17, P = 0.02), IL-6 (0.1, P = 0.17), TNF-α (0.97, P = 0.011), and CRP(0.17, P = 0.003) after exercise in the high pollution environment. Additionally, only eosinophils exhibited a significant increase (25.6, p = 0.022) in the medium-pollution environment, while the percentage change in other inflammatory cells after exercise in the medium-pollution environment did not significantly differ from that in the low pollution environment. The results above suggest that high levels of AP significantly increase inflammation in the body, while the difference in inflammation levels between medium and low pollution levels is smaller.
Table 4.
Differences in inflammatory marker changes among three different levels of AP
| WBC | Neutrophils | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p Value* | Coefficient | 95% CI | p Value* | ||||
| M | 1.77 | -7.75 | 11.40 | 0.721 | -3.51 | -18.44 | 11.74 | 0.653 | |
| H | 27.0 | 17.48 | 36.63 | 0.000 | 26.76 | 12.06 | 41.90 | 0.000 | |
| Lymphocytes | Monocytes | ||||||||
| Coefficient | 95% CI | p Value* | Coefficient | 95% CI | p Value* | ||||
| M | 3.35 | -9.03 | 15.34 | 0.594 | 2.11 | -6.90 | 10.97 | 0.647 | |
| H | 32.22 | 19.85 | 44.22 | 0.000 | 28.23 | 19.28 | 36.95 | 0.000 | |
| Eosinophils | Basophils | ||||||||
| Coefficient | 95% CI | p Value* | Coefficient | 95% CI | p Value* | ||||
| M | 25.64 | 3.93 | 46.41 | 0.022 | 14.33 | -4.89 | 32.78 | 0.142 | |
| H | 48.93 | 27.25 | 70.12 | 0.000 | 11.5 | -8.00 | 29.79 | 0.236 | |
| IL-1β | IL-10 | ||||||||
| Coefficient | 95% CI | p Value* | Coefficient | 95% CI | p Value* | ||||
| M | 0.39 | -0.09 | 0.86 | 0.116 | 0.05 | -0.09 | 0.19 | 0.503 | |
| H | 0.76 | 0.29 | 1.23 | 0.003 | 0.17 | 0.03 | 0.32 | 0.020 | |
| IL-6 | TNF-α | ||||||||
| Coefficient | 95% CI | p Value* | Coefficient | 95% CI | p Value* | ||||
| M | 0.05 | -0.02 | 0.11 | 0.174 | -0.00 | -0.08 | 0.07 | 0.920 | |
| H | 0.10 | 0.04 | 0.17 | 0.004 | 0.97 | 0.02 | 0.17 | 0.011 | |
| CRP | |||||||||
| Coefficient | 95% CI | p Value* | |||||||
| M | 0.05 | -0.06 | 0.16 | 0.370 | |||||
| H | 0.17 | 0.06 | 0.28 | 0.003 | |||||
Mixed effect models adjusted for gender, age, and BMI. Exposure scenario with reference to ‘Low level AP and PE exposure’. *: Statistical significance in the results of the linear mixed-effects model. M: Medium level AP and PE exposure; H: High level AP and PE exposure. Abbreviations WBC. White Blood Cell; IL, interleukin; TNF-α, tumour necrosis factor α; CRP, C reactive protein
Discussion
This study conducted a self-controlled crossover design to assess the effects of moderate-intensity PE on the cardiorespiratory health of healthy young adults in environments with low, medium, and high AP concentrations. Our findings indicate that PE in medium and low-level AP environments seems relatively safe for cardiorespiratory health among healthy young adults. However, PE in high-level AP environments can be detrimental to cardiorespiratory health, significantly increasing the body’s inflammatory response.
Pollution levels
Reviewing previous studies, it is evident that different studies have varied definitions for low- and high-concentration ranges. Some studies classify pollution levels as high, while other studies might consider them low, potentially contributing to inconsistent research conclusions [22]. For instance, a significant discrepancy in the classification of pollutant levels was observed between the study by Kocot et al. [12] and the study by Matt et al. Kocot et al. primarily categorize pollutant levels based on low and high traffic flow, whereas Matt et al. define low and high pollutant levels using a PM10 threshold of 50 µg/m³. In the research conducted by Matt et al. [13], the difference between low and high levels of exposure was 43 µg/m³ for PM2.5 and 58 µg/m³ for PM10. In contrast, the study by Kocot et al. reported a discrepancy of 49 µg/m³ for PM2.5 and 156 µg/m³ for PM10 between low and high levels of exposure. Consequently, the findings of the two studies were markedly different. Our study clearly distinguishes between low, medium, and high levels of AP concentrations. For instance, in the study by Kocot et al. [12], a threshold of PM10 at 50 µg/m3 was used to differentiate between good and poor air quality, categorizing pollution levels as poor that aligns with our high pollution exposure levels. However, our PM2.5 exposure concentrations are higher than those reported by Kocot et al. Additionally, in our study, medium pollution exposure levels are comparable to high pollution exposure levels in other experimental studies [13, 17]. Therefore, our classification of AP levels is more refined, accurately reflecting the impact of PE on health benefits under different levels of AP environments.
Effects of combined exercise and AP exposure on cardiorespiratory function
Our experimental findings indicate that exercise can reduce blood pressure, which is consistent with previous research results. Moreover, compared to exposure at medium to high concentrations, exercising at lower concentrations significantly lowers SBP. Similarly, in a crossover trial conducted in Barcelona, Spain, Kubesch et al. [23] found that intermittent PA was associated with lower SBP compared to resting, particularly following exposure to lower traffic-related air pollution (TRAP). Additionally, Kubesch et al. [23] demonstrated that exposure to higher TRAP was associated with higher DBP compared to lower TRAP. Kocot et al. [12] conducted a crossover study on healthy adult males, which also showed significant differences in the relative changes of DBP between pollution exposure experiments and control experiments, with a greater increase during pollution exposure experiments. This finding aligns with ours. This suggests that even though exercise can regulate blood pressure, exercising in environments with higher pollution levels may still increase DBP. This could be attributed to the increased concentration of PM2.5, which may weaken the blood pressure-lowering effect of exercise. Evidence suggests that inhaling PM may trigger acute autonomic imbalance, leading to acute endothelial/vascular dysfunction, favoring vasoconstriction and a sharp decline in aortic compliance, as well as increased bioactivity of endothelin or renin-angiotensin-aldosterone system activation [24, 25]. These factors, individually or collectively, may contribute to an elevation in blood pressure within hours of exposure to air particles. Therefore, elevated blood pressure may be a biomarker of adverse pathways leading to increased cardiovascular risk [24].
Regarding changes in lung function, Kubesch et al. [17] conducted a study involving 28 healthy adults, and their findings regarding low levels of AP concentration align with ours. They found that following PA during periods of low TRAP exposure, there was a significant increase in FEV1 and FEF25 − 75%. They also demonstrated that PA was associated with increases in FEV1, FVC, and FEF25 − 75% compared to rest, and even exercise in high TRAP environments had beneficial effects on lung function. Our research results also indicate that PE remains beneficial for lung function in environments with medium to low AP concentrations. Similarly, in a crossover study by Matt et al. [13] involving 30 healthy adults, immediate post-exercise comparisons with baseline showed significant increases in FEV1 (48.5 mL, p = 0.02), FEV1/FVC (0.64%, p = 0.01), and FEF25 − 75% (97.8 mL, p = 0.02). However, in our study, the magnitude of respiratory responses was small, and these responses were observed only in healthy young adults.
Although exercise improves lung function, the benefits diminish with increasing pollutant concentrations. Kocot et al. [12] conducted a crossover experiment involving 15 min of submaximal exercise in healthy young adult males under conditions of poor and good air quality. The pollutant concentrations in their exposure group were similar to our medium-concentration pollutant levels. They compared the relative changes between the exposure and control groups and found no differences in FVC, FEV1, and FEV1/FVC after exercise, which is consistent with our findings. Unlike Kocot et al., we also compared the changes in cardiorespiratory health indicators after exercise in high and low AP concentration environments, finding significant decreases in FVC, FEV1, and PEF. Kocot et al. concluded that acute respiratory changes following exercise under exposure conditions depend on pollutant concentrations, with only participants exposed to particularly high levels showing acute decreases in FEV1/FVC post-exercise, and the relative changes in FEV1/FVC were significantly negatively correlated with pollutant concentrations. Strak et al. [26] investigated the effects of AP on the respiratory health of healthy cyclists and found a slight increase in lung function immediately after cycling, but a negative correlation with AP emerged six hours after cycling. Matt et al.‘s study [13] also indicated that PA mitigates the negative effects of PM on the upper and lower respiratory tracts, with substantial evidence of interaction between PM and physical exercise’s respiratory effects. In healthy participants, exercise usually has bronchodilatory effects, most likely due to the activation of β2-receptors by endogenous catecholamines. However, regardless of whether they engage in PE, individuals experience a significant decline in the function of both the upper and lower respiratory airways following an increase in PMcoarse concentrations [13].
Changes in inflammation after combined exposure to exercise and AP
Previous studies have demonstrated that higher levels of AP, particularly PM, can induce the production of nitric oxide by epithelial cells, leading to a significant increase in FeNO levels [27, 28], resulting in local inflammatory responses. Furthermore, research has indicated a correlation between changes in FeNO levels after exercise and the concentration of air pollutants. For instance, Kubesch et al. [17]observed a significant association between coarse particulate matter and increased FeNO levels. Additionally, they noted a modest increase in FeNO levels after PA compared to rest. In contrast to their findings, our study reveals a significant decrease in mean FeNO levels after exercise in environments with medium to low AP, while in high AP environments, mean FeNO levels increase but without significant differences. This difference may be attributed to variations in study design; our study only collected FeNO levels within 30 min post-exercise and did not gather information on longer-term reactions following combined exercise and AP exposure. Inflammatory responses following AP exposure may require more time, as PA has been shown to increase nitric oxide production through epigenetic changes, with the association between PM2.5 and FeNO being most significant with a one-day lag time [29]. Kocot et al. [30] conducted exercise sessions with 76 healthy university students under conditions of high AP and good air quality, finding that increased FeNO levels were associated with higher levels of PA and higher concentrations of air pollutants. Moreover, the statistical significance of the difference in FeNO levels between pollution exposure and control experiments was observed 15 min after exercise cessation, rather than immediately post-exercise. Hence, the duration of the inflammatory response is also a factor to consider in this study. Additionally, Bos et al. [31] found that FeNO levels increased after training in urban environments, while aerobic training in rural environments did not affect FeNO levels. Consistent with their findings, the linear mixed-effects analysis in our study also suggests a significant increase in FeNO levels after exercise in high AP environments relative to low concentrations. This indicates that engaging in PA in highly polluted air environments may increase respiratory tract inflammation levels, triggering local inflammatory responses.
There is limited research on the systemic inflammatory response to combined exposure to AP and PA. However, previous reports have indicated [17] that engaging in PA in environments with AP can increase the count of inflammatory cells, leading to a systemic inflammatory response. Acute PA increases the number of inflammatory cells in the body, and exercise also increases the dose of inhaled particles, resulting in an increase in systemic inflammatory biomarkers. The combined effect of these factors leads to an increase in systemic inflammatory markers in the body. Kubesch et al. [17] found that compared to no exercise in low TRAP conditions, there was a significant increase in white blood cells after exercise during high TRAP exposure. They also observed associations between PM10 and PM2.5 with increased white blood cells and between coarse PM and increased neutrophils. Bos et al. [31] investigated the changes in inflammatory markers following moderate exercise conducted on a bicycle ergometer in urban and rural environments. They found that after training in the urban setting, there was an increase in white blood cells and neutrophils, whereas no change was observed in the rural group. Consistent with our findings, acute exercise led to an increase in most inflammatory cells regardless of the AP concentration, with a more pronounced increase observed after exercise in environments with higher concentrations. This phenomenon can be ascribed to the high concentration of PM capable of stimulating cells via the activation of toll-like receptors (TLRs), which in turn triggers immune responses. Furthermore, the presence of polycyclic aromatic hydrocarbons (PAHs) and heavy metals within PM can also activate immune cells, augmenting their responses, and initiating pro-inflammatory intracellular signaling pathways, thereby exacerbating inflammation and detrimental to health [32]. This also serves as a reminder that individuals should refrain from engaging in PE in environments with high levels of AP. In contrast, acute PE in settings with medium to low AP levels has a relatively minor impact on the body.
Limitations
Our study has several limitations. Firstly, the sample size is small, and the study population consists of healthy young adults, thus the findings may not be generalizable to other populations. Secondly, we only focused on short-term exercise and acute responses. Longer duration exercises or repeated measurements over longer periods post-exercise could provide more substantial insights. Additionally, during low-level exposure, participants were exposed to unfiltered air. Therefore, we primarily compared the effects of pollution at three different concentration levels rather than comparing AP to clean air. Lastly, due to the extended duration of the experiments, variations in environmental temperature and relative humidity may have introduced some confounding effects into the results.
Conclusions
Acute PE in medium and low-level AP environments is generally secure concerning short-term effects on cardiorespiratory health among healthy young adults. However, acute PE in high-level AP environments can be detrimental to cardiorespiratory health, significantly increasing the body’s inflammatory response.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank M. Min He for the English language editorial services.
Author contributions
Xingsheng Jin: Writing – original draft, Writing - review & editing. Weiyi Wang: Writing - review & editing. Qian Sun: Writing - review & editing. Yang Chen: Writing - review & editing. Bingxiang Xu: Supervision, Funding acquisition, Writing - review & editing. Haili Tian: Supervision, Funding acquisition, Writing - review & editing. All authors reviewed the manuscript.
Funding
This work was supported by grants from the Humanities and Social Sciences Youth Fund of the Ministry of Education (grant number 21YJC890030), the National Natural Science Foundation of China (grant number 32400957), the Shanghai Natural Science Foundation (grant number 23ZR1403700), the National Natural Science Foundation of China (grant number 32200515), and the Open Research Fund of the National Key Laboratory of Genetic Engineering (grant number SKLGE-2315).
Data availability
All data generated and analyzed during the current study are included in this article. Datasets of the study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethics approval for the study was obtained from the Ethics Committee of Shanghai University of Sport (Ethics approval no: 102772019RT001) and registered in the Chinese Clinical Trial Registry (Registered No: ChiCTR2000031851). Written informed consent was obtained from participants before they participated in the study.
Consent for publication
All participants provided informed consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xingsheng Jin, Weiyi Wang and Qian Sun contributed equally to this work.
Contributor Information
Bingxiang Xu, Email: xubingxiang@sus.edu.cn.
Haili Tian, Email: tianhaili123@163.com.
References
- 1.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and Health impacts of Air Pollution: a review. Front Public Health. 2020;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holgate S. Air pollution is a public health emergency. BMJ. 2022;378:o1664. [DOI] [PubMed] [Google Scholar]
- 3.Schraufnagel DE, Balmes JR, De Matteis S, Hoffman B, Kim WJ, Perez-Padilla R, Rice M, Sood A, Vanker A, Wuebbles DJ. Health benefits of Air Pollution reduction. Ann Am Thorac Soc. 2019;16(12):1478–87. [DOI] [PubMed] [Google Scholar]
- 4.Miller MR. The cardiovascular effects of air pollution: Prevention and reversal by pharmacological agents. Pharmacol Ther. 2022;232:107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KK, Bing R, Kiang J, Bashir S, Spath N, Stelzle D, Mortimer K, Bularga A, Doudesis D, Joshi SS, et al. Adverse health effects associated with household air pollution: a systematic review, meta-analysis, and burden estimation study. Lancet Glob Health. 2020;8(11):e1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahad O, Kuntic M, Frenis K, Chowdhury S, Lelieveld J, Lieb K, Daiber A, Munzel T. Physical activity in Polluted Air-Net Benefit or Harm to Cardiovascular Health? A Comprehensive Review. Antioxid (Basel) 2021, 10(11). [DOI] [PMC free article] [PubMed]
- 7.Ruegsegger GN, Booth FW. Health benefits of Exercise. Cold Spring Harb Perspect Med 2018, 8(7). [DOI] [PMC free article] [PubMed]
- 8.Chastin SFM, Abaraogu U, Bourgois JG, Dall PM, Darnborough J, Duncan E, Dumortier J, Pavon DJ, McParland J, Roberts NJ, et al. Effects of regular physical activity on the Immune System, Vaccination and Risk of Community-Acquired Infectious Disease in the General Population: systematic review and Meta-analysis. Sports Med. 2021;51(8):1673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson T, Olds T, Curtis R, Blake H, Crozier AJ, Dankiw K, Dumuid D, Kasai D, O’Connor E, Virgara R, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. 2022;4(8):e615–26. [DOI] [PubMed] [Google Scholar]
- 10.An R, Shen J, Ying B, Tainio M, Andersen ZJ, de Nazelle A. Impact of ambient air pollution on physical activity and sedentary behavior in China: a systematic review. Environ Res. 2019;176:108545. [DOI] [PubMed] [Google Scholar]
- 11.Sinharay R, Gong J, Barratt B, Ohman-Strickland P, Ernst S, Kelly FJ, Zhang JJ, Collins P, Cullinan P, Chung KF. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: a randomised, crossover study. Lancet. 2018;391(10118):339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocot K, Zejda JE. Acute cardiorespiratory response to ambient air pollution exposure during short-term physical exercise in young males. Environ Res. 2021;195:110746. [DOI] [PubMed] [Google Scholar]
- 13.Matt F, Cole-Hunter T, Donaire-Gonzalez D, Kubesch N, Martinez D, Carrasco-Turigas G, Nieuwenhuijsen M. Acute respiratory response to traffic-related air pollution during physical activity performance. Environ Int. 2016;97:45–55. [DOI] [PubMed] [Google Scholar]
- 14.Wagner DR, Brandley DC. Exercise in Thermal inversions: PM(2.5) Air Pollution effects on pulmonary function and aerobic performance. Wilderness Environ Med. 2020;31(1):16–22. [DOI] [PubMed] [Google Scholar]
- 15.Giles LV, Carlsten C, Koehle MS. The pulmonary and autonomic effects of high-intensity and low-intensity exercise in diesel exhaust. Environ Health. 2018;17(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park HY, Gilbreath S, Barakatt E. Respiratory outcomes of ultrafine particulate matter (UFPM) as a surrogate measure of near-roadway exposures among bicyclists. Environ Health. 2017;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubesch NJ, de Nazelle A, Westerdahl D, Martinez D, Carrasco-Turigas G, Bouso L, Guerra S, Nieuwenhuijsen MJ. Respiratory and inflammatory responses to short-term exposure to traffic-related air pollution with and without moderate physical activity. Occup Environ Med. 2015;72(4):284–93. [DOI] [PubMed] [Google Scholar]
- 18.Pasqua LA, Damasceno MV, Cruz R, Matsuda M, Martins MAG, Marquezini MV, Lima-Silva AE, Saldiva PHN, Bertuzzi R. Exercising in the urban center: inflammatory and cardiovascular effects of prolonged exercise under air pollution. Chemosphere. 2020;254:126817. [DOI] [PubMed] [Google Scholar]
- 19.Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz CN, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: the st. James women take heart project. Circulation. 2010;122(2):130–7. [DOI] [PubMed] [Google Scholar]
- 20.Organization WH. WHO global air quality guidelines: particulate matter (PM2. 5 and PM10), ozone, nitrogen dioxide. sulfur dioxide and carbon monoxide: World Health Organization; 2021. [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 22.DeFlorio-Barker S, Lobdell DT, Stone SL, Boehmer T, Rappazzo KM. Acute effects of short-term exposure to air pollution while being physically active, the potential for modification: a review of the literature. Prev Med. 2020;139:106195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubesch N, De Nazelle A, Guerra S, Westerdahl D, Martinez D, Bouso L, Carrasco-Turigas G, Hoffmann B, Nieuwenhuijsen MJ. Arterial blood pressure responses to short-term exposure to low and high traffic-related air pollution with and without moderate physical activity. Eur J Prev Cardiol. 2015;22(5):548–57. [DOI] [PubMed] [Google Scholar]
- 24.Hudda N, Eliasziw M, Hersey SO, Reisner E, Brook RD, Zamore W, Durant JL, Brugge D. Effect of reducing Ambient Traffic-Related Air Pollution on blood pressure: a randomized crossover trial. Hypertension. 2021;77(3):823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giorgini P, Di Giosia P, Grassi D, Rubenfire M, Brook RD, Ferri C. Air Pollution exposure and blood pressure: an updated review of the literature. Curr Pharm Des. 2016;22(1):28–51. [DOI] [PubMed] [Google Scholar]
- 26.Strak M, Boogaard H, Meliefste K, Oldenwening M, Zuurbier M, Brunekreef B, Hoek G. Respiratory health effects of ultrafine and fine particle exposure in cyclists. Occup Environ Med. 2010;67(2):118–24. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Liu F, Niu Z, Mao S, Tang H, Li N, Chen G, Liu S, Lu Y, Xiang H. The association between short-term exposure to ambient air pollution and fractional exhaled nitric oxide level: a systematic review and meta-analysis of panel studies. Environ Pollut. 2020;265Pt A:114833. [DOI] [PubMed] [Google Scholar]
- 28.Anand A, Castiglia E, Zamora ML. The Association between Personal Air Pollution exposures and Fractional exhaled nitric oxide (FeNO): a systematic review. Curr Environ Health Rep 2024, 11(2):210-224. [DOI] [PMC free article] [PubMed]
- 29.Chen R, Qiao L, Li H, Zhao Y, Zhang Y, Xu W, Wang C, Wang H, Zhao Z, Xu X, et al. Fine particulate matter constituents, nitric oxide synthase DNA methylation and exhaled nitric oxide. Environ Sci Technol. 2015;49(19):11859–65. [DOI] [PubMed] [Google Scholar]
- 30.Kocot K, Baranski K, Melaniuk-Wolny E, Zajusz-Zubek E, Kowalska M. Acute FeNO and blood pressure responses to Air Pollution exposure in young adults during physical activity. Int J Environ Res Public Health 2020, 17(23). [DOI] [PMC free article] [PubMed]
- 31.Bos I, De Boever P, Vanparijs J, Pattyn N, Panis LI, Meeusen R. Subclinical effects of aerobic training in urban environment. Med Sci Sports Exerc. 2013;45(3):439–47. [DOI] [PubMed] [Google Scholar]
- 32.Jin X, Chen Y, Xu B, Tian H. Exercise-mediated protection against Air Pollution-Induced Immune damage: mechanisms, challenges, and future directions. Biology 2024, 13(4). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during the current study are included in this article. Datasets of the study are available from the corresponding author upon reasonable request.