Abstract
Background
The belzutifan is a hypoxia inducible factor-2 alpha (HIF-2α) inhibitor for the treatment of advanced or metastatic clear cell renal cell carcinoma (mccRCC) and has exhibited good safety and efficacy in clinical trials. We conducted a meta-analysis of relevant studies to further clarify the efficacy and safety of belzutifan for the treatment of mccRCC.
Methods
Multiple databases and abstracts from major scientific meetings were systematically reviewed for eligible articles published before June 1, 2024. The following outcomes were analyzed: objective response rate (ORR), disease control rate (DCR), median duration of response (mDOR), median progression-free survival (mPFS), median overall survival (mOS), and treatment-related adverse events (TRAes). 426 records were reviewed, and data were extracted by at least two individuals.
Results
Seven studies involving 715 patients were included in this meta-analysis. The pooled ORR was 34% (95% confidence interval [CI]: 23–46%), the DCR was 79% (95% CI: 66–90%), the mDOR was 21.8 months (95% CI: 14.82–28.78), and the mPFS time was 8.8 months (95% CI: 6.15–11.44). The pooled incidence of grade 3–5 TRAes was 46%, and the most common TRAe was anemia. Further subgroup analysis revealed that, compared with belzutifan monotherapy, the combination of belzutifan with tyrosine kinase inhibitors (TKIs) as second- or later-line therapy was associated with a statistically significant increase in the ORR. Toxicity was also greater with combined inhibition therapy.
Conclusions
Our meta-analysis revealed moderate antitumor activity and a manageable safety profile of the inhibitor belzutifan in patients with mccRCC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40360-024-00828-5.
Keywords: Clear cell renal cell carcinoma, Belzutifan, Efficacy, Adverse events, Meta-analysis
Background
Renal cell carcinoma (RCC) is the most prevalent kidney cancer type, with the clear cell RCC (ccRCC) subtype representing the most common form [1]. The latest Global Cancer Statistics (2020) estimated around 430,000 new RCC diagnoses in that year, of which 25–30% were advanced or metastatic ccRCC (mccRCC) [2], which has only a 12% five-year survival rate, despite significant improvements by immunotherapy and tyrosine kinase inhibitors (TKIs) [3]. For such patients, additional therapeutic options are critically needed.
One important oncoprotein that is crucial for the progression of ccRCC is Hypoxia Inducible Factor-2 Alpha (HIF-2α) [4, 5]. With promising safety and efficacy characteristics, PT2385 and PT2399, first-generation HIF-2α inhibitors, demonstrate that HIF-2α inhibition offers a novel approach for treating mccRCC [6–8]. Belzutifan (PT2977, often referred to as MK-6482) is a second-generation HIF-2α) inhibitor. It is designed to target HIF-2α more efficiently than first-generation inhibitors. Belzutifan (Welireg, Merck & Co., Inc.) was permitted for use by the US FDA on December 14, 2023, for treating patients with advanced RCC after ineffective therapy with a programmed cell death-1 (PD-1) or PD-ligand 1 (PD-L1) inhibitor along with a TKI [9].
To our knowledge, there has been no synthesis of the evidence of the efficacy and safety of belzutifan. We, therefore, conducted this meta-analysis to assess the potential therapeutic value of this pharmaceutical in treating mccRCC.
Methods
Search strategy and study selection
Under the reference number CRD42024559760, this meta-analysis was filed on the Prospective International Registry of Systematic Reviews—PROSPERO (http://www.crd.york.ac.uk/ [accessed on June 1, 2024]), according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) standards. Searches of online databases (PubMed, EMBASE, Web of Science, and the Cochrane Library) from their creation until June 1, 2024, were used to find relevant studies. The following main search terms were used: (“renal cell carcinoma” OR “renal cell cancer” OR “clear cell renal cell carcinoma”) AND (metastatic OR advanced) AND (“HIF-2α” OR “HIF-2α inhibitor” OR “Belzutifan” OR “PT2977” OR “MK-6482”). In order to find relevant clinical trials, abstracts from conferences conducted via the European Society of Medical Oncology and the American Society of Clinical Oncology up until June 1, 2024, were also searched.
The selected studies followed these inclusion criteria: (I) the specific inclusion of individuals with pathologically proven mccRCC; (II) treatment regimens, including belzutifan either as monotherapy or in conjunction with another therapy, irrespective of the presence of a comparative analysis; and (III) publication in English. The reviews, letters, editorials, author comments, and case reports were omitted. In cases of numerous publications concerning the same cohort, the most current data for the intended outcome analysis was collected.
Extraction of data
The following data was independently gathered by two investigators: clinical trial identification (ID), first author, phase, publication year, line of treatment, regimen, and number of patients. The median overall survival (mOS), median progression-free survival (mPFS), disease control rate (DCR), treatment-related adverse events (TRAEs), objective response rate (ORR), and median duration of response (mDOR) were also noted. The disease control responses comprised partial response, complete response, and stable disease, and the complete and partial responses were among the objective responses. For studies not reporting a specific number of adverse events, we estimated the corresponding number of cases on the basis of percentages and the total cohort size. Any differences among the investigators were resolved by reviewing the original texts and discussing until a consensus was reached.
Assessment of study quality
The quality of the randomized controlled trials (RCTs) was evaluated using the Cochrane risk of bias assessment approach. Following this, studies were assessed in terms of randomization, blinding to outcomes, concealment of allocation, blinding of staff and participants, selective reporting, incomplete data on outcomes, and other biases, assessing each as either “high risk,” “low risk,” or “unclear.” The methodology of noncomparative studies was examined using the Methodological Index for Nonrandomized Studies (MINORS) checklist. The checklist has 12 components, each evaluated on a scale from 0 to 2, with 0 indicating ‘not reported,’ 1 representing ‘reported but inadequate,’ and 2 indicating ‘reported and adequate’ [10]. A MINORS score > 15 indicates good quality for a noncomparative study. Two reviewers independently assessed each included study’s quality.
Statistical analysis
The Stata SE12.0 (version 12.0; Stata Corporation) software was employed to perform the meta-analysis. The calculated effect size was combined effect sizes (ESs) and 95% confidence intervals (CIs). The rate was converted and adjusted to a value less than 0.2 or equal to 1 using the double arcsine method [11]. The I2 statistic was used to quantify inconsistency, and Cochran’s Q test (chi-square distribution) was used to evaluate statistical heterogeneity in the included trials. High heterogeneity across trials was indicated by an I2 > 50% or p < 0.10 in a Q test. Since the majority of the studies were single-arm (noncomparative), lacking control groups, a random effect model was employed for analysis [12]. Subgroup analyses were performed for investigating potential causes of the heterogeneity. Two-tailed p-values < 0.05 were considered statistically significant.
Results
Search results
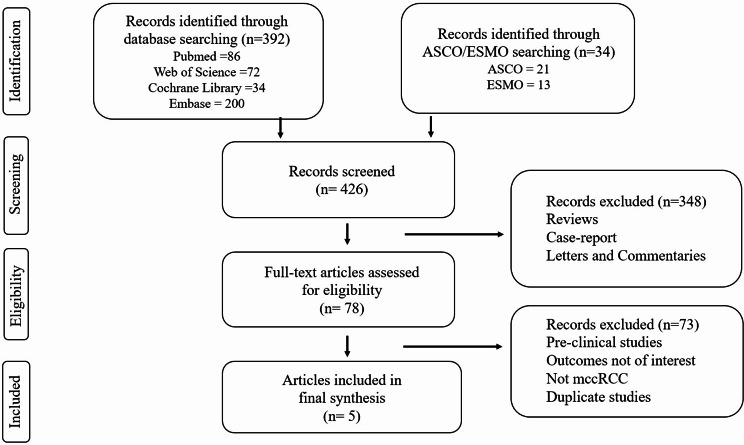
The search identified 426 articles, of which 78 were available for further assessment after eliminating article type. Following the evaluation of titles and abstracts, 73 papers were eliminated. We excluded the articles on LITESPARK-004 clinical trial because the patients enrolled had localized RCC, conflicting with the inclusion criteria for our meta-analysis. Ultimately, 5 articles were included in the final analysis [13–17]. One was published as a full article; the other four, as meeting abstracts. The method of selecting articles is shown in Fig. 1.
Fig. 1.
Flow diagram of literature search and study selection
Study characteristics and quality assessments
Two cohorts (cohorts 1 and 2) with varying numbers of participants were enrolled in each of the two relevant articles. Each of these cohorts was regarded as an independent research. Thus, a total of 715 patients from 7 studies (1 RCT and 6 noncomparative studies) were available for the meta-analysis. Belzutifan was evaluated as the first-line regimen in one study and as the second- or later-line regimen in the other six. Patients were treated with belzutifan alone in four studies and with belzutifan-based combination therapy in the remaining three. Table 1 provides comprehensive details on the listed research. Bias was low in one RCT. The remaining studies had good quality, as shown by their quality scores, which ranged from 15 to 22.
Table 1.
Main characteristics of the studies included in meta-analysis
| Study name | Clinical trial identifier | Phase | Study design | Treatment | Line of therapy | No. patients | ORR (%) |
DCR (%) |
Median DOR Months (95% CI) |
Median PFS Months (95% CI) |
Median OS Months (95% CI) |
Grade 3–5 TRAes (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LITESPARK-001 | NCT02974738 | I/II | NS | belzutifan (120 mg QD) | ≥ 2 | 55 | 25 | 80 |
NR (3.1–38.0) |
14.5 (7.3–22.1) |
- | 40 |
|
LITESPARK-003 -cohort 1 |
NCT03634540a | II | NS |
belzutifan (120 mg QD) + cabozantinib |
1 | 50 | 70 | 98 |
28.6 (1.9–35.8) |
30.3 (16-NR) |
NR (NR-NR) |
46 |
|
LITESPARK-003 -cohort 2 |
II | NS |
belzutifan (120 mg QD) + cabozantinib |
≥ 2 | 52 | 31 | 92.3 |
31.5 (4.2–36.8) |
13.8 (9–19) |
26.7 (20–41) |
64 | |
| LITESPARK-005 | NCT04195750 | III | RCT |
belzutifan (120 mg QD) VS everolimus |
≥ 2 | 374 | 22.7 | 61.3b |
19.5 (1.9–31.6) |
5.6 (3.8–6.5) |
21.4 (18.2–24.3) |
38.5 |
|
LITESPARK-013 -(120 mg) |
NCT04489771a | II | NS | belzutifan (120 mg QD) | ≥ 2 | 76 | 23.7 | 75 |
NR (2.6–16.1) |
7.3 (5.6–9.5) |
NR (,22.0-NR) |
46.1 |
|
LITESPARK-013 -(200 mg) |
II | NS | belzutifan (200 mg QD) | ≥ 2 | 78 | 23.1 | 78.2 |
16.1 (2.1–23.5) |
9.1 (5.5–12.0) |
NR (20.6-NR) |
46.2 | |
| KEYMAKER-U03B | NCT04626518 | I/II | NS |
belzutifan (120 mg QD) + lenvatinib |
≥ 2 | 30 | 50 | 54 |
NR (1.4–14.0) |
11.2 (4-NR) |
- | 50 |
Note aThis research contains two cohorts. b at interim analysis 1
Abbreviation NS noncomparative study, RCT randomized controlled trial, VS versus, QD quaque die, ORR objective response rate, DCR disease control rate, DOR duration of response, PFS progression-free survival, OS overall survival, TRAes treatment-related adverse events, NR not reached
Efficacy
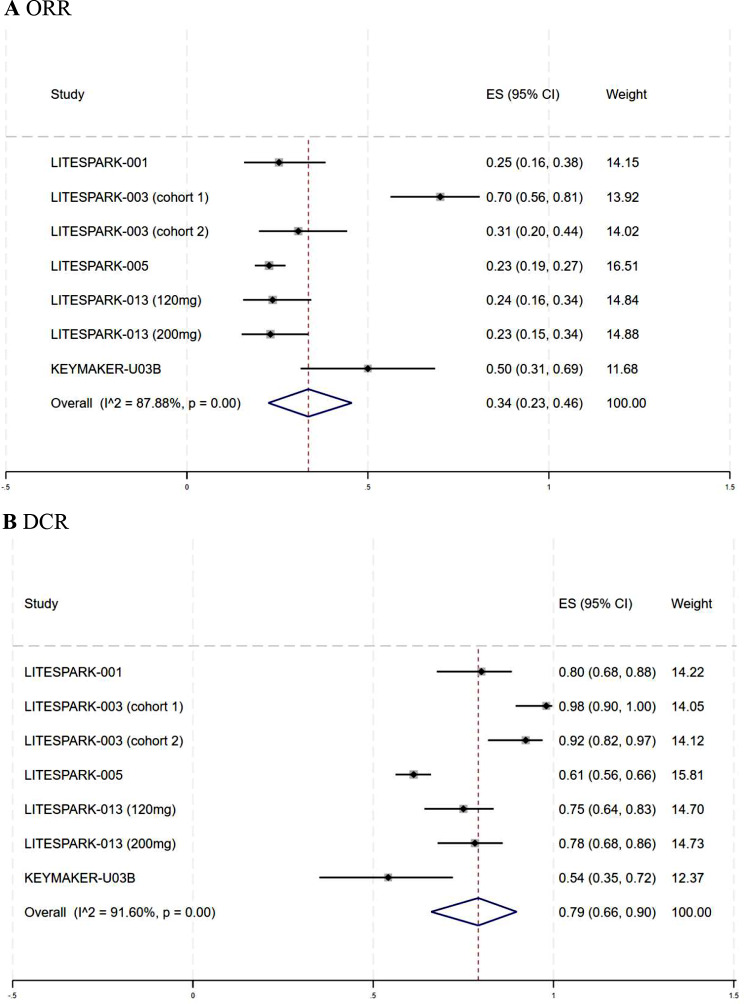
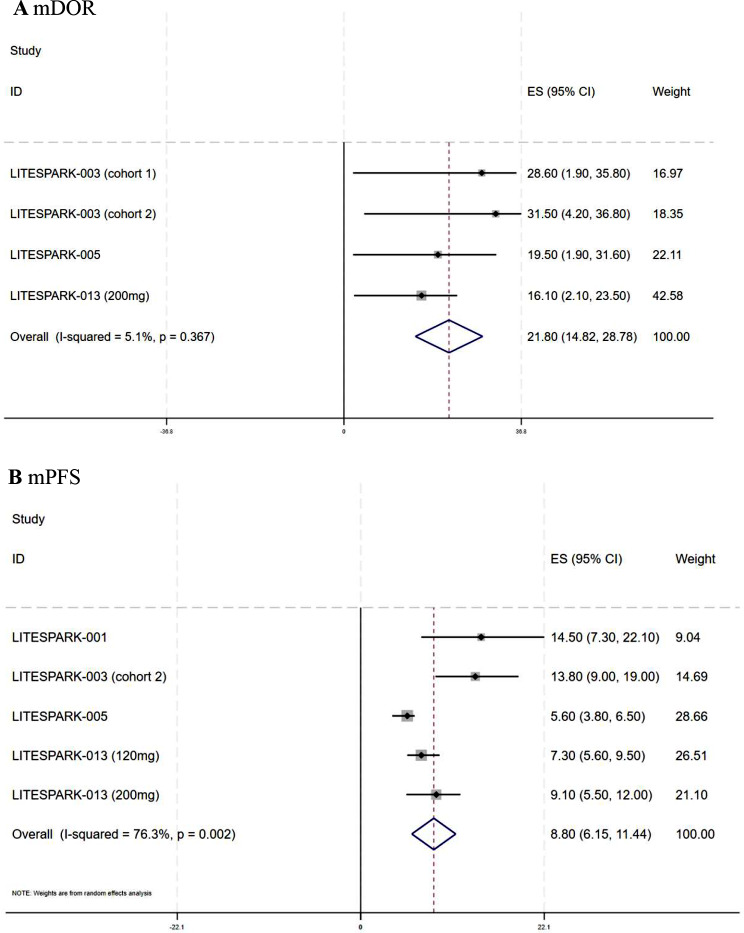
The combined ORR and DCR of the seven trials that reported drug response were 34% (95% CI: 23–46%, I2 = 87.88%) and 79% (95% CI: 66–90%, I2 = 91.6%), respectively. Figure 2 displays the forest plots for ORR and DCR. The pooled mDOR was 21.8 months (95% CI: 14.82–28.78, I2 = 5.1%), with four studies reporting the mDOR and its CI (Fig. 3A). The aggregated mPFS was 8.8 months (95% CI: 6.15–11.44, I2 = 76.3%), based on five studies that provided the mPFS and its CI (Fig. 3B). Owing to the limited number of studies reporting complete data, we did not calculate the pooled mOS or survival rates.
Fig. 2.
Forest plots of the response rates for the meta-analysis. A Forest plots of objective response rate (ORR) in mccRCC; B Forest plots of disease control rate (DCR) in mccRCC. ES effect size, CI confidence interval, mccRCC advanced or metastatic clear cell renal cell carcinoma
Fig. 3.
Forest plots of the survival outcomes for the meta-analysis. A Forest plots of median duration of response (mDOR) in mccRCC; B Forest plots of median progression-free survival (mPFS) in mccRCC. ES effect size, CI confidence interval, mccRCC advanced or metastatic clear cell renal cell carcinoma
Treatment-related adverse events
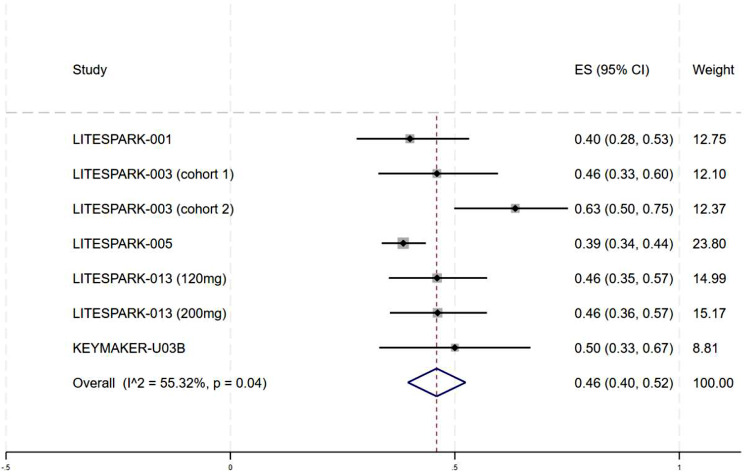
Table 2 summarizes all TRAes. The most common TRAes were anemia (84.1%), hypertension (65.91%), dysgeusia (50%), palmar–plantar erythrodysesthesia (50%), fatigue (45.72%), proteinuria (42.35%), hypophosphatemia (28.43%), thrombocytopenia (26%), nausea (25.39%) and hypothyroidism (22%). The aggregated incidence of grade 3–5 TRAes was 46% (95% CI: 40–52%, I2 = 55.32%) (Fig. 4).
Table 2.
Total treatment-related adverse effects in mccRCC patients
| Adverse events | Studies involved | Event/Total | % |
|---|---|---|---|
| Circulatory system AEs | |||
| Anemia | 7 | 599/713 | 84.01 |
| Hypertension | 3 | 87/132 | 65.91 |
| Thrombocytopenia | 1 | 13/50 | 26 |
| Edema | 5 | 92/581 | 15.83 |
| Decreased lymphocyte count | 1 | 4/55 | 7.27 |
| Decreased platelet count | 1 | 3/55 | 5.45 |
| Digestive system AEs | |||
| Dysgeusia | 1 | 25/50 | 50 |
| Nausea | 7 | 181/713 | 25.39 |
| Diarrhoea | 6 | 151/713 | 21.18 |
| Decreased appetite | 7 | 134/713 | 18.79 |
| Increased alanine aminotransferase | 6 | 111/683 | 16.25 |
| Increased aspartate aminotransferase | 6 | 104/683 | 15.23 |
| Vomiting | 3 | 64/477 | 13.42 |
| Constipation | 3 | 68/526 | 12.93 |
| Stomatitis | 2 | 23/422 | 5.45 |
| Endocrine system AEs | |||
| Hypophosphataemia | 2 | 29/102 | 28.43 |
| Hypothyroidism | 1 | 11/50 | 22 |
| Increased blood creatinine | 2 | 35/427 | 8.2 |
| Hemoglobin decreased | 2 | 12/154 | 7.79 |
| Hypertriglyceridemia | 1 | 14/372 | 3.76 |
| Increased blood alkaline phosphatase | 1 | 3/55 | 5.45 |
| Hypercalcemia | 1 | 2/55 | 3.64 |
| Hyperglycemia | 1 | 10/372 | 2.69 |
| Skin AEs | |||
| Palmar–plantar erythrodysesthesia | 2 | 51/102 | 50 |
| Pruritus | 3 | 35/526 | 6.65 |
| Rash | 1 | 17/372 | 4.57 |
| Nervous system AEs | |||
| Fatigue | 7 | 326/713 | 45.72 |
| Headache | 4 | 67/581 | 11.53 |
| Dizziness | 4 | 65/581 | 11.19 |
| Respiratory system AEs | |||
| Dyspnea | 4 | 87/526 | 16.54 |
| Cough | 4 | 41/581 | 7.06 |
| Pneumonitis | 2 | 6/427 | 1.41 |
| Locomotor system AEs | |||
| Arthralgia | 1 | 54/372 | 14.52 |
| Myalgia | 3 | 11/209 | 5.26 |
| Muscular weakness | 1 | 2/55 | 3.64 |
| Others AEs | |||
| Proteinuria | 2 | 36/85 | 42.35 |
| Weight decreased | 1 | 11/50 | 22 |
| Hypoxia | 4 | 105/526 | 19.96 |
| Back pain | 1 | 55/372 | 14.78 |
| Asthenia | 4 | 61/581 | 10.5 |
| Pyrexia | 1 | 22/372 | 5.91 |
| Increased weight | 2 | 7/133 | 5.26 |
| Flushing | 1 | 2/55 | 3.64 |
| Malaise | 1 | 2/55 | 3.64 |
Abbreviations AEs adverse effects, mccRCC advanced or metastatic clear cell renal cell carcinoma
Fig. 4.
Forest plots of the incidence of treatment-related grade ≥ 3 adverse events in mccRCC. ES effect size, CI confidence interval, mccRCC advanced or metastatic clear cell renal cell carcinoma
Subgroup analysis
A subgroup analysis was performed to provide further insights depending on the therapy line, drug regimen in ≥ second-line, dose, and IMDC risk (Table 3). The aggregated ORR and DCR for first-line therapy were 70% (95% CI: 56–81%) (Fig. S1A) and 98% (95% CI: 90–100%) (Fig. S2A), respectively, both above those for second- and later-line therapies (p = 0.00, p = 0.00). For ≥ second-line treatment, the pooled ORR and DCR for the belzutifan-based combination group was greater than that for the belzutifan monotherapy group (ORR: 37% vs. 23%; DCR: 83% vs. 73%) (Fig. S1B and Fig. S2B). Moreover, the rates of grade 3–5 TRAes in the combination group were higher than those in the monotherapy group (59% vs. 41%, p = 0.002) (Fig. S3). In the subgroup analysis based on IMDC risk, superior antitumor activity was observed in the favorable group, with an ORR of 41% (18–66%) (Fig. S4A) and a DCR of 100% (94–100%) (Fig. S4B).
Table 3.
Subgroup analysis of ORR, DCR, mDOR, mPFS and TRAes in mccRCC
| Subgroups | ORR | DCR | mDOR | mPFS | Grade 3–5 TRAes | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | ES (95% CI) |
I2(P) | P a | N | ES (95% CI) |
I2(P) | P a | N | ES (95% CI) |
I2(P) | P a | N | ES (95% CI) |
I2(P) | P a | N | ES (95% CI) |
I2(P) | P a | |
| Line of therapy | <0.01 | <0.01 | 0.388 | - | 0.988 | |||||||||||||||
| First-line | 1 |
70% (56–81%) |
1 |
98% (90–100%) |
1 |
28.6 (11.65–45.55) |
0 | 1 |
46% (33–60%) |
|||||||||||
| ≥ Second-line | 6 |
26% (21–32%) |
43.51% (0.12) |
6 |
75% (63–85%) |
86.95% (0.00) |
3 |
20.41 (12.75–28.07) |
17.2% (0.299) |
5 |
8.8 (6.15–11.44) |
- | - | 6 |
46% (39–54%) |
62.22% (0.02) |
||||
| Regimen in ≥ Second-line | 0.014 | 0.177 | 0.131 | - | 0.002 | |||||||||||||||
| ≥ Second Single | 4 |
23% (20–27%) |
0.00% (0.96) |
4 |
73% (62–83%) |
82.13% (0.00) |
2 |
17.26 (8.58–25.94) |
0.0% (0.716) |
4 |
7.64 (5.36–9.91) |
67.6% (0.026) |
4 |
41% (37–45%) |
0.00% (0.45) |
|||||
| ≥ Second Combination | 2 |
37% (26–48%) |
2 |
83% (73–91%) |
1 |
31.5 (15.2–47.8) |
1 |
13.8 (8.8–18.8) |
2 |
59% (48–69%) |
||||||||||
| Drug dosage | 0.137 | 0.916 | 0.168 | - | 0.996 | |||||||||||||||
| 120 mg | 6 |
36% (22–50%) |
89.72% (0.00) |
6 |
79% (64–92%) |
92.82% (0.00) |
3 |
26.02 (16.81–35.24) |
0.0% (0.532) |
4 |
8.85 (5.68–12.04) |
79.9% (0.002) |
6 |
46% (39–54%) |
61.79% (0.02) |
|||||
| 200 mg | 1 |
23% (15–34%) |
1 |
78% (68–86%) |
1 |
16.10 (2.10–23.50) |
1 |
9.1 (5.85–12.35) |
1 |
46% (36–57%) |
||||||||||
| IMDC risk group | 0.374 | 0.041 | ||||||||||||||||||
| Favorable | 5 |
41% (18–66%) |
78.11% (0.00) |
3 |
100% (94–100%) |
|||||||||||||||
| Intermediate/poor | 5 |
29% (19–40%) |
65.85% (0.02) |
3 |
87% (75–96%) |
|||||||||||||||
Note Pa Subgroup difference P value
Abbreviations mccRCC advanced or metastatic clear cell renal cell carcinoma, ORR objective response rate, DCR disease control rate, mDOR median duration of response, mPFS median progression-free survival, TRAes treatment-related adverse events, IMDC International Metastatic Renal Cell Carcinoma Database Consortium, ES effect size, 95% CI 95% confidence interval
Sensitivity analysis
Independent sensitivity analyses were undertaken to assess the effects of specific studies on ORR, mPFS, DCR, and grade 3–5 AEs. The results remained steady, according to sensitivity analysis (Fig. S5).
Discussion
HIF-2α is a transcription factor that mediates oxygen homeostasis and promotes carcinogenesis in mccRCC by controlling the expression of genes involved in angiogenesis, erythropoiesis, glycolysis, tumor growth, and cell cycle progression [18]. In many clinical studies, Belzutifan, a second-generation inhibitor of HIF-2α, has demonstrated a good safety profile and significant antitumor efficacy. Despite belzutifan’s FDA approval for mccRCC therapy, a thorough analysis of the benefits and risks associated with its usage is needed. Our study revealed that belzutifan treatment was associated with an ORR and DCR of 34% (95% CI: 23–46%) and 79% (95% CI: 66–90%), respectively. The mDOR was 21.8 months (95% CI: 14.82–28.78), and the mPFS was 8.8 months (95% CI: 6.15–11.44). The rate of grade 3–5 TRAes was 46% (95% CI: 40–52%).
Immune checkpoint inhibitors (ICIs) and TKIs are recognized first-line combination treatments for mccRCC, with ORRs ranging from 39 to 71% and a median OS of 4 years [19–24]. An ongoing phase II trial called LITESPARK-003-cohort 1 is currently assessing belzutifan and cabozantinib as first-line therapies for advanced ccRCC. Preliminary evidence for the efficacy of this strategy seems promising, with an ORR of 70%, a CBR of 98% and an mPFS time of 30.3 months [14]. However, whether these outstanding response rates translate to a notable OS benefit needs to be proven in the final results and additional successive clinical trials.
Currently, it is unknown which second and later-line therapy choices are the most suitable. Several studies have shown response rates ranging from 10 to 66% and mPFS times between 4.7 and 9.3 months for previously treated patients receiving TKI monotherapy [25–31]. A 2015 phase II study revealed that compared with monotherapy, TKI combination treatment led to longer median PFS (14.6 months) [32]. ICI and ICI combination therapy have also received attention. Compared with everolimus, nivolumab monotherapy prolonged OS (25 months) in the phase III Checkmate 025 trial, with an acceptable ORR (25%) [33]. As a salvage treatment, adding ipilimumab, an additional ICI targeting the cytotoxic T-lymphocyte-associated protein 4, to nivolumab monotherapy after an insufficient response to nivolumab alone has demonstrated limited efficacy [34–36]. Two open-label phase Ib/II trials (KEYNOTE-146 and TiNivo) assessing the effectiveness of ICI/TKI combinations in the second-line context showed potential for use as antitumor treatments, with an ORR of 62% and a mOS of 18.9 months [37, 38]. Belzutifan demonstrated modest anticancer efficacy in the current research in patients undergoing second- or later-line treatment, with a DCR of 75%, an ORR of 26%, and a mPFS of 8.8 months, indicating its potential as a therapeutic alternative for this patient group. Significantly, a number of variables may influence the efficacy of second-line treatments, including patient preferences, comorbidities, and the kind of first-line therapy used. This study did not perform a matching subgroup analysis to look into how the aforementioned factors affected belzutifan’s effectiveness because pertinent data were absent.
Belzutifan treatment is hampered by drug resistance [39]. A method used to address treatment resistance is to integrate therapeutic drugs with various molecular-targeted treatments. The subgroup analysis showed that the ORR was considerably higher when belzutifan and a TKI were used together in the second or later line of treatment, as opposed to belzutifan monotherapy. Moreover, the relatively high toxicity burden of combination therapies must be considered. Additionally, HIF-2α inhibitor resistance is associated with the immunosuppressive tumor environment [40, 41]. Previous studies have shown that in hypoxic ccRCC cells, the levels of PD-L1 are correlated with those of HIF-2α [42]. It was discovered that both protein and mRNA levels of PD-L1 were decreased by targeting HIF-2α. HIF-2α’s impact on checkpoint regulation underscores its potential application as a treatment target in conjunction with PD-1/PD-L1 inhibitors. First-generation HIF-2α inhibitors, like PT2385, showed synergistic inhibition of tumorigenesis when paired with an anti-PD-1 antibody in a phase I study [43]. Clinical investigations of the potential therapeutic benefits of belzutifan in combination with the PD-1 inhibitor pembrolizumab (NCT04736706, NCT05239728) for mccRCC are awaited.
The most effective methods for risk assessment and the most supportive clinical guidelines for therapeutic direction are currently the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) models for advanced or metastatic RCC. According to current guidelines, patients with favorable IMDC risk should consider ICI-TKI combinations as their preferred alternative [44, 45]. Manneh et al. verified the advantage of ICI-TKIs over sunitinib as the first-line therapy in terms of PFS and ORR in a meta-analysis [46]. Belzutifan significantly increased the DCR for favorable-risk individuals relative to those with intermediate/poor risk, according to the subgroup analysis based on IMDC risk. While the total response rate in the favorable-risk group was positive, the difference was non-significant. Unfortunately, we could not quantitatively synthesize PFS and OS data in this regard because most studies did not report such data. Moreover, notably, IMDC models were developed at a time when ICIs/TKIs were considered first-line therapy, and their use in with belzutifan is still emerging. Thus, reliable biomarkers associated with the response to belzutifan must still be identified.
Regarding safety, belzutifan has a toxicity profile distinct from those of current therapeutic modalities, including TKIs and ICIs. Therefore, we analyzed the most common and grade 3–5 TRAes of belzutifan. The pooled incidence of grade 3–5 TRAes was 46%, suggesting that belzutifan was tolerable. Additionally, the most common AE was a hematological -AE, anemia, with an incidence of 84.1%. Anemia is an expected on-target toxicity of belzutifan, possibly because of the downstream action of HIF-2α suppression, which reduces erythropoietin (EPO) synthesis [47]. Many patients may find it intolerable to have transfusions more than once, and EPO-stimulating drug treatment is usually limited to those undergoing systemic therapy for palliative purposes. Therefore, improving anemia management, especially allowing for treatment pauses, may make treatment more tolerable and improve the individual’s quality of life [48]. It is important to note that due to the lack of detailed data, we are unable to extract the specific number of people who experienced anemia with belzutifan monotherapy and those who experienced anemia with combination therapy. Therefore, we cannot perform a more detailed analysis to determine which drug combination has a higher incidence of anemia.
It is essential to recognize a few of this meta-analysis’s limitations. Firstly, no direct group comparisons were made because the included research was phase II single-arm trials without control data. Secondly, the primary source of data used in this study was directly taken from published conference abstracts. Some trials included in our analysis did not provide complete or final data on OS, PFS, or toxicity. An insufficient amount of data might influence the analysis. Third, individual-level patient features, including age, sex, and status of performance, were not included. Thus, we could not conduct more detailed subgroup analyses, primarily analyses based on IDMC status. Therefore, in light of the aforementioned factors, further research is required, and results must be evaluated cautiously.
Conclusions
Belzutifan’s safety and effectiveness in treating patients with mccRCC are being shown for the first time with this meta-analysis. Belzutifan with a TKI has therapeutic advantages in second and later-line therapy, according to subgroup analysis. Belzutifan also seems to increase the DCR and ORR in patients in the favorable risk category. However, additional prospective research is required to validate these results.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- RCC
Renal cell carcinoma
- ccRCC
clear cell RCC
- mccRCC
Advanced or metastatic ccRCC
- TKIs
Tyrosine kinase inhibitors
- HIF-2α
Hypoxia Inducible Factor-2 Alpha
- FDA
Food and Drug Administration
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death-ligand 1
- ORR
Objective response rate
- DCR
Disease control rate
- mDOR
median duration of response
- mPFS
median progression-free survival
- mOS
median overall survival
- TRAes
Treatment-related adverse events
- RCTs
Randomized controlled clinical trials
- Cis
Confidence intervals
- ICIs
Immune checkpoint inhibitors
- IMDC
International Metastatic Renal Cell Carcinoma Database Consortium
- EPO
Erythropoietin
Author contributions
GS drafted the backgroundand discussion, interpreted the data, and critically revised the remainder of the manuscript. GS and SX conducted the analysis and drafted the methods and results of the manuscript and critically revised the remainder of the manuscript. YZ and XJ substantially contributed to the design of the study and have critically revised the manuscript. CW and XJ contributed to the design of included studies and the interpretation of the data and have critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Jinan Municipal Health Commission’s in hospital big data technology plan [grant numbers 2022-YBD-01].
Data availability
Data will be made available on request.
Declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ge Song and Song Xue contributed equally and are joint first authors.
Chunling Wu and Xiaowei Ji contributed equally and are joint corresponding authors.
Contributor Information
Chunling Wu, Email: 524042824@qq.com.
Xiaowei Ji, Email: Dove1990ge@outlook.com.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. 10.3322/caac.21763. [DOI] [PubMed]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Institute NC. Cancer stat facts: kidney and renal pelvis cancer. 2020. Available from: Accessed 13 Mar 2024. https://seer.cancer.gov/statfacts/html/kidrp.html
- 4.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas S, Troy H, Leek R, Chung YL, Li JL, Raval RR, Turley H, Gatter K, Pezzella F, Griffiths JR, et al. Effects of HIF-1alpha and HIF2alpha on growth and metabolism of clear-cell renal cell carcinoma 786-0 xenografts. J Oncol. 2010;2010:757908. 10.1155/2010/757908. [DOI] [PMC free article] [PubMed]
- 6.Yu T, Tang B, Sun X. Development of inhibitors targeting hypoxia-inducible factor 1 and 2 for Cancer Therapy. Yonsei Med J. 2017;58:489–96. 10.3349/ymj.2017.58.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Y, Yu Q, Zhang X. Allosteric inhibition of HIF-2α as a novel therapy for clear cell renal cell carcinoma. Drug Discovery Today. 2019;24:2332–40. 10.1016/j.drudis.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Courtney KD, Infante JR, Lam ET, Figlin RA, Rini BI, Brugarolas J, Zojwalla NJ, Lowe AM, Wang K, Wallace EM, et al. Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible Factor-2α antagonist in patients with previously treated Advanced Clear Cell Renal Cell Carcinoma. J Clin Oncology: Official J Am Soc Clin Oncol. 2018;36:867–74. 10.1200/jco.2017.74.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S.Food&Drug. FDA approves belzutifan for advanced renal cell carcinoma. 2023. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belzutifan-advanced-renal-cell-carcinoma. Accessed 1 June 2024.
- 10.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 11.Spittal MJ, Pirkis J, Gurrin LC. Meta-analysis of incidence rate data in the presence of zero events. BMC Med Res Methodol. 2015;15:42. 10.1186/s12874-015-0031-0. [DOI] [PMC free article] [PubMed]
- 12.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Commun Health. 2013;67:974–8. 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 13.Jonasch E, Bauer TM, Papadopoulos KP, Plimack ER, Merchan JR, McDermott DF, Dror Michaelson M, Appleman LJ, Roy A, Perini RF. et al. Phase I LITESPARK-001 study of belzutifan for advanced solid tumors: extended 41-month follow-up in the clear cell renal cell carcinoma cohort. Eur J Cancer. 2024;196:113434. 10.1016/j.ejca.2023.113434. [DOI] [PubMed]
- 14.UroToday. ESMO 2023: LITESPARK-003 Phase 2 study of belzutifan in combination with cabozantinib for advanced clear cell renal cell carcinoma (ccRCC). 2023. Available from: https://www.urotoday.com/conference-highlights/esmo-2023/esmo-2023-kidney-cancer/147495-esmo-2023-phase-2-litespark-003-study-of-belzutifan-in-combination-with-cabozantinib-for-advanced-clear-cell-renal-cell-carcinoma-ccrcc-lba87.html. Accessed 1 June 2024.
- 15.UroToday. ESMO 2023: LITESPARK-005 Belzutifan Versus Everolimus in participants with previously treated advanced clear cell renal cell carcinoma: randomized open-label Phase 3. 2023. Available from: https://www.urotoday.com/conference-highlights/esmo-2023/esmo-2023-kidney-cancer/147501-esmo-2023-litespark-005-belzutifan-versus-everolimus-in-participants-with-previously-treated-advanced-clear-cell-renal-cell-carcinoma-randomized-open-label-phase-3.html. Accessed 1 June 2024.
- 16.UroToday. ESMO 2023: LITESPARK-013 Phase 2- safety and efficacy of two doses of belzutifan in patients with advanced Renal Cell Carcinoma (RCC). 2023. Available from: https://www.urotoday.com/conference-highlights/esmo-2023/esmo-2023-kidney-cancer/147500-esmo-2023-safety-and-efficacy-of-two-doses-of-belzutifan-in-patients-pts-with-advanced-rcc-results-of-the-randomized-phase-2-litespark-013-study.html. Accessed 1 June 2024.
- 17.Albiges L, Beckermann K, Miller WH, Goh JC, Gajate P, Harris CA, Suárez C, Peer A, Park SH, Stadler WM. Belzutifan plus Lenvatinib for patients (pts) with advanced clear cell renal cell carcinoma (ccRCC) after progression on a PD-1/L1 and VEGF inhibitor: preliminary results of arm B5 of the phase 1/2 KEYMAKER-U03B study. J Clin Oncol. 2023;41:4553–4553. [Google Scholar]
- 18.Haase VH. The VHL tumor suppressor: master regulator of HIF. Curr Pharm Design. 2009;15:3895–903. 10.2174/138161209789649394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navani V, Heng DYC. Treatment selection in first-line metastatic renal cell carcinoma-the Contemporary Treatment paradigm in the age of combination therapy: a review. JAMA Oncol. 2022;8:292–9. 10.1001/jamaoncol.2021.4337. [DOI] [PubMed] [Google Scholar]
- 20.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116–27. 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1103–15. 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U, Shah AY, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2021;384:829–41. 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ, et al. Lenvatinib plus Pembrolizumab or Everolimus Adv Ren Cell Carcinoma. 2021;384:1289–300. 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, McDermott DF, Escudier B, Burotto M, Choueiri TK, Hammers HJ, Barthélémy P, Plimack ER, Porta C, George S, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022;128:2085–97. 10.1002/cncr.34180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albiges L, Fay AP, Xie W, Krajewski K, McDermott DF, Heng DY, Dariane C, DeVelasco G, Lester R, Escudier B, et al. Efficacy of targeted therapies after PD-1/PD-L1 blockade in metastatic renal cell carcinoma. Eur J cancer (Oxford England: 1990). 2015;51:2580–6. 10.1016/j.ejca.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Nadal R, Amin A, Geynisman DM, Voss MH, Weinstock M, Doyle J, Zhang Z, Viudez A, Plimack ER, McDermott DF, et al. Safety and clinical activity of vascular endothelial growth factor receptor (VEGFR)-tyrosine kinase inhibitors after programmed cell death 1 inhibitor treatment in patients with metastatic clear cell renal cell carcinoma. Annals Oncology: Official J Eur Soc Med Oncol. 2016;27:1304–11. 10.1093/annonc/mdw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells JC, Dudani S, Gan CL, Stukalin I, Azad AA, Liow E, Donskov F, Yuasa T, Pal SK, De Velasco G, et al. Clinical effectiveness of second-line Sunitinib following Immuno-Oncology Therapy in patients with metastatic renal cell carcinoma: a real-world study. Clin Genitourin Cancer. 2021;19:354–61. 10.1016/j.clgc.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1814–23. 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Procopio G, Claps M, Pircher C, Porcu L, Sepe P, Guadalupi V, De Giorgi U, Bimbatti D, Nolè F, Carrozza F, et al. A multicenter phase 2 single arm study of cabozantinib in patients with advanced or unresectable renal cell carcinoma pre-treated with one immune-checkpoint inhibitor: the BREAKPOINT trial (Meet-Uro trial 03). Tumori. 2023;109:129–37. 10.1177/03008916221138881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J, Hariharan S, et al. Axitinib versus Sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–62. 10.1016/s1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 31.Ornstein MC, Pal SK, Wood LS, Tomer JM, Hobbs BP, Jia XS, Allman KD, Martin A, Olencki T, Davis NB, et al. Individualised axitinib regimen for patients with metastatic renal cell carcinoma after treatment with checkpoint inhibitors: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2019;20:1386–94. 10.1016/s1470-2045(19)30513-3. [DOI] [PubMed] [Google Scholar]
- 32.Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–82. 10.1016/s1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 33.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–13. 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKay RR, McGregor BA, Xie W, Braun DA, Wei X, Kyriakopoulos CE, Zakharia Y, Maughan BL, Rose TL, Stadler WM, et al. Optimized management of Nivolumab and Ipilimumab in Advanced Renal Cell Carcinoma: a response-based phase II study (OMNIVORE). J Clin Oncology: Official J Am Soc Clin Oncol. 2020;38:4240–8. 10.1200/jco.20.02295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimm MO, Esteban E, Barthélémy P, Schmidinger M, Busch J, Valderrama BP, Schmitz M, Schumacher U, Baretton GB, Duran I. Efficacy of nivolumab/ipilimumab in patients with initial or late progression with nivolumab: updated analysis of a tailored approach in advanced renal cell carcinoma (TITAN-RCC). J Clin Oncol. 2021;39:4576–4576. [Google Scholar]
- 36.Choueiri TK, Kluger HM, George S, Tykodi SS, Escudier B. FRACTION-RCC: innovative, high-throughput assessment of nivolumab + ipilimumab for treatment-refractory advanced renal cell carcinoma (aRCC). J Clin Oncol. 2020;38:5007–5007. [Google Scholar]
- 37.Lee CH, Shah AY, Rasco D, Rao A, Taylor MH, Di Simone C, Hsieh JJ, Pinto A, Shaffer DR, Girones Sarrio R, et al. Lenvatinib plus Pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 2021;22:946–58. 10.1016/s1470-2045(21)00241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albiges L, Barthélémy P, Gross-Goupil M, Negrier S, Needle MN, Escudier B. TiNivo: safety and efficacy of tivozanib-nivolumab combination therapy in patients with metastatic renal cell carcinoma. Annals Oncology: Official J Eur Soc Med Oncol. 2021;32:97–102. 10.1016/j.annonc.2020.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, Oudard S, Else T, Maranchie JK, Welsh SJ, et al. Belzutifan for Renal Cell Carcinoma in Von Hippel-Lindau Disease. N Engl J Med. 2021;385:2036–46. 10.1056/NEJMoa2103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shurin MR, Umansky V. Cross-talk between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy. J Clin Investig. 2022;132. 10.1172/jci159473. [DOI] [PMC free article] [PubMed]
- 41.Wu Q, You L, Nepovimova E, Heger Z, Wu W, Kuca K, Adam V. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol. 2022;15:77. 10.1186/s13045-022-01292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lequeux A, Noman MZ, Xiao M, Sauvage D, Van Moer K, Viry E, Bocci I, Hasmim M, Bosseler M, Berchem G, et al. Impact of hypoxic tumor microenvironment and tumor cell plasticity on the expression of immune checkpoints. Cancer Lett. 2019;458:13–20. 10.1016/j.canlet.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Rini BI, Appleman LJ, Figlin RA, Plimack ER, Merchan JR, Wang K, Thamake S, Zojwalla NJ, Choueiri TK, Mcdermott DF. Results from a phase I expansion cohort of the first-in-class oral HIF-2α inhibitor PT2385 in combination with nivolumab in patients with previously treated advanced RCC. J Clin Oncol. 2019;37:558–558. [Google Scholar]
- 44.Motzer RJ, Jonasch E, Boyle S, Carlo MI, Manley B, Agarwal N, Alva A, Beckermann K, Choueiri TK, Costello BA, et al. NCCN guidelines insights: kidney Cancer, Version 1.2021. J Natl Compr Cancer Network: JNCCN. 2020;18:1160–70. 10.6004/jnccn.2020.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powles T. Recent eUpdate to the ESMO Clinical Practice guidelines on renal cell carcinoma on cabozantinib and nivolumab for first-line clear cell renal cancer: renal cell carcinoma: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Annals Oncology: Official J Eur Soc Med Oncol. 2021;32:422–3. 10.1016/j.annonc.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Manneh R, Lema M, Carril-Ajuria L, Ibatá L, Martínez S, Castellano D, de Velasco G. Immune checkpoint inhibitor combination therapy versus sunitinib as first-line treatment for favorable-IMDC-risk advanced renal cell carcinoma patients: a meta-analysis of randomized clinical trials. Biomedicines. 2022;10. 10.3390/biomedicines10030577. [DOI] [PMC free article] [PubMed]
- 47.Choi WW, Boland JL, Kalola A, Lin J. Belzutifan (MK-6482): Biology and Clinical Development in Solid tumors. Curr Oncol Rep. 2023;25:123–9. 10.1007/s11912-022-01354-5. [DOI] [PubMed] [Google Scholar]
- 48.Shepherd STC, Drake WM, Turajlic S. The road to systemic therapy in von Hippel-Lindau (VHL) disease: Are we there yet? Eur J Cancer. 2023;182:15–22. 10.1016/j.ejca.2022.12.011. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.