Abstract
Background
Blake’s pouch cyst (BPC) is a midline cystic anomaly of the posterior fossa. BPC has been shown to have a risk of aneuploidy prenatally. Copy number variation (CNV) and/or genetic syndromes have been reported in a few prenatal/postnatal cases with BPC. The purpose of this study is to determine the additional value of CNV analysis for prenatal diagnosis and prognosis evaluation of BPC.
Methods
We reviewed the sonographic findings and genetic results of BPC diagnosed within 6 years at our center. Patients were classified into the isolated and non-isolated groups based on the prenatal and postnatal imaging. We analyzed the chromosomal abnormalities by conventional karyotype analysis combined with chromosomal microarray analysis (CMA) or CNV sequencing (CNV-seq).
Results
We recruited 467 low-risk fetuses as the control group to establish normal references of vermian area and brainstem-vermis (BV) angle. Prenatal/postnatal MRI or neonatal neurosonography was used as diagnostic criteria. 34 patients were diagnosed as BPC, including 21 (61.8%) patients with non-isolated and 13 (38.2%) with isolated. Twenty-two patients underwent CMA/CNV-seq, among them 14 patients were performed both CMA/CNV-seq and karyotype analysis. Seven (7/22, 31.8%) patients with BPC had chromosomal abnormalities, including 3 (3/22, 13.6%) patients with chromosomal aneuploidy - trisomy 21, 18 and 13, and 4 (4/22, 18.2%) patients had pathogenic CNVs located at 3p, 9p, Xp/Xq and 7p. Anomalies in fetal heart (35.3%), central nervous system (CNS) (26.5%) and limb (14.7%) were the three top anomalies accompanying BPC.
Conclusions
CNV analysis could provide some additional information for prenatal diagnosis and prognosis counseling for patients with non-isolated BPC. And, it adds less value for patients with isolated BPC, however, isolated BPC can be a soft marker for aneuploidy.
Keywords: Blake’s pouch cyst, Copy number variation, Genetics, Ultrasound, Fetus, Posterior fossa anomaly
Background
Blake’s pouch cyst (BPC), first described as a separate entity by Tortori-Donati in 1996 [1], is a midline cystic anomaly of the posterior fossa. It represents an outpouching of the fourth ventricle into the cisterna magna, which is thought to result from delayed or failed perforation of the roof of the fourth ventricle [2]. The persistent cyst stays with an open fourth ventricle until the lateral foramina of Luschka is opened and normal cerebrospinal fluid circulation is established. On neuroimaging, BPC is characterized by upward rotation of the normal vermis with a normal posterior fossa and tentorium [3]. The classic characteristic in axial transcerebellar plane is the “keyhole” sign [2]. The accurate incidence of BPC remains unknown.
A few studies have reported that BPC is associated with a risk of chromosomal anomalies, mainly trisomy 21, 18 and 13 [4–6]. These studies are either with a small sample size or merely with karyotype analysis. Copy number variation (CNV) and/or genetic syndromes are associated with postnatal BPC [7–9]. However, data on CNV and/or genetic syndromes in prenatal cases are lacking, and the genetic etiology of BPC has not been sufficiently documented.
Isolated BPC is defined as BPC without any associated malformations, and some researchers have speculated that it is a normal anatomical variant [10]. Approximately 95–100% of fetuses with isolated BPC have normal neurodevelopmental outcomes [3, 11], however, BPC may also associate with some malformations. A limited number of studies have shown that BPC was accompanied by other malformations [4, 6, 11, 12], the risk of chromosomal anomalies was estimated to be higher for non-isolated BPC. However, for the small sample size, the associated anomalies and the rate of chromosomal abnormalities were not fully described in these publications.
Therefore, we aimed to determine the role of CNV analysis in prenatal BPC patients with a relatively large sample size and describe the associated anomalies in detail.
Methods
This single-center retrospective study was conducted between August 2015 to November 2021 with permission of the Institutional Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University (Approval No. 2017-KY-076-01).
The inclusion criteria for 467 low-risk fetuses were as follows. The gestational age (GA) was calculated from the last menstrual period and confirmed using the crown-rump length at 11–14 gestational weeks. All pregnancies were singleton and all patients did not have any medical complications or congenital malformations. None of the fetuses had any structural malformations or genetic disorders; all infants had an Apgar score of 10 at birth and were not associated with any abnormality.
Moreover, 34 cases of BPC were included, the diagnoses were confirmed by prenatal/postnatal magnetic resonance imaging (MRI) and/or neonatal neurosonography. The definition of BPC was based on that provided by D’Antonio [13] as follows (Fig. 1): (1) the “keyhole” sign in the axial plane of the cerebellum; (2) intact or nearly intact vermis with visualization of at least two anatomical landmarks (primary fissure and fastigium), the brainstem-vermis (BV) angle above the 95th percentile and the vermian area above the 5th percentile of the control values in the midsagittal plane.
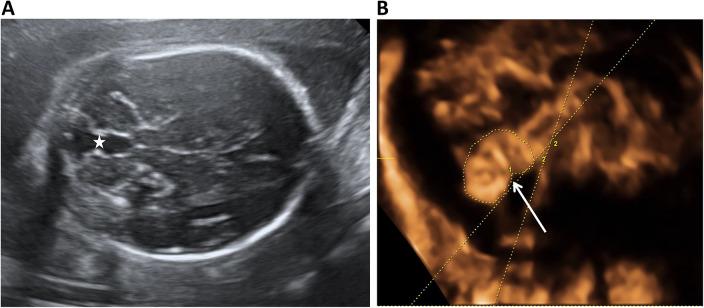
Fig. 1.
Classic ultrasonographic features of a Blake’s pouch cyst (BPC) (A) The “keyhole” sign (☆) in the axial plane (B) Mild rotation of an intact vermis (arrow) with a normal tentorium and posterior fossa. Measurements of the brainstem-vermis angle (angle between two lines) and surface area of vermis (manual tracing by calliper) are demonstrated in (B)
All sonographic examinations were performed using the Voluson E8 (C1-4, RAB-6D and RIC5-9-D) and Samsung WS80A (CA1-7 A, CV1-8 A and V5-9) ultrasound devices with trans-abdominal and trans-vaginal transducers. All patients underwent three-dimensional (3D) ultrasonography and neurosonography. The examinations and assessments were conducted by two sonographers (Dr. Guo and Dr. Sun) with more than 5-year experience on 3D ultrasound and neurosonography. Transvaginal approach was conducted when the fetuses were in cephalic presentation with patients’ permission. The surface area of the vermis was measured by tracing the contour of the vermis, BV angle and brainstem-tentorium (BT) angle were measured using Volpe’s method [14] in the midsagittal view. All measurements were conducted thrice and the average value was recorded. The measurement techniques and 15 BPC cases have been reported in our previous study [15].
Conventional karyotype analysis and CNV analysis including chromosomal microarray analysis (CMA) or CNV sequencing (CNV-seq) were conducted prenatally using an invasive procedure or by abortion tissue after termination. Samples of amniotic fluid or umbilical cord blood were acquired by an obstetrician with informed consent from the mother and her partner.
We followed up the patients who chose to continue their pregnancies every 2–4 weeks with neurosonography until birth. Fetal MRI and/or postnatal MRI or neonatal neurosonography were performed when possible. Perinatal and postnatal outcomes were collected from the electronic medical records or telephone interviews. Postnatal development was determined according to the Chinese version of the Gesell Development Diagnosis Scale that incorporated five principal domains, viz. adaptability, gross motor, fine motor, language and social-emotional responses. A development quotient (DQ) ≥ 85 was deemed normal and a DQ<75 was considered as deficient. The follow-up period ranged from 1 to 3 years.
Data were analyzed using IBM SPSS statistics 24.0 (Inc., Chicago, IL). Continuous data were presented as the median/mean ± standard deviation (SD), and categorical data were presented as numbers (n) and percentages (%). The 5th, 50th, and 95th percentiles of normal vermian measurements were also calculated in the controls.
Results
A total of 467 fetuses were included as the control group. The mean ± SD, 5th, 50th, and 95th percentiles of the surface area of the vermis in the control group were presented in Table 1. The BV and BT angles were relatively stable during pregnancy with 95% confidence intervals (CIs) of 2–12° and 27–42°, respectively. BPC was defined as a BV angle exceeding 12° and surface area of the vermis above the 5th percentile of the normal value.
Table 1.
Vermian measurements of 467 fetuses in the control group by 3D ultrasound and neurosonography
| Gestational age (weeks) | Number of Fetuses (n) | Mean ± SD | Vermian Area, cm2 | ||
|---|---|---|---|---|---|
| 5% | 50% | 95% | |||
| 20 | 13 | 0.59 ± 0.06 | 0.50 | 0.60 | 0.69 |
| 21 | 12 | 0.84 ± 0.13 | 0.59 | 0.83 | 1.05 |
| 22 | 59 | 1.14 ± 0.19 | 0.86 | 1.12 | 1.48 |
| 23 | 70 | 1.24 ± 0.15 | 0.96 | 1.24 | 1.54 |
| 24 | 18 | 1.43 ± 0.17 | 1.24 | 1.39 | 1.78 |
| 25 | 21 | 1.58 ± 0.18 | 1.38 | 1.54 | 1.97 |
| 26 | 22 | 1.66 ± 0.26 | 1.27 | 1.85 | 2.14 |
| 27 | 20 | 1.99 ± 0.31 | 1.62 | 1.92 | 2.71 |
| 28 | 15 | 2.25 ± 0.20 | 1.97 | 2.22 | 2.67 |
| 29 | 21 | 2.40 ± 0.25 | 1.99 | 2.35 | 2.78 |
| 30 | 50 | 2.67 ± 0.25 | 2.36 | 2.65 | 3.09 |
| 31 | 44 | 2.84 ± 0.24 | 2.50 | 2.84 | 3.27 |
| 32 | 22 | 2.88 ± 0.31 | 2.53 | 2.84 | 3.26 |
| 33 | 29 | 3.08 ± 0.27 | 2.80 | 3.00 | 3.52 |
| 34 | 25 | 3.08 ± 0.33 | 2.70 | 2.98 | 3.80 |
| 35 | 26 | 3.29 ± 0.40 | 2.70 | 3.29 | 3.86 |
The background characteristics of 34 fetuses with BPC were demonstrated in Table 2. Chromosomal abnormalities were observed in 7 (7/22, 31.8%) patients, including 3 patients with aneuploidy and 4 with pathogenic CNVs (Table 3). Patient 1 had isolated BPC with trisomy 21. Patient 2 had trisomy 18 + mar, with choroid plexus cysts, partial agenesis of the corpus callosum (pACC), small for gestational age (SGA), and club feet. Patient 3 had trisomy 13, with exomphalos, SGA, and coarctation of the aorta. Pathogenic CNVs were detected in patients 4, 5, 6, and 7, including duplications and deletions located at loci3p, 9p, Xp/Xq (Figs. 2) and 7p (Fig. 3). Common ultrasonographic characteristics such as ventricular septal defect (VSD) were found in patients 4 and 6, and mild ventriculomegaly and abnormal cavum septi pellucidi were observed in patients 5 and 7. However, no pathogenic CNV in the same location were found.
Table 2.
Clinical characteristics of all participants diagnosed with BPC
| Variables | Isolated group | Non-isolated Group |
|---|---|---|
| Number (n%) | 13 (38.2%) | 21 (61.8%) |
| Maternal age (median, range) | 31.7 (21–43) | 31.4 (26–44) |
| Nulliparous (n%) | 54.5 | 61.9 |
| Gestational age at diagnosis (median, range) | 25.6 (22–32) | 24.1 (21–29) |
| Testing items (n) | ||
| CMA/CNV-seq | 2 | 6 |
| Karyotyping + CMA/CNV-seq | 3 | 11 |
Abbreviations: CNV-seq, copy number variation sequencing; CMA, chromosomal microarray analysis
Table 3.
Positive genetic results in 7 of 22 patients with BPC in this study
| Case N | Maternal age | GA | Associated anomalies | Testing items | Genetic results | Size | Genetic categorization | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 22 | none | Karyotype analysis + CMA | 47, XN, + 21 | - | - | TOP |
| 2 | 37 | 23 | pACC, choroid plexus cysts, club foot, SGA | Karyotype analysis + CNV-seq | 48, XN, + 18, +mar | - | - | TOP |
| 3 | 32 | 29 | CoA, PLSVC, exomphalos, SGA | Karyotype analysis + CMA | 47, XN, + 13 | - | - | TOP |
| 4 | 36 | 22 | VSD and polyhydramnios | CMA |
dup(3p26.3p22.1) del(9p24.3.3p24.1) |
41.2 Mb 7.9 Mb |
pathogenic pathogenic |
TOP |
| 5 | 29 | 23 | ACC, ventriculomegaly | CNV-seq |
del(Xp11.23p22.33) del(Xq13.2q28) |
45.0 Mb 82.11 Mb |
pathogenic pathogenic |
TOP |
| 6 | 31 | 23 | VSD, protruded forehead, adducted and fixed thumbs | Karyotype analysis + CNV-seq | del(7)(p21.1p13) | 26.78 Mb | pathogenic | TOP |
| 7* | 33 | 23 | narrow CSP, ventriculomegaly | CNV-seq | dup(9)(p24.3-13.1) | 38.58 Mb | pathogenic | TOP |
Abbreviations: CoA, coarctation of aorta; VSD, ventricular septal defect; pACC, partial agenesis of corpus callosum; SGA, small for gestational age; TOP, termination of pregnancy; CSP, cavity of septum pellucidum; CMA, chromosomal microarray analysis; CNV-seq, copy number variation sequencing
* Case 7 had 6 pathogenic duplications located at chr9, the segment sizes ranged from 0.56 Mb to 17.42 Mb. The size listed was the total size
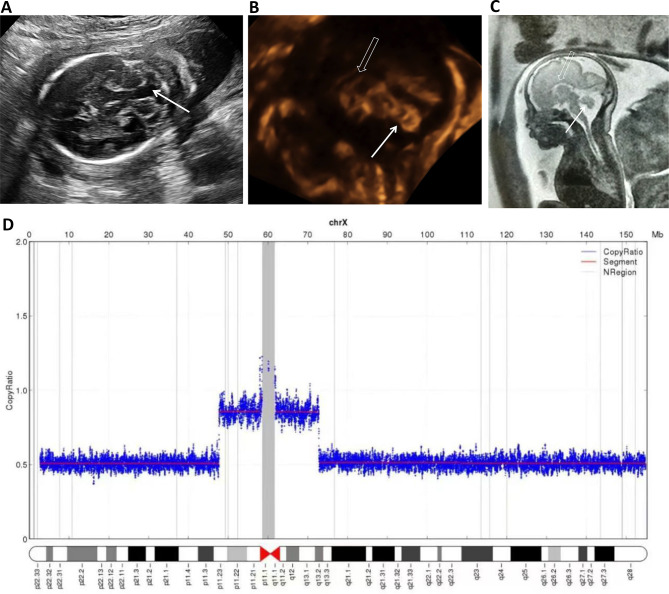
Fig. 2.
A BPC associated with ACC. (A) At 22 weeks, the “keyhole” sign (arrow) was observed in the axial plane. (B) In the midsagittal plane, the normal-sized vermis (arrow) was elevated with a surface area of 1.2 cm2, and a normal corpus callosum was not seen (hollow arrow). (C) Fetal MRI showed the similar vermis (arrow) and agenesis of corpus callosum (hollow arrow) at 24 gestational weeks. (D) CNV analysis showed deletions in Xp11.23p22.33 and Xq13.2q28, probably it was a ring of X chromosome, and mosaic monosomy X (45,X/46,X, r(X)(p11.23q13.2)) may be present. Karyotype analysis was not performed. BPC: Blake’s pouch cyst, MRI: magnetic resonance imaging, ACC: agenesis of the corpus callosum
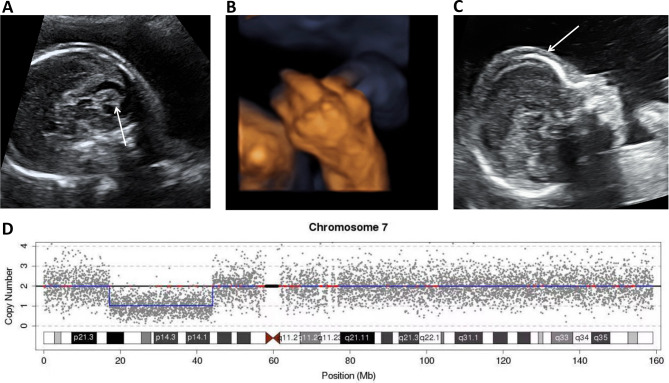
Fig. 3.
A BPC associated with adducted thumbs and a protruding forehead at 23 weeks. (A) In the midsagittal plane, a normal-sized vermis (arrow) appeared elevated with a surface area of 1.1 cm2. (B) Three-dimensional ultrasound showed adducted and fixed thumbs. (C) The sagittal profile showed a protruding forehead and edema. (D) Genetic testing showed deletion in the 7p21.1p13 segment, indicating pathogenic variance. BPC: Blake’s pouch cyst
The associated malformations of BPC included cardiac anomalies in 12 (35.3%), other cerebral abnormalities in 9 (26.5%), limb abnormalities in 5 (14.7%), and other anomalies (Table 4). VSD was the most common extra-CNS anomaly, and pACC/ACC was the most common anomaly in CNS. Interestingly, we noted that limb abnormalities ranked the third among associations, especially club foot. And SGA/FGR was not unusual in other associated anomalies.
Table 4.
Associated findings in 34 fetuses with BPC
| Associated anomalies | n (%) |
|---|---|
| Presence of associated anomalies | |
| Isolated | 13 (38.2) |
| Non-isolated | 21 (61.8) |
| Cardiac anomalies | 12 (35.3) |
| VSD | 8 |
| CoA or aortic valve stenosis | 5 |
| PLSVC | 3 |
| Suspected cardiomyopathy | 2 |
| Pulmonary stenosis | 2 |
| Persistent truncus arteriosus | 1 |
| CNS anomalies | 9 (26.5) |
| pACC/ACC | 6 |
| Ventriculomegaly/hydrocephaly | 6 |
| Choroid plexus cyst | 3 |
| Midline cyst | 2 |
| Limb abnormalities | 5 (14.7) |
| club foot | 3 |
| Polydactyly/Syndactyly | 1 |
| Adducted and fixed thumbs | 1 |
| Overlapping fingers | 1 |
| Other | 5 (14.7) |
| SGA/FGR | 3 |
| Renal dysplasia | 2 |
| Cleft lip and palate | 1 |
| Exomphalos | 1 |
| Single umbilical artery | 1 |
Abbreviations: BPC, Blake’s pouch cyst; CoA, coarctation of aorta; VSD, ventricular septal defect; PLSVC, persistent left superior vena cava; pACC, partial agenesis of corpus callosum; SGA, small for gestational age; FGR, fetal growth restriction
Twenty patients with BPC underwent fetal MRI; the results of 18 patients were consistent with BPC. One fetus (Fig. 4) was diagnosed with DWM by the first fetal MRI due to the elevated tentorium and slightly smaller vermis, but the diagnosis was corrected as BPC by second fetal MRI. Another case was diagnosed as BPC associated with vermian hypoplasia by fetal MRI; however, follow-up sonography revealed that the cyst had regressed in utero. Both babies did well after birth. Postnatal MRI was performed in 2 babies and neonatal neurosonography was conducted in 4 babies, all confirming an intact vermis. BPC was absorbed in 10 fetuses in utero.
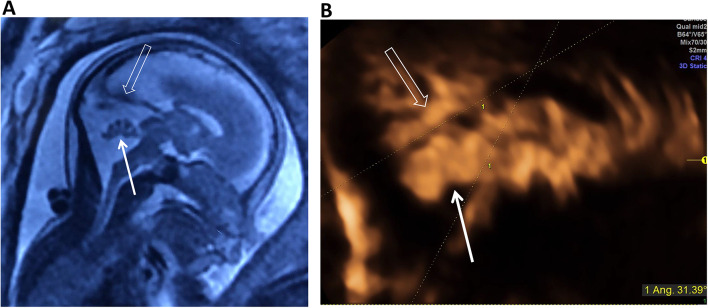
Fig. 4.
An isolated BPC with elevated tentorium at 22 gestational weeks. (A) an intact vermis (arrow) moderately rotated with brainstem-vermis (BV) angle of 34°and the tentorium (hollow arrow) was elevated with brainstem-tentorium (BT) angle of 46°at 22 weeks. (B) vermis (arrow) was still rotated with BV angle of 33°and the tentorium returned in normal position with BT angle of 31.4° at 30 weeks. Results of karyotype analysis, CNV analysis, and WES were all negative. The baby did well at the followup of 1.5 years old. BPC, Blake’s pouch cyst; DWM, Dandy-Walker malformation; CNV, copy number variation; WES, whole-exome sequencing
Seventeen fetuses were terminated due to severe malformations and/or genetic anomalies. Sixteen fetuses including 12 isolated and 4 non-isolated BPC, were born and thrived well, except one baby. The baby was prenatally diagnosed as isolated BPC, however, postnatal examinatons revealed that he had polydactyly and syndactyly. Postnatal MRI confirmed an intact vermis and suspected kernicterus, the DQ was all below 75 for five items at 11 months, and he could not walk at 18 months. Regarding the genetic testing of the baby, both prenatal CNV analysis by amniocentesis and postnatal Trio-WES by peripheral blood did not show any pathogenic or likely pathogenic variants. One fetus with trisomy 13 suffered intrauterine death at 33 weeks GA.
Discussion
CNV analysis could provide some additional information for prenatal diagnosis and prognosis counseling for patients with non-isolated BPC. The overall rate of chromosomal aberrations was 31.8% (7/22), which was higher than that reported in the literature [13]. Firstly, in previous studies authors mainly conducted karyotype analysis, and in our study we performed karyotype analysis and/or CNV analysis. Moreover, most cases in our study had multiple malformations, those isolated BPC especially those without classic ‘keyhole’ sign were not included. The karyotype analysis seemed inadequate for non-isolated BPC. Although in previous studies [6, 16], aneuploidy can be present in isolated BPC, we also detected an isolated BPC with trisomy 21. By reviewing the literature, we found two fetuses with non-isolated BPC had pathogenic CNVs located at 6p and 9p [5], and 1q21 [17]. BPC has been reported in some postnatal chromosomal aberrant syndromes [7–9], including deletions in 13q, 5q, and 11p. In our study, the distribution of pathogenic CNVs were scattered including duplications and deletions located at loci3p, 9p, Xp, Xq, and 7p, which indicated the genetic heterogeneity of BPC. Although most pathogenic CNVs detected in our study are large fragments, which can also be detected by karyotyping. CNV analysis has the advantage of being fast and efficient comparing with karyotype analysis. The value of CNV analysis in prenatal BPC has not been sufficiently explored.
CNV analysis may add less value with isolated BPC cases. In our study, no pathogenic CNVs were detected in isolated BPC. We agree with the authors who deem isolated BPC as a normal anatomical variant. Here we assume that BPC is an underlying ultrasonographic soft marker in prenatal diagnosis. It can disappear in utero, and the presence of BPC may indicate a combination of some structural abnormalities and increase the risk of chromosomal abnormalities. When a BPC is suspected prenatally, systematic ultrasonography and targeted neurosonography should be provided to exclude the associated anomalies. Although the value of CNV analysis seems limited for isolated BPC, karyotype analysis is always recommended, for isolated BPC can be a marker for aneuploidy. Based on the results of the previous studies [18, 19], the patients and their physicians should also discuss the possibilities of abnormal CMA/ WES (whole-exome sequencing) findings in normal pregnancies and especially with apparently benign findings, such as BPC.
In this study, the incidence of associated anomalies was calculated to be 61.8% (21/34), which seemed higher than the previous studies. For one thing, just a small number of cases (n = 4–19) were included in previous studies which mentioned the associated anomalies [4–6, 20]. There was one study with relatively a large sample size (n = 32) showing the similar result with our study [11]. On the other hand, the cases we included were all with typical ‘keyhole’ sign, and some atypical cases may have a less risk of combination with other anomalies. Consistent with the previous study [6], cardiac anomalies were the most commonly detected associations, among them, VSD ranked first. And CNS anomalies, mainly ACC/pACC, were also common in patients with BPC. Notably, limb abnormalities, mainly club foot, were the third most associated malformations, which has rarely been mentioned in the literature. BPC may be not as isolated as we believed before, since some associated anomalies could be detected after birth.
Sometimes, it may be not easy to distinguish BPC from other posterior fossa anomalies. BPC could result in compression and elevation of the vermis, which resembles vermian hypoplasia on imaging [21, 22]. The tentorium is usually located in the normal position in patients with BPC, but can be also elevated [23]. When the compression of the cyst is eliminated, the vermis can be fully displayed, and the tentorium returns to a normal position on imaging. That was why we included nearly intact vermes rather than normal vermes in the study group. Under these circumstances, genetic testing, especially CNV analysis, plays an important role in prognosis evaluation and parental counseling.
As reported, isolated BPC has favorable outcomes [10]. In our study, 12 in 13 patients with isolated BPC showed normal neurodevelopment. One of isolated BPC cases turned out to be trisomy 21, the maternal age was 40 years old. We should keep in mind that isolated BPC may be a soft marker for aneuploidy, especially in the high-risk pregnancies. Consistent with the previous studies [4, 10, 11], approximately one-third cases with BPC undergo resolution in utero. Accordingly, regular ultrasonographic follow-ups in utero are strongly recommended. Corresponding with other study [2], one in four babies with non-isolated BPC showed abnormal neurodevelopment in our study. Prenatally diagnosed BPC may not be as benign as believed. Genetic studies including karyotype analysis and CNV analysis should be offered for non-isolated BPC.
The strengths of our study are as follows. This is the first study to investigate CNV analysis for prenatal BPC obtained from a relatively large sample population in China. And, the anomalies associated with BPC were analyzed in detail. Our study also has limitations. Firstly, it did not include a pathological analysis of BPC from fetuses which were terminated, although fetal MRI was performed. Moreover, the follow-up periods were mainly limited to the first year after birth, although some clinical symptoms have been reported in childhood or even in adulthood.
Conclusions
CNV analysis is helpful for prenatal diagnosis and prognosis counseling for patients with non-isolated BPC. While for isolated BPC cases, CNV analysis has less value. However, BPC may be a soft marker for aneuploidy. The malformations associated with BPC are common on prenatal imaging, which mainly occur in the CNS, cardiovascular system, and limbs.
Acknowledgements
We thank all patients participating the study.
Abbreviations
- BPC
Blake’s pouch cyst
- CNV
Copy number variation
- CMA
Chromosomal microarray analysis
- CNV-seq
CNV sequencing
- WES
Whole-exome sequencing
- CNS
Central nervous system
- GA
Gestational age
- BV
Brainstem-vermis
- 3D
Three-dimensional
- BT
Brainstem-tentorium
- DQ
Development quotient
- CIs
Confidence intervals
- pACC
Partial agenesis of the corpus callosum
Author contributions
GCX collected the data and wrote the main manuscript. SLJ and WQQ revised the manuscript. LY and YYS analyzed and interpreted the patient data regarding the genetic test. WL collected the data. WXL analyzed and interpreted the MR imaging of the patients. All authors read and approved the final manuscript.
Funding
The study was supported by Beijing Natural Science Foundation (7204263), National Natural Science Foundation of China (81701704) and Beijing Municipal Science & Technology Commission (Z211100002921017).
Data availability
The data are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed with the permission of the Institutional Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Written informed consent was obtained from the parents/guardians of all participants.
Consent for publication
Written informed consent was obtained from the patients for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lijuan Sun and Qingqing Wu contributed equally to this work.
Contributor Information
Lijuan Sun, Email: sunlijuan@ccmu.edu.cn.
Qingqing Wu, Email: qingqingwu@ccmu.edu.cn.
References
- 1.Tortori-Donati P, Fondelli MP, Rossi A, Carini S. Cystic malformations of the posterior cranial fossa originating from a defect of the posterior membranous area. Child’s Nerv Syst. 1996;6(12):303–8. 10.1007/BF00301017. [DOI] [PubMed] [Google Scholar]
- 2.Post A, Norton ME, Monteagudo AA. Blake’s Pouch Cyst. Am J Obstet Gynecol. 2020;B47–50. 10.1016/j.ajog.2020.08.187. [DOI] [PubMed]
- 3.D’Antonio F, Khalil A, Garel C, Pilu G, Rizzo G, Lerman-Sagie T, et al. Systematic review and meta-analysis of isolated posterior fossa malformations on prenatal imaging (part 2): neurodevelopmental outcome. Ultrasound Obst Gyn. 2016;48(1):28–37. 10.1002/uog.15755. [DOI] [PubMed] [Google Scholar]
- 4.Behram M, Oğlak SC, Ölmez F, Gedik Özköse Z, Süzen Çaypınar S, Başkıran Y, et al. Blake’s pouch cyst: prenatal diagnosis and management. J Turkish Soc Obstetric Gynecol. 2021;18(1):44–9. 10.4274/tjod.galenos.2020.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei T, Feng J, Xie Y, Xie H, Zheng J, Lin M. Chromosomal aneuploidies and copy number variations in posterior fossa abnormalities diagnosed by prenatal ultrasonography. Prenatal Diag. 2017;37(11):1160–8. 10.1002/pd.5159. [DOI] [PubMed] [Google Scholar]
- 6.Paladini D, Quarantelli M, Pastore G, Sorrentino M, Sglavo G, Nappi C. Abnormal or delayed development of the posterior membranous area of the brain: anatomy, ultrasound diagnosis, natural history and outcome of Blake’s pouch cyst in the fetus. Ultrasound Obst Gyn. 2012;39(3):279–87. 10.1002/uog.10138. [DOI] [PubMed] [Google Scholar]
- 7.Gardiner K, Chitayat D, Choufani S, Shuman C, Blaser S, Terespolsky D, et al. Brain abnormalities in patients with Beckwith-Wiedemann syndrome. Am J Med Genet a. 2012;158A(6):1388–94. 10.1002/ajmg.a.35358. [DOI] [PubMed] [Google Scholar]
- 8.Myers KA, Wallis MJ, Fitt GJ, Sarnat HB, Newton MR. Blake’s pouch cyst in 13q deletion syndrome: posterior fossa malformations may occur due to disruption of multiple genes. Am J Med Genet a. 2017;173(9):2442–5. 10.1002/ajmg.a.38346. [DOI] [PubMed] [Google Scholar]
- 9.Shohoud SA, Azab WA, Alsheikh TM, Hegazy RM. Blake’s pouch cyst and Werdnig-Hoffmann disease: report of a new association and review of the literature. Surg Neurol Int. 2014;5:S282–8. 10.4103/2152-7806.139390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salsi G, Volpe G, Montaguti E, Fanelli T, Toni F, Maffei M, et al. Isolated Upward Rotation of the fetal cerebellar Vermis (Blake’s Pouch Cyst) is a normal variant: an analysis of 111 cases. Fetal Diagn Ther. 2021;48(6):485–92. 10.1159/000516807. [DOI] [PubMed] [Google Scholar]
- 11.Gandolfi Colleoni G, Contro E, Carletti A, Ghi T, Campobasso G, Rembouskos G, et al. Prenatal diagnosis and outcome of fetal posterior fossa fluid collections. Ultrasound Obst Gyn. 2012;39(6):625–31. 10.1002/uog.11071. [DOI] [PubMed] [Google Scholar]
- 12.Hirono S, Ito D, Murai H, Kobayashi M, Suyama M, Fujii K, et al. Postnatal development of Blake’s pouch cyst: a case report and new insight for its pathogenesis. Child’s Nerv Syst. 2014;30(10):1767–71. 10.1007/s00381-014-2458-8. [DOI] [PubMed] [Google Scholar]
- 13.D’Antonio F, Khalil A, Garel C, Pilu G, Rizzo G, Lerman-Sagie T, et al. Systematic review and meta-analysis of isolated posterior fossa malformations on prenatal ultrasound imaging (part 1): nomenclature, diagnostic accuracy and associated anomalies. Ultrasound Obst Gyn. 2016;47(6):690–7. 10.1002/uog.14900. [DOI] [PubMed] [Google Scholar]
- 14.Volpe P, Contro E, De Musso F, Ghi T, Farina A, Tempesta A, et al. Brainstem-vermis and brainstem-tentorium angles allow accurate categorization of fetal upward rotation of cerebellar vermis. Ultrasound Obst Gyn. 2012;39(6):632–5. 10.1002/uog.11101. [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Guo C, Yao L, Zhang T, Wang J, Wang L, et al. Quantitative diagnostic advantages of three-dimensional ultrasound volume imaging for fetal posterior fossa anomalies: preliminary establishment of a prediction model. Prenatal Diag. 2019;39(12):1086–95. 10.1002/pd.5549. [DOI] [PubMed] [Google Scholar]
- 16.Martinez Ten P, Illescas T, Adiego B, Estevez M, Bermejo C, Wong AE, et al. Non-visualization of choroid plexus of fourth ventricle as first‐trimester predictor of posterior fossa anomalies and chromosomal defects. Ultrasound Obst Gyn. 2018;51(2):199–207. 10.1002/uog.17445. [DOI] [PubMed] [Google Scholar]
- 17.Volpe P, Persico N, Fanelli T, De Robertis V, Alessandro D, Boito J. Prospective detection and differential diagnosis of cystic posterior fossa anomalies by assessing posterior brain at 11–14 weeks. Am J Obstet Gynecol MFM. 2019;1(2):173–81. 10.1016/j.ajogmf.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Michal L, Shira L, Mirela G, Lily B, Rayna Joy G, Uri H, et al. Exome sequencing in every pregnancy? Results of trio exome sequencing in structurally normal fetuses. Prenat Diagn. 2024;010.1002/pd.6585. [DOI] [PMC free article] [PubMed]
- 19.Hagit D, Shira S, Shiri S. Is it time for prenatal chromosomal-microarray analysis to all women? A review of the diagnostic yield in structurally normal fetuses. Curr Opin Obstet Gynecol. 2021;33(2). 10.1097/GCO.0000000000000690. [DOI] [PubMed]
- 20.Wüest A, Surbek D, Wiest R, Weisstanner C, Bonel H, Steinlin M, et al. Enlarged posterior fossa on prenatal imaging: differential diagnosis, associated anomalies and postnatal outcome. Acta Obstet Gyn Scan. 2017;96(7):837–43. 10.1111/aogs.13131. [DOI] [PubMed] [Google Scholar]
- 21.Kau T, Marterer R, Kottke R, Birnbacher R, Gellen J, Nagy E, et al. Blakeʼs pouch cysts and Differential diagnoses in prenatal and postnatal MRI. Clin Neuroradiol. 2020;30(3):435–45. 10.1007/s00062-019-00871-4. [DOI] [PubMed] [Google Scholar]
- 22.Kau T, Birnbacher R, Schwärzler P, Habernig S, Deutschmann H, Boltshauser E. Delayed fenestration of Blake’s pouch with or without vermian hypoplasia: fetal MRI at 3 tesla versus 1.5 tesla. Cerebellum Ataxias. 2019;6(0):4. 10.1186/s40673-019-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson MD, Maher K, Gilles FH. A different approach to cysts of the posterior fossa. Pediatr Radiol. 2004;34(9):720–32. 10.1007/s00247-004-1253-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.