Abstract
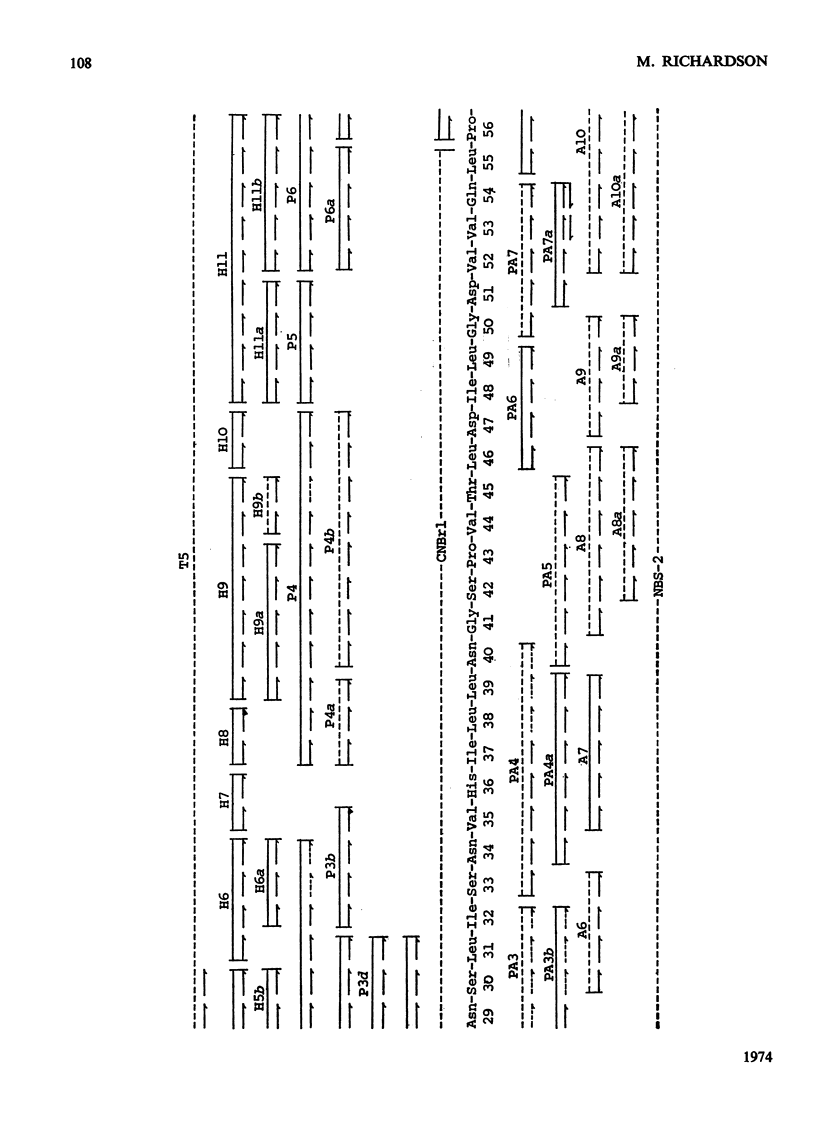
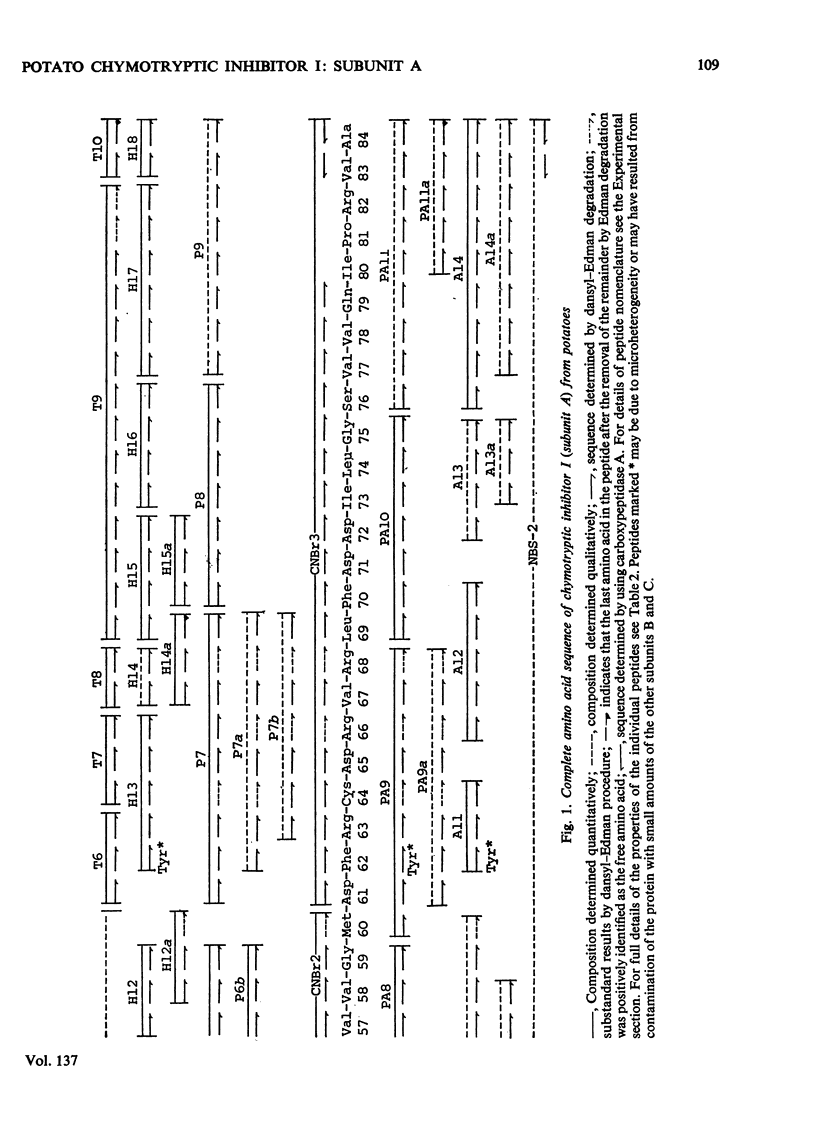
The amino acid sequence of subunit A of the potato chymotryptic inhibitor I was determined. The sequence was deduced from analysis of fragments and peptides derived from the protein by cleavage with cyanogen bromide, N-bromosuccinimide and dilute acid, and by digestion with trypsin, thermolysin, pepsin and papain. The molecule consists of a single polypeptide chain of 84 residues, which contains two homologous regions each of 13 amino acids. The protein does not appear to be homologous with any other known proteinase inhibitors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLS A. K., RYAN C. A. CONCERNING A CRYSTALLINE CHYMOTRYPTIC INHIBITOR FROM POTATOES, AND ITS BINDING CAPACITY FOR THE ENZYME. J Biol Chem. 1963 Sep;238:2976–2982. [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- Hochstrasser K., Werle E., Siegelmann R., Schwarz S. Uber pflanzliche Proteasenhibitorem. V. Isolierung und Charakterisierung einiger polyvalenter Proteaseninhibitoren aus Solanum tuberosum. Hoppe Seylers Z Physiol Chem. 1969 Jul;350(7):897–902. [PubMed] [Google Scholar]
- Hojima Y., Moriya H., Moriwaki C. Studies of kallikrein inhibitors in potatoes. I. Partial purification and some properties. J Biochem. 1971 Jun;69(6):1019–1025. doi: 10.1093/oxfordjournals.jbchem.a129554. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Kiyoara T., Yoshikawa M. Chemical and physicochemical characterization of two different types of proteinase inhibitors (inhibitors II-a and II-b) from potatoes. J Biochem. 1972 Oct;72(4):1029–1035. doi: 10.1093/oxfordjournals.jbchem.a129964. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Kiyohara T., Yoshikawa M. Purification and partial characterization of two different types of proteinase inhibitors (inhibitors II-a and II-b) from potatoes. J Biochem. 1971 Nov;70(5):817–826. doi: 10.1093/oxfordjournals.jbchem.a129699. [DOI] [PubMed] [Google Scholar]
- Kiyohara T., Iwasaki T., Yoshikawa M. Chemical and physicochemical characterization of potato proteinase inhibitor I and comparison of its specificity with those of inhibitors II-a and II-b. J Biochem. 1973 Jan;73(1):89–95. [PubMed] [Google Scholar]
- Koide T., Tsunasawa S., Ikenaka T. The amino acid sequence of soybean trypsin inhibitor (Kunitz). J Biochem. 1972 Jan;71(1):165–167. doi: 10.1093/oxfordjournals.jbchem.a129740. [DOI] [PubMed] [Google Scholar]
- Krahn J., Stevens F. C. Antitrypsin site of lima bean protease inhibitor. Biochemistry. 1972 May 9;11(10):1804–1808. doi: 10.1021/bi00760a011. [DOI] [PubMed] [Google Scholar]
- MCDOWALL M. A., SMITH E. L. DILUTE ACID HYDROLYSIS OF PAPAIN. KINETIC STUDIES AND PEPTIDES FROM THE CARBOXYMETHYLATED PROTEIN. J Biol Chem. 1965 Jan;240:281–289. [PubMed] [Google Scholar]
- McLachlan A. D. Repeating sequences and gene duplication in proteins. J Mol Biol. 1972 Mar 14;64(2):417–437. doi: 10.1016/0022-2836(72)90508-6. [DOI] [PubMed] [Google Scholar]
- Melville J. C., Ryan C. A. Chymotrypsin inhibitor 1 from potatoes: a multisite inhibitor composed of subunits. Arch Biochem Biophys. 1970 Jun;138(2):700–702. doi: 10.1016/0003-9861(70)90399-1. [DOI] [PubMed] [Google Scholar]
- Melville J. C., Ryan C. A. Chymotrypsin inhibitor I from potatoes. Large scale preparation and characterization of its subunit components. J Biol Chem. 1972 Jun 10;247(11):3445–3453. [PubMed] [Google Scholar]
- Odani S., Ikenaka T. Studies on soybean trypsin inhibitors. IV. Complete amino acid sequence and the anti-proteinase sites of Bowman-Birk soybean proteinase inhibitor. J Biochem. 1972 May;71(5):839–848. doi: 10.1093/oxfordjournals.jbchem.a129833. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Ramshaw J. A., Richardson M., Boulter D. The amino-acid sequence of the cytochrome c of Ginkgo biloba L. Eur J Biochem. 1971 Dec 10;23(3):475–483. doi: 10.1111/j.1432-1033.1971.tb01643.x. [DOI] [PubMed] [Google Scholar]
- Rancour J. M., Ryan C. A. Isolation of a carboxypeptidase B inhibitor from potattoes. Arch Biochem Biophys. 1968 Apr;125(1):380–383. doi: 10.1016/0003-9861(68)90675-9. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Chymotrypsin inhibitor I from potatoes: reactivity with mammalian, plant, bacterial, and fungal proteinases. Biochemistry. 1966 May;5(5):1592–1596. doi: 10.1021/bi00869a020. [DOI] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Seidl D. S., Liener I. E. Identification of the trypsin-reactive site of the Bowman-Birk soybean inhibitor. Biochim Biophys Acta. 1971 Oct;251(1):83–93. doi: 10.1016/0005-2795(71)90063-8. [DOI] [PubMed] [Google Scholar]
- Tan C. G., Stevens F. C. Amino acid sequence of lima bean protease inhibitor component IV. 2. Isolation and sequence determination of the chymotryptic peptides and the complete amino acid sequence. Eur J Biochem. 1971 Feb;18(4):515–523. doi: 10.1111/j.1432-1033.1971.tb01271.x. [DOI] [PubMed] [Google Scholar]
- Thompson E. W., Laycock M. V., Ramshaw J. A., Boulter D. The amino acid sequence of Phaseolus aureua L. (mung-bean) cytochrome c. Biochem J. 1970 Mar;117(1):183–192. doi: 10.1042/bj1170183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. A., Laskowski M., Sr Isolation of three isoinhibitors of trypsin from garden bean, Phaseolus vulgaris, having either lysine or arginine at the reactive site. J Biol Chem. 1973 Feb 10;248(3):756–762. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


