Abstract
Chimeric antigen receptor T (CAR-T) cell therapy has shown remarkable success in hematologic malignancies but has encountered challenges in effectively treating solid tumors. One major obstacle is the presence of the immunosuppressive tumor microenvironment (TME), which is mainly built by myeloid-derived suppressor cells (MDSCs). Recent studies have shown that MDSCs have a detrimental effect on CAR-T cells due to their potent immunosuppressive capabilities. Targeting MDSCs has shown promising results to enhance CAR-T immunotherapy in preclinical solid tumor models. In this review, we first highlight that MDSCs increase tumor proliferation, transition, angiogenesis and encourage circulating tumor cells (CTCs) extravasation leading to tumor progression and metastasis. Moreover, we describe the main characteristics of the immunosuppressive activities of MDSCs on T cells in TME. Most importantly, we summarize targeting therapeutic strategies of MDSCs in CAR-T therapies against solid tumors. These strategies include (1) therapeutic targeting of MDSCs through small molecule inhibitors and large molecule antibodies; (2) CAR-T targeting cancer cell antigen combination with MDSC modulatory agents; (3) cytokine receptor antigen-targeted CAR-T indirectly or directly targeting MDSCs reshapes TME; (4) modified natural killer (NK) cells expressing activating receptor directly targeting MDSCs; and (5) CAR-T directly targeting MDSC selective antigens. In the near future, we are expected to witness the improvement of CAR-T cell therapies for solid tumors by targeting MDSCs in clinical practice.
Keywords: Chimeric antigen receptor T (CAR-T), Myeloid-derived suppressor cells (MDSCs), Tumor microenvironment (TME), Solid tumor
Background
The International Agency for Research on Cancer (IARC) projects that close to 20 million new cases of cancer has been diagnosed globally in 2022, which most of these cases are caused by solid tumors [1]. Although immunotherapy of immune checkpoint blockade (ICB) has achieved good therapeutic effects in 20% of cancer patients of a few limited solid tumor types, most patients are distraughtly waiting for a new immunotherapy to treat cancer [2]. A major obstacle faced by tumor immunotherapy is the presence of tumor microenvironment (TME), which consists of a complex network of non-immune microenvironment and immune microenvironment that contains effector immune cells such as effector CD4+ T cells, cytotoxic CD8+ T cells (CTLs), B cells, and NK cells, as well as immunosuppressive cell types such as myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and tumor-associated macrophages (TAMs) [3]. In a few immunologically ‘hot’ tumors, TME highly infiltrated CTLs, which have significant responses to ICBs [4]. But in the most poorly immunogenic ‘cold’ tumors, TME lacks infiltrating CTLs and is full of immunosuppressive myeloid cells [5]. The immunosuppressive TME presents a variety of difficulties, including hypoxia, metabolic reprogramming circumstances, and immunosuppressive signaling that support tumor cell survival, expansion, and metastasis, thus promoting cancer development and progression [6–8]. Therapeutic targeting of TME is becoming a new hope for treating solid cancers.
CAR-T cell therapy is a promising treatment method that is expected to not only supplement CTLs but also target the TME [9]. CAR-T cells are T cells that have been genetically modified to express chimeric antigen receptors on their surface, allowing them to recognize and target specific antigens on cancer cells [10]. CAR-T cell therapy has achieved great advances in hematological tumor treatment but has not had a satisfactory effect on solid tumors [11]. One of the reasons for the significant difference in treatment effectiveness is the presence of immunosuppressive TME in solid tumors [12]. Firstly, the immunosuppressive TME will cause CAR-T cells to infiltrate into solid tumors inefficiently, resulting in inadequate activation of CAR-T cells. Secondly, suppressive immune cells in TME can deplete essential amino acids like L-arginine, cystine, and cysteine, release reactive oxygen species (ROS) and reactive nitrogen species (RNS), secrete large amounts of immunosuppressive cytokines like prostaglandin E2 (PGE2), transforming growth factor beta (TGF-β), interleukin 10 (IL-10), and indole amine 2, 3-dioxygenase (IDO), or express programmed cell death protein 1 (PD-1), programmed death ligand 1 (PD-L1) to inhibit CAR-T cell proliferation and activation, and induce CAR-T cell exhaustion. The development of new CAR-T therapies or combination therapies that can target both tumor cells and TME is currently underway [13–15].
In recent years, targeting MDSCs has gradually become a new research direction and hot field in developing new TME-targeted CAR-T immunotherapy strategies [16]. MDSCs are a heterogeneous population of myeloid cells, which are classified into two main subsets based on their phenotype and function [17]. In mice, polymorphonuclear (PMN)-MDSCs can be defined as CD11b+Ly6G+Ly6Clow cells. Monocytic (M)-MDSCs are defined as CD11b+Ly6G−Ly6Chi cells [18]. MDSCs can positively promote proliferation, survival, and migration of tumor cells but negatively inhibit immunological responses of T cells, NK cells and B cells in TME [19]. Tumor cells drive MDSCs to produce a refuge for tumor growth within the TME as a result of their bidirectional interaction. The accumulation of MDSC was directly associated with cancer outcomes that were not favorable [20]. Meanwhile, MDSCs suppress the immune system and encourage angiogenesis and metastasis, leading to cancer development and progression [21]. Finally, when CAR-T cells enter into tumor tissues, MDSCs directly block CAR-T cell proliferation and activation, and induce CAR-T cell exhaustion through the formation of immunosuppressive TME, thus blocking the cytotoxic attack of CAR-T cells against tumor cells and formation a protective shield for tumor cells [22]. The development of new MDSC-targeted CAR-T immunotherapy is extremely urgent for breaking through the bottleneck of treating solid tumors. In this review, we discuss why MDSCs, as a major component of TME, contribute to tumor progression and metastasis, how MDSCs inhibit T cells and CAR-T cells, and what strategies of targeting MDSCs improve the efficacy of anti-cancer CAR-T therapy.
MDSCs represent a major component of the TME and play a critical role in the regulation of solid tumor progression and metastasis
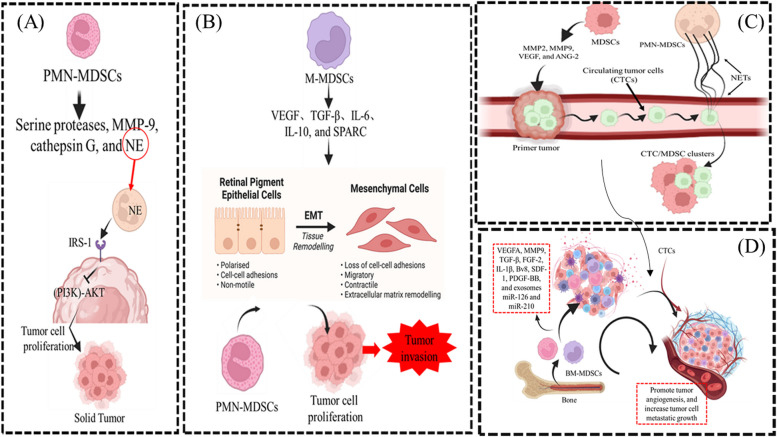
TME is made up of both tumor and non-tumor cells, such as mesenchymal stromal cells, fibroblasts, endothelial cells, Tregs, TAMs, and MDSCs, as well as soluble components. Within the TME, the vast majority of MDSCs (approximately 80%) are PMN-MDSCs, while M-MDSCs only account for 20% of all the MDSCs [23]. However, M-MDSCs rapidly differentiate from TAMs in tumor tissue because hypoxia-inducible factor 1α (HIF-1α) of TME induces the downregulation of signal transducer and activator of transcription 3 (STAT3) phosphorylation in MDSCs [24]. Actually, MDSCs and TAMs constitute the main tumor-associated myeloid cells, accounting for more than 50% of non-tumor cells, thus contributing to the immunosuppressive TME and promoting tumor progression and metastasis [25]. First, MDSCs directly enhance tumor proliferation. PMN-MDSCs release serine proteases, neutrophil elastase (NE), matrix metalloprotease-9 (MMP-9), and cathepsin G to directly upregulate tumor proliferation. Specifically, PMN-MDSCs secrete NE into TME, which is subsequently endocytosed by tumor cells through binding to insulin receptor substrate-1 (IRS-1). This ligation removes the inhibitory effect of IRS-1 on the phosphatidylinositol 3-kinase (PI3K)-AKT signaling pathways of tumor cells, thus enhancing tumor cell proliferation [26, 27]. Second, MDSCs promote tumor migration and invasion by regulating the epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET) of tumor cells. Vascular endothelial growth factor (VEGF), TGF-β, IL-6, IL-10, and secreted protein acidic and rich in cysteine (SPARC) produced by MDSCs induce EMT of tumor cells, and then mesenchymal tumor cells lose their intercellular junctions to acquire high migratory and invasive abilities [28, 29]. However, deletion of SPARC rendered MDSCs with reduced suppressive function and restored the EMT of tumor cells. In addition to participating in the induction of EMT, when circulating tumor cells (CTCs) reach the host target organ, MDSCs will induce the MET process of tumor cells by secreting versican to attenuate Smad2 phosphorylation levels [30]. In this way, induction of EMT by M-MDSCs promotes tumor invasion from the primary site to a distant site, whereas induction of MET by PMN-MDSCs promotes tumor cell proliferation to support metastatic growth. Third, MDSCs promote the extravasation of CTCs. After CTCs detach from the primary tumor into the blood vessels, the mechanical and shear forces present inside the vessels will block their extravasation. MDSCs-derived MMP2, MMP9, VEGF, and angiopoietin-2 (ANG-2) effectively increase the permeability of blood vessels and are conducive to the extravasation of CTCs [31, 32]. At the same time, PMN-MDSCs induce the formation of neutrophil extracellular traps (NETs), which capture CTCs through vascular cell adhesion molecule-1 (VCAM-1) to form CTC/MDSC clusters. NETs damage endothelial cells and promote the adhesion and extravasation of CTCs [33–35]. Fourth, MDSCs induce angiogenesis in order for CTCs to colonize the pre-metastasis niche (PMN) efficiently. The PMN is characterized by increased vascular permeability, extracellular matrix remodeling, bone marrow-derived cell recruitment, angiogenesis, and immunosuppression, all of which contribute to creating a microenvironment that supports the colonization and growth of tumor cells. PMN generates new blood vessels to provide nutrients for CTCs to proliferate. MDSCs release MMP9 or VEGF-A, which promotes neovascularization via peroxisome proliferator-activated receptor-gamma (PPARγ) signaling [36]. In addition, TGF-β, fibroblast growth factor-2 (FGF-2), interleukin-1β (IL-1β), bombina variegata peptide 8 (Bv8), stromal cell-derived factor-1 (SDF-1), platelet-derived growth factor-BB (PDGF-BB) and exosomes miR-126a and miR-210 derived from MDSCs can also promote tumor angiogenesis and increase tumor cell metastatic growth [37–43] (Fig. 1).
Fig. 1.
MDSCs promote tumor progression and metastasis via formation of TME. A MDSCs directly enhance tumor proliferation. B MDSCs promote tumor migration and invasion by regulating the epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET) of tumor cells. C MDSCs promote the extravasation of circulating tumor cells (CTCs). D MDSCs induce angiogenesis in order for CTCs to colonize the pre-metastasis niche (PMN) efficiently
MDSCs play a tumor-promoting role by antagonizing T cell activity
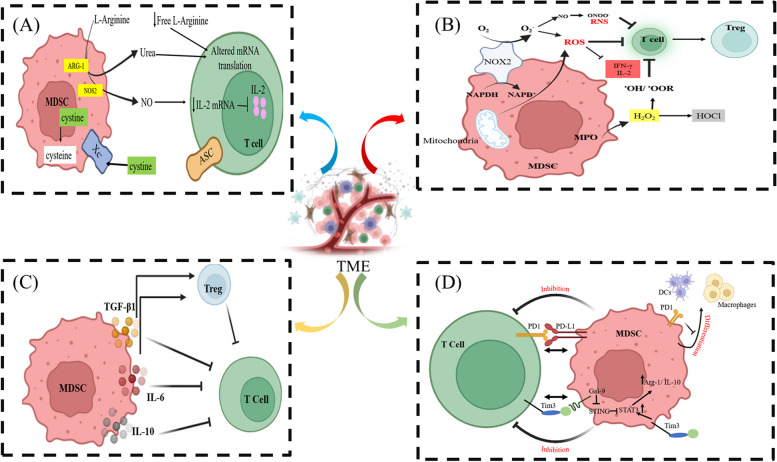
MDSCs, on one hand, enhance tumor growth and metastasis by increasing tumor proliferation, boosting EMT and MET, protecting and encouraging CTC extravasation, and stimulating angiogenesis. On another hand, MDSCs' immunosuppressive role on T cells promotes tumor cell survival. The following section describes the main characteristics of the immunosuppressive activities of MDSCs on T cells in TME (Fig. 2).
Fig. 2.
MDSCs play a tumor-promoting role by antagonizing T cell activity. The function of T cells in the TME is significantly impeded by MDSCs via (A) Exhaustion of L-arginine, cystine, and cysteine. B Production of ROS and RNS. C Production of immunosuppressive cytokines such as IL-10, TGFβ1, and IL-6 and induction of Tregs. D Expression of inhibitory receptors such as, PD-L1
Depletion of L-arginine, cystine, and cysteine
MDSCs can induce T cell suppression by depleting the extracellular availability of L-arginine via arginase 1 (Arg-1) and inducible nitric oxide synthase (NOS2)-dependent metabolic pathway [44].
The cationic amino acid transporter (CAT)-2B transfers L-arginine from the extracellular to the intracellular compartment of MDSCs, and then overexpression of Arg-1 in MDSCs hydrolyses L-arginine to L-ornithine and urea, or upregulation of NOS2 in MDSCs catalyzes L-arginine to L-citrulline and nitric oxide (NO). Thus, Arg-1 induction of decreased L-arginine and increased urea concentration in TME causes translational blockade of the ζ-chain of CD3 in T cells, or NOS2 release of NO reduces the stability of IL-2 mRNA and then interferes with the IL-2 receptor (IL-2R)-signalling pathway in T cells, thereby blocking T-cell activation and proliferation. In a breast cancer study, L-arginine was depleted by the production of NOS2 and Arg-1 by MDSCs. However, L-arginine supplementation significantly decreased tumor growth and increased survival time in 4T1 tumor-bearing mice, which was related to a decrease in MDSCs and boosted innate and adaptive immune responses [45].
MDSCs can also limit T-cell antitumor activity by consuming cystine and sequestering cysteine, thereby depleting these essential amino acids [46]. Cysteine is essential for T-cell DNA and protein synthesis, proliferation, and cytokine secretion upon antigen stimulation. Cysteine is produced by cells in two ways: one way is that cells express the plasma membrane cystine transporter xc− system which imports and converts disulfide-bonded cystine from the oxidizing extracellular environment into intracellular reduced cysteine; another way is that cells have cystathionase which can convert methionine to cysteine. T cells do not have cystathionase or the xc− transporter for producing their own cysteine, but T cells can import extracellular cysteine through their ASC neutral amino acid transporter, which is released by macrophages or dendritic cells (DCs) through their xc− transporter or cystathionase. On the other hand, MDSCs have the xc− transporter but lack ASC-neutral amino acid transporter, so they can acquire cystine from extracellular environment but do not export cysteine to their surroundings [47]. In tumor microenvironment, the competition between MDSCs, DCs and macrophages for cystine leads to the concomitant decrease in cysteine released by DCs and macrophages, which result in the local depletion of cysteine. Thus, tumor-specific T cells cannot be activated because of lack cysteine, and T-cell-mediated antitumor immunity is inhibited.
ROS and RNS production
ROS are important markers for immunosuppressive activity of MDSCs [48, 49]. MDSCs produce ROS through two major sources: NADPH oxidase 2 (NOX2) and mitochondria. Different enzymes such as, NOX2 and NOX4 are responsible for generating superoxide anion (O2−) from molecular oxygen (O2) using NADPH as an electron donor. NOX2 is primarily involved in the production of ROS in phagocytes, while NOX4 is more commonly associated with ROS generation in non-phagocytic cells [50]. The O2− is highly active and prone to convert to the relatively stable hydrogen peroxide (H2O2) as the main ROS state. In addition, MDSCs also express myeloperoxidase (MPO), which catalyzes H2O2 and chloride ions to form highly oxidative hypochlorous acid (HOCl) as another ROS state. Metabolic alterations in MDSCs, such as MPO increased glycolysis and alterations in the tricarboxylic acid (TCA) cycle and fatty acid metabolism, can contribute to ROS production leading to increased oxidative stress in the tumor microenvironment [51]. ROS affect T cell receptor (TCR) signaling by modifying critical signaling molecules [52]. ROS-mediated oxidation of protein tyrosine phosphatases (PTPs) can inhibit their activity, resulting in dysregulated TCR signaling [53]. This impairment can lead to decreased T cell activation and altered cytokine production. ROS-induced DNA damage in T cells can activate DNA damage response pathways, leading to cell cycle arrest and apoptosis. ROS can affect T cell differentiation and effector functions. For example, ROS can influence the differentiation of CD4+ T cells into Tregs, which have immunosuppressive properties [54]. Additionally, ROS can impair the production of cytokines, such as IFN-γ and IL-2, which are crucial for T cell effector functions. ROS can upregulate immune checkpoint molecules, such as programmed cell death PD-1, on T cells [55]. Increased expression of these inhibitory receptors can lead to T cell exhaustion and decreased anti-tumor responses.
MDSCs can upregulate the expression of NOS2, which produces NO through the conversion of L-arginine into L-citrulline. Furthermore, NO and O2− can interact to form two kinds of RNS: peroxynitrite (ONOO−) and dinitrogen trioxide (N2O3), which have protein nitration and S-nitrosylation effect, respectively [56]. MDSCs induce T cell tolerance mainly through secretion of peroxynitrite in antigen-specific manner [57]. In one way, peroxynitrite triggers nitration of MHC class I-peptide complex on tumor cells. In another way, it nitrates TCR α and β subunits, CD8 molecule and lymphocyte-specific protein tyrosine kinase (LCK) [58]. Nitration of TCR-CD8-LCK complex can reduce its binding to MHC-peptide complex and promote dissociation of the ζ-chain of CD3, thus disrupting TCR signaling cascade leading to inhibiting of T cell activation and proliferation.
Production of immunosuppressive cytokines
Numerous immunosuppressive cytokines that MDSCs produce support their immunosuppressive activities. Complex regulatory mechanisms controlled by the tumor microenvironment and different signaling pathways are necessary for MDSCs to produce these cytokines. MDSCs can produce TGF-β1, a potent immunosuppressive cytokine that inhibits T cell responses [59]. TGF-β1 can be secreted in its latent form and requires activation to become biologically active. Activation of TGF-β1 can occur through proteolytic cleavage. TGF-β1 activation suppresses T cell proliferation, differentiation, and effector functions while promoting the generation of Tregs [60].
In addition, MDSCs produce IL-10, an immunosuppressive cytokine with pleiotropic effects [61]. IL-10 suppresses the activation and function of various immune cells, including T cells, antigen-presenting cells (APCs), and NK cells. IL-10 inhibits the production of pro-inflammatory cytokines and chemokines, reduces antigen presentation by APCs, and impairs T cell proliferation and effector functions.
Moreover, MDSCs can secrete IL-6, which plays a complex role in immunosuppression [62]. IL-6 can promote the expansion and survival of MDSCs themselves, contributing to their accumulation. Additionally, IL-6 can promote the generation of Tregs and directly attenuate CD4+ T cell differentiation into Th1 cells and IFN-γ production, which decreases their ability to help CD8+ T cells, resulting in impaired adaptive immune responses against the tumors.
MDSCs can also produce PGE2, a lipid mediator with immunosuppressive properties [63]. PGE2 produced by MDSCs, acting via the EP2 and EP4 receptors on T cells, strongly inhibits T cell development, proliferation, function and IL-12 production, but promotes the expansion of Tregs, thus directly suppresses antitumor immune responses.
Expression of inhibitory receptors
MDSCs can express a range of inhibitory receptors and ligands on their cell surface. In one side, the interactions between inhibitory ligands expressed on MDSCs and receptors expressed on T cells dampen T cell activation. In another side, the expression of inhibitory receptors triggers MDSCs immunosuppressive function or blocks their differentiation to antigen-presenting myeloid cells. PD-1/PD-L1 is a pair of inhibitory receptor/ligand expressed on the surface of MDSCs. Binding of the ligand PD-L1 on MDSCs to its receptor PD-1 on T cells delivers inhibitory signals that result in T cell exhaustion and inhibits T cell activation, proliferation, cytokine production, and cytotoxic activity, leading to decreased anti-tumor responses [64]. PD-1 is also expressed on MDSCs in cancer context. However, PD-1 ablation in MDSCs induces differentiation of inflammatory macrophages and DC and promotes antigen-presenting function [65].
T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) and its ligand galectin-9 are expressed on MDSCs. Extra-cellular galectin-9 on MDSCs can interact with TIM-3 expressed on activated T cells which induces the apoptosis of effector T cells in 4T1 tumor model [66]. Additionally, intra-cellular galectin-9 on MDSCs can accelerate STING protein degradation and reactivate STAT3 signaling to up-regulation of Arg-1 and IL-10 in nasopharyngeal carcinoma model [67]. Recently, Houbao Qi et al. found that TIM-3 expressed in MDSCs interacted with tyrosine-protein kinase Fyn to cause STAT3 phosphorylation, which in turn increases the production of Arg-1 and IL-10 in MDSCs in Toxoplasma gondii infected model [68]. Considering that TIM-3 is expressed in MDSCs of tumor model, TIM-3 may contribute to MDSC expansion and suppreesive activity through TIM-3-STAT3 signaling, thus leading to deficient T cell function.
Induction of T regulatory cells
MDSC can induce the generation and expansion of Tregs, which are a subset of T cells with immunosuppressive properties. The interaction between MDSCs and Tregs has important implications for immune regulation. MDSCs can promote the conversion of conventional T cells into Tregs [69]. MDSCs secrete immunosuppressive cytokines such as TGF-β, IL-10, and IL-6, which play critical roles in Tregs induction. These cytokines, along with other factors produced by MDSCs, create an immunosuppressive microenvironment that favors the differentiation of Tregs from naïve T cells. Tregs induced by MDSCs exhibit suppressive functions and can further contribute to immune suppression. MDSCs can also support the expansion and maintenance of pre-existing Tregs [70]. This expansion of Tregs by MDSCs further strengthens the immunosuppressive network in the microenvironment. Tregs expanded by MDSCs exert immunosuppressive effects on other T cells.
Therapeutic strategies of MDSC targeting in solid tumor is a new hope for enhanced CAR-T efficacy
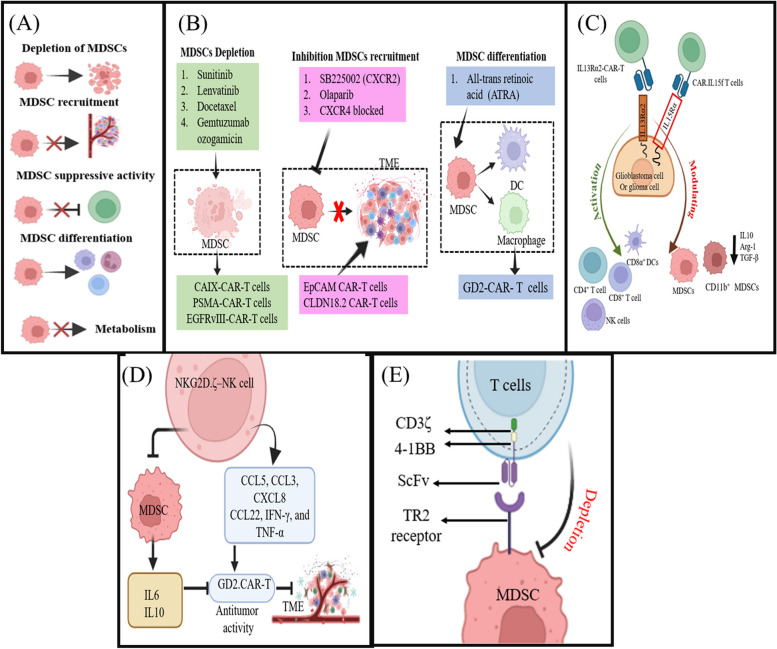
MDSCs create an antagonistic and immunosuppressive TME that can reduce the effectiveness of CAR-T cell treatment for solid tumors. To address the obstacles, therapeutic strategies of MDSC targeting in solid tumor treatment have been developed in order for enhanced CAR-T anti-tumor effect. These strategies include indirect approaches that CAR-T targets tumor cell antigen combined treatment with selectively MDSC targeting small molecule inhibitors and large molecule antibodies, or Cytokine receptor antigen-targeted CAR-T indirectly or directly targeting MDSCs shapes TME from immunosuppressive to immunostimulatory, and direct strategies including modified NK cells expressing activating receptor directly targeting MDSCs, CAR-T targets and depletes MDSCs (Fig. 3).
Fig. 3.
Target MDSCs in solid tumor for effective CAR-T immunotherapy. Therapeutic strategies include (A) therapeutic targeting of MDSCs through small molecule inhibitors and large molecule antibodies; (B) CAR-T targeting cancer cell antigen combination with MDSC modulatory agents; (C) cytokine receptor antigen-targeted CAR-T indirectly or directly targeting MDSCs reshapes TME; (D) modified natural killer (NK) cells expressing activating receptor directly targeting MDSCs; and (E) CAR-T directly targeting MDSC selective antigens
Therapeutic targeting of MDSCs through small-molecule inhibitors and large-molecule antibodies
The strategies to target MDSCs in solid tumors with small-molecule inhibitors and large-molecule antibody treatment can be broadly classified into five categories: (1) Depletion of MDSCs. This strategy mainly induces MDSC cell death in tumor-bearing mice or cancer patients treated with chemotherapeutic medicines such as gemcitabine [71], 5-fluorouracil [72], carboplatin, paclitaxel [73], cisplatin [74], doxorubicin [75], and artemisinin [76], or with tumor necrosis factor-related apoptosis-induced ligand-receptor (TRAIL-R) agonists like death receptor 5 (DR5) antibody DS-8273a [77, 78], or with peptibody generated by fusing S100A9-derived peptides with the antibody Fc portion [79], or anti-CD33 antibody conjugated calicheamicin immunotoxin such as Gemtuzumab ozogamicin (GO) [80] and GTB-3550 [81], or liver X receptor (LXR) agonists such as GW3965 and RGX-104 [82], which successfully reduces MDSC numbers in the peripheral organs and tumor tissues. (2) Inhibition of MDSC recruitment. This strategy involves preventing MDSC migration to the tumor site by targeting chemokine receptors such as C–C motif chemokine receptor 2 (CCR2) inhibitors PF-04136309 [83] and CCX872 [84], CCR5 inhibitor maraviroc [85], CCR2/CCR5 co-inhibitor BMS-687681 [86], C-X-C motif chemokine receptor 2 (CXCR2) inhibitors SB225002 [87] and AZD5069 [88], or using epigenetic therapy such as DNA methyltransferase inhibitor 5-azacytidine (5-Aza) and histone deacetylase inhibitor entinostat [89] or valproic acid (VPA) [90]. (3) Blockade of MDSC suppressive activity. This strategy involves blocking MDSC immunosuppressive machinery molecules such as Arg-1 inhibitor N-hydroxylnor-l-Arg (nor-NOHA) [91], iNOS inhibitor l-NG-monomethyl-l-arginine (l-NMMA), phosphodiesterase-5 (PDE5) inhibitor tadalafil (inhibition of Arg-1 and iNOS enzymatic activity) [92], nitroaspirin (inhibition of Arg-1, iNOS, and PNT enzymatic activity) [93], ROS scavengers N-acetylcysteine (NAC) [94] and catalase [95], NF erythroid 2-related factor 2 (NRF2) agonist triterpenoid (activation of the antioxidant response to reduce production of ROS) [96], NO scavenger carboxy-PTIO (C-PTIO) [97], and MPO inhibitor 4-ABAH [98]. (4) Promotion of MDSC differentiation. This strategy involves encouraging MDSCs to differentiate into mature myeloid cells by targeting myeloid differentiation signal pathways such as retinoic acid receptor activator all-trans retinoic acid (ATRA) [99], vitamin D receptor activator 25-hydroxyvitamin D3 [100], STAT3 inhibitor JSI-124 (cucurbitacin I) [101], or FLLL32 [102]. (5) MDSC reprogramming. This strategy entails targeting metabolic pathways, immunoinhibitory receptors, endoplasmic reticulum (ER) stress responses, or polarization signals that switch MDSC from an immunosuppressive phenotype toward an inflammatory one. For instance, inhibition of lipid metabolism pathways like carnitine palmitoyltransferase 1 (CPT1) inhibitor etomoxir (fatty acid oxidation (FAO) inhibition) [103, 104], cyclooxygenase-2 (COX-2) inhibitor celecoxib (inhibition of PGE2 production) [105], FATP2 inhibitor lipofermata (selective inhibition of PGE2 synthesis and ROS production) [106, 107]; blocking immunoinhibitory receptor like leukocyte immunoglobulin-like receptor subfamily B member 2 (LILRB2) antagonist antibody [108], or PD-1 /its ligand PD-L1 blockade [64, 65]; deletion ER stress response or polarization signal molecules such as the cellular stress sensor C/EBP-homologous protein (CHOP) [109], PKR-like endoplasmic reticulum kinase (PERK) [110], tumor necrosis factor-α–induced protein 8-like 2 (TIPE2) [111], toll interacting protein (TOLLIP) [112].
CAR-T targeting cancer cell antigen combination with MDSC modulatory agents improves anti-tumor effect
First, treatment with MDSC depletion agents strengthens the anti-tumor activity of tumor antigen-redirected CAR-T cells. Sunitinib, a clinically available multitargeted tyrosine kinase inhibitor, induced MDSC apoptosis and reduced the frequency of MDSCs at the tumor site by inhibiting the STAT3 signal, but increased the carbonic anhydrase IX (CAIX) expression on the surface of renal tumor cells while enhancing the proliferation and infiltration of CAIX-CAR-T cells. Sunitinib treatment increased the efficacy of CAIX-CAR-T cells against the mouse lung metastasis model of human renal cancer [113]. In addition, lenvatinib, a tyrosine kinase inhibitor, decreased the frequency and immunosuppressive activity of MDSCs but increased the proliferation, tumor infiltration, and antitumor activity of T cells. Lenvatinib administration enhanced the therapeutic effect of CAIX-CAR T cells against the xenograft model of murine renal cell carcinoma [114]. Moreover, docetaxel, an inhibitor of cellular mitosis, decreased the frequency of MDSCs, decreased immune checkpoint molecules (PD-1, CTLA-4, and TIM-3) and exhaustion, but increased the percentage and proliferation of prostate-specific membrane antigen (PSMA)-CAR-T cells [115]. Docetaxel therapy enhanced the curative efficacy of PSMA-CAR-T cells against the mouse liver metastasis model of human prostate cancer and the xenograft model of human prostate cancer [116]. Recently, Livingstone Fultang et al. found that human M-MDSCs and PMN-MDSCs are transcriptomically different, but CD33 is a common surface marker and a therapeutic target on peripheral and infiltrating MDSCs across cancer subtypes [80]. Because GO contains an immunotherapeutic module anti-CD33 antibody that can recognize and bind to CD33 surface expression cells, exactly MDSCs represent the most myeloid cells in cancer patients. The anti-CD33 antibody mainly targets the MDSCs in tumor tissue and chemotherapeutic unit calicheamicin, which can internalise and increase p-ATM levels, leading to MDSC DNA damage. Thus, treatment with GO led to specific targeting of MDSCs and inducing MDSC cell death, but enhanced disialoganglioside (GD2)-/mesothelin-/epidermal growth factor receptor variant III (EGFRvIII)-CAR-T cell proliferation and cytotoxicity against neuroblastoma and mesothelioma.
Second, treatment with MDSC recruitment agents boosts the anti-tumor capacity of tumor antigen-redirected CAR-T cells. SB225002, a CXCR2 (a prominent chemokine receptor expressed on PMN-MDSC but not M-MDSC) antagonist. CXCR2 on tumor-infiltrated MDSCs were significantly elevated after hypo fractionated radiotherapy (HFRT) initiation, which induced MDSCs migration to tumor site [117]. SB225002 treatment showed a significant reduction of MDSCs in the tumor, but significantly enhanced intra tumor epithelial cell adhesion molecule (EpCAM) CAR-T cells infiltration. A triple combination therapy with a SB225002, HFRT, and EpCAM CAR-T cells showed significantly increased antitumor efficacy and prolonged mice survival, compared with the treatment regimen with HFRT and EpCAM CAR-T cells. Overall, after HFRT, CXCR2 blockade significantly promoted the efficacy of EpCAM CAR-T cells by impairing MDSC accumulation within the tumor site and enhancing infiltration of CAR-T cells. Olaparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, suppressed the secretion of SDF-1α from cancer-associated fibroblasts (CAFs) through HIF-1α inhibition and decreased the expression of CXCR4 in breast cancer cells and MDSCs, inhibiting the recruitment of MDSCs to the tumor microenvironment [118]. However, olaparib increased the infiltration and IFN-γ secretion of EGFRvIII-targeting CAR-T cells and finally significantly improved EGFRvIII-targeting CAR-T cell anti-tumor efficacy against mouse breast cancers.
In addition to blocking MDSC recruitment by small molecular inhibitors, CAR-T cells can also target MDSC recruitment factors. CAFs can recruit MDSCs to tumor tissues by releasing CXCL12, CCL2, and CXCL1 chemokines. Fibroblast activation protein (FAP), a vital characteristic of active CAFs, usually upregulates expression in CAFs. In the sequential two CAR-T cell treatment against pancreatic ductal adenocarcinoma (PDAC) mouse models, FAP-targeted CAR-T cells directly eliminated CAFs and decreased CXCL12 levels in tumor tissues, finally inhibiting MDSC recruitment and accumulation but promoting claudin18.2 (CLDN18.2) targeted CAR-T cell infiltration and survival in tumor sites [119]. Thus, this improved the CLDN18.2 CAR-T cells for treating CLDN18.2-positive pancreatic cancer. Beside sequential two CAR-T cell treatment, designing CAR-T cells targeting tumor antigens and MDSC recruitment factors in one cell is another choice. In a recent report, Sun et al. developed CLDN18.2 CAR-T cells with CXCR4 co-expression for treating CLDN18.2-positive PDAC [120]. Expression of CXCR4 led to blocking the release of TNF-α, IL-6, and IL17A in CLDN18.2 CAR-T cells, which resulted in STAT3/NF-κB/SDF-1α signaling pathway inhibition of CAFs. Through blockade of this recruitment signal cycle between CAFs and MDSCs, it decreased the migration of MDSCs into tumor sites but inversely increased CAR-T cell infiltration and therapeutic efficacy in CLDN18.2-positive pancreatic cancer.
Third, treatment with MDSC differentiation agents boosts the efficacy of anti-tumor antigen CAR-T cells. ATRA, a clinically approved drug, promotes immature myeloid cells to differentiate into a nonsuppressive subtype. ATRA reduced the number of M-MDSCs and diminished the suppressive potency of PMN-MDSCs through increased expression of glutathione synthase and neutralization of ROS, leading to inhibiting MDSC differenation in sarcoma xenograft models. In addition, ATRA administration increased the frequency of CD8+ CAR-T cells in the peripheral blood and improved the antitumor efficacy of GD2-CAR-T cells against sarcoma tumors [121].
Cytokine receptor antigen-targeted CAR-T indirectly or directly targeting MDSCs reshapes TME from immunosuppressive to immunostimulatory, leading to anti-tumor efficacy enhancement
Interleukin-13 receptor alpha2 (IL13Rα2) is a high-affinity membrane receptor for the anti-inflammatory cytokine IL-13, which has higher affinity for IL-13Rα2 than its other receptor, the IL-13 receptor alpha 1/interleukin 4 receptor alpha (IL-13Rα1/IL-4Rα) heterodimer [122, 123]. Given IL-13Rα2 has a short cytoplasmic tail, activation of IL-13Rα2 inhibits IL-13 signaling through canonical JAK/STAT6 signaling pathway but promotes activator protein 1 (AP-1) and extracellular signal-related kinase (ERK) signaling pathway. Actually, IL13Rα2 is a cancer testis antigen, and is overexpressed in the majority of GBM tumors but not expressed on normal brain tissue, making it a highly selective immunotherapy target, this provides the rationale for development CAR T cells targeting IL13Rα2+ GBM [124, 125]. Katarzyna C. Pituch et al. created mouse T cells that expressed IL13Rα2-CARs with a CD28.ζ (IL13Rα2-CAR.CD28.ζ) or a shortened signaling domain (IL13Rα2-CAR.Δ) [126]. IL13Rα2-CAR.CD28.ζ T cells can more efficiently kill IL13Rα2+ glioblastoma (GBM) in vitro and in vivo compared to IL13Rα2-CAR.Δ.T cells. IL13Rα2-CAR.CD28.ζ T cells produced proinflammatory cytokines (IFN-γ, TNF-α) and inducing a significant increase in CD4+ and CD8+ T cells and CD8α+ DCs and a decrease in Ly6G+ MDSCs contributing to reshaping immunosuppressive to proinflammatory TME.
Interleukin-15 receptor alpha (IL15Rα) is expressed on MDSCs, macrophages, DCs, B cells and glioma cells, but IL15Rβγ receptor complex expressed on T cells. In fact, IL-15 can bind to both IL15Rα and IL15Rβγ. Although most preclinical studies using immunodeficient xenograft mouse models had confirmed CAR-T-expressing IL-15 enable to specific kill IL15Rα+ GBM, these models lacking the complete immune system ignored if IL-15-expressing CAR-T cells also targeted killing IL15Rα+ tumor-infiltrating immune cells such as MDSCs and IL15Rβγ+ T cells [127]. Markella Zannikou et al. generated murine T cells expressing IL13Rα2-CAR.CD28.ζ linked secretory murine IL15 at the C-terminal part of the CAR construct (CAR. IL15s) or IL13Rα2-CAR.CD28.ζ linked murine IL15 fusion protein at the N-terminal part of the CAR construct (CAR.IL15f) [128]. CAR.IL15s and CAR.IL15f T cells can deplete MDSCs in vitro, but CAR.IL15f T cells are more potent than CAR.IL15s T cells in killing IL13Rα2+ glioma cells. CAR.IL15f T cells more efficaciously decreased their secretion of immunosuppressive molecules (IL10, Arg-1 and TGF-β) than CAR.IL15s T cells. CAR-IL15f T cells are superior to CAR.IL15s T cells in mediating survival of mice in syngeneic models of glioma. Treatment with CAR.IL15f T cells showed higher frequencies of CD8+ T cells, NK and B cells, but a decrease in CD11b+ myeloid cells in tumors of mouse syngeneic glioma model, which suggested CAR.IL15f T cells reversed the immunosuppressive to immunostimulatory TME and IL15-modified CAR T cells act as a dual targeting agent against tumor cells and MDSCs in GBM.
Modified NK cells expressing activating receptor directly targeting MDSCs improves anti-tumor antigen CAR-T cells efficacy
The activating receptor NKG2D is activated by nonclassic MHC molecules expressed on NK cells stressed by DNA damage, hypoxia, or viral infection, which induce NK cells cytotoxicity against tumor or infected cells via secreting proinflammatory cytokines and chemokines. However, the NKG2D cytotoxic adapter molecule DAP10 is inhibited by suppressive molecules TGF-β of the solid tumors and tumor-infiltrating MDSCs, leading to limit the antitumor functions of NK cells [129]. Interestingly, NKG2D ligands are overexpressed on many tumor cells and MDSCs. This provides an opportunity to target and clear MDSCs by modifying NK cells, in order to relieve the immunosuppressive TME and enhance the therapeutic effect of CAR-T cells on solid tumors [130]. Robin Parihar et al. developed gene-modified NK cells with a chimeric NKG2D receptor fused to the cytotoxic ζ-chain of the T-cell receptor (NKG2D.ζ) [131]. This NKG2D.ζ–NK cells maintain NKG2D.ζ expression which is unaffected by TGF-β or soluble NKG2D ligands, and kill NKG2D ligand-expressing MDSCs with a shift in the cytokine milieu from immune-suppressive (more IL6 and IL10; less IFN-γ and TNF-α) to immune stimulatory (less IL6 and IL10; more IFN-γ and TNF-α) in the TME. Additionally, NKG2D.ζ–NK cells secrete chemokines (CCL5, CCL3, CXCL8 and CCL22) that recruit GD2.CAR-T cells trafficking to tumor sites and increase antitumor activity of GD2.CAR-T cells resulting in tumor regression and prolonged survival compared with treatment with GD2.CAR-T cells alone.
CAR-T directly targeting MDSC specific antigens is a new therapeutically strategy for enhancing anti-tumor antigen CAR-T cells efficacy
TRAIL-R2 (TR2) is expressed on MDSCs, TAMs, tumor cells like breast cancer (BC) cells, and T cells [132–134]. When its ligand TRAIL or a TR2 agonistic antibody (DS-8273a) engaged this receptor TR2, the cells including MDSCs, TAMs, tumor cells were induced TR2-mediated apoptosis via the caspase-8-regulated extrinsic death receptor pathway, but neither resting nor activated T cells underwent increased apoptosis because of upregulated levels of the protein cellular FLICE-like inhibitory protein (cFLIP) which resembles caspase-8 but lacks the protease activity necessary for apoptosis, and thus inhibit caspase-8 activation in a dominant negative manner through competitive binding of cFLIP to Fas-associated death domain [135, 136]. Based on CAR. Mucin 1 (MUC1) transduced T cells (CAR.MUC1 T cells) which contained a single chain variable fragment (scFv) derived from the MUC1 (HMFG2) monoclonal antibody (mAb) fused to a 41BB costimulatory endodomain and CD3 ζ-chain specifically eliminate MUC1 expressing BC cell lines [137]. Saisha A Nalawade et al. generated the expression of two scFv dual CAR-T cells (CAR.MUC1.TR2.41BB T cells) against MUC1+ BC cell lines, which coexpressed another scFv derived from the TR2 (DS-8273a) mAb fused to a 41BB costimulatory endodomain and CD3ζ chain [138]. In the same way, they generated CAR.HER2.TR2.41BB T cells against HER2+ BC cells. On one hand, coexpressing TR2.41BB receptor on CAR-T cells augmented CAR-T cell responses targeting either MUC1 or HER2 against orthotopic tumors in three distinct BC models. Due to high expression of TR2 on their surface of MDSCs, CAR.MUC1.TR2.41BB T cells or CAR.HER2.TR2.41BB T cells can express the single chain TR2 agonistic antibody (DS-8273a) binding to TR2 on MDSCs which facilitated formation of TR2 clusters through CAR-T cell-MDSC cell interactions so as to confirm specifically targeting and eliminating MDSCs without toxicity on normal myeloid cells. On another hand, co-expressing TR2.41BB receptor on CAR-T cells delivered a second costimulatory signal to the CAR T cells through a 41BB endodomain. Exactly, CAR.MUC1 or CAR.HER2 already had signals 1 and 2 on encountering a TAA (tumor-associated antigen) and a CD28 costimulatory signal. So CAR.MUC1.TR2.41BB T cells or CAR.HER2.TR2.41BB T cells included two costimulatory domains, CD28 for the CAR construct and 41BB for the costimulatory receptor, along with upregulated levels of cFLIP, thereby optimally activating the T cells, which improved T cell survival, proliferation, and persistence at the tumor site. All in all, CAR-T cells targeting tumor-associated antigen with a novel chimeric TR2.41BB costimulatory receptor achieved the magical effect of one arrow and three sculptures which targeted immunosuppressive and tumor promoting MDSCs (resulting in TME remodeling), tumor cells and improved T cell proliferation at the tumor site, thus exhibited superior antitumor potential.
Clinical trials of MDSC targeting therapy
Clinical trials investigating MDSC targeting strategies mainly focus on controlling MDSC expansion and survival, blocking MDSC recruitment and inhibiting MDSC immunosuppressive function. Unlike preclinical studies which targeting MDSCs alone can show apparent anti-tumor effect, clinical trials targeting MDSCs as a monotherapy displayed inferior therapy outcomes in solid cancer patients. For example, DS-8273a (agonistic DR5 antibody) selectively eliminated MDSCs with prolonged progression-free survival (PFS) in advanced stage solid cancer patients in a phase I trial (NCT02076451), but no objective clinical responses were noted [77]. ATRA eliminated MDSCs, improved dendritic cell ratio and function, and antigen-specific T-cell response in patients with metastatic renal cell carcinoma in a phase II trial (NCT00100906), but ATRA did not directly inhibit the growth of tumors [139]. Tasquinimod treatment alone reduced the recruitment of MDSCs by targeting S100A9, and improved PFS in metastatic castration-resistant prostate cancer (mCRPC) patients with 7.0 months as compared to placebo treatment with 4.4 months in a phase III trial (NCT01234311), but no overall survival (OS) benefit was observed between tasquinimod with 21.3 months and placebo with 24.0 months [140]. Tadalafil treatment as a single agent in a phase II trial (NCT00894413) reduced peripheral MDSCs numbers, inhibited MDSC function by decreasing ARG1 and iNOS, and thus enhanced systemic and tumor-specific immunity in patients with head and neck squamous cell carcinoma (HNSCC), but no clinical anti-tumor activity was evident [92].
However, clinical trials targeting MDSCs combined with targeted chemotherapy or immunotherapy drugs have better therapeutic efficacy than monotherapy. For instance, the combination of ATRA with pembrolizumab (anti-PD-1 antibody) in patients with stage IV melanoma in a phase Ib/II clinical trial (NCT03200847) effectively lowered the frequency of circulating MDSCs, and achieved overall response rate of 71%, a complete response of 50% and the 1-year overall survival of 80% [141]. DS-8273a administration augmented the clinical efficacy of nivolumab (anti-PD-1 antibody) in subjects with unresectable stage III or stage IV melanoma in a phase I clinical trial (NCT02983006). Moreover, CCX872 (CCR2 inhibitor) co-treated with 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) in pancreatic cancer patients in a phase I clinical trial (NCT02345408) had longer overall survival compared with those in FOLFIRINOX monotherapy group [23]. The combination regimen of anakinra (an inhibitor of IL- 1beta), 5-fluorouracil, and bevacizumab (a VEGF blocking antibody) in patients with colorectal cancer in a phase II clinical trial (NCT02090101) elicited a decrease of PMN-MDSCs in 15 of 25 patients with a better survival outcome [142]. The combination therapy of tadalafil and nivolumab showed augmentation of effector T cells in the periphery, and had a pathologic treatment response of ≥ 20% in 54% of the patients with HNSCC in phase I clinical trial (NCT03238365) [143].
Although a few clinical cases report positive therapeutic effect, anti-tumor activity of CAR-T cells has generally been limited in most clinical trials. These encouraging clinical results have been described using multiple doses of an IL-13Ra-targeted CAR in a patient with glioblastoma in phase I clinical trial (NCT02208362) [144], using GD2-specific CAR T cells in four paediatric patients with pontine or midline glioma in phase I clinical trial (NCT04196413) [145], using a claudin18.2-targeted CAR in 98 patients with gastrointestinal tumours in phase I clinical trial (NCT03874897) [146] and a GD2-specific CAR in 27 patients with neuroblastoma in phase I/II clinical trial (NCT03373097) [147]. Most of these clinical experiments are focused on phase I, mainly to evaluate the safety of CAR-T therapy and explore the dosage of administration. As more experiments enter phase II, which focuses more on anti-tumor efficacy, it is highly likely that more combination therapies will be introduced. This is mainly because the obstacles to CAR-T cell therapy for solid tumors are not only related to CAR-T cells themselves, but also to external factors such as tumor type and tumor microenvironment. The immunosuppressive microenvironment represented by MDSC cells is a barrier that must be addressed, so it can be foreseen that targeted MDSC therapy and CAR-T therapy combination therapy will inevitably emerge in the future.
Future research avenues
As the limitations of CAR-T therapy in solid tumors become increasingly apparent, innovative approaches that integrate MDSC targeting with next-generation CAR-T strategies offer a promising path forward. MDSCs, with their potent immunosuppressive capabilities, represent a critical barrier to effective immunotherapy. Therefore, identifying and refining combinatorial strategies to simultaneously target MDSCs and enhance CAR-T cell efficacy is an urgent priority. One promising avenue is the integration of bispecific T-cell engagers (BiTEs), which link T cells directly to tumor-associated antigens. While BiTEs have primarily been used to redirect T cells to tumor cells, recent studies suggest their potential in modulating MDSC activity [148]. BiTEs could be designed to engage T cells with MDSCs through MDSC-specific surface molecules, thereby reducing their immunosuppressive effects in the TME. For example, targeting S100A9 or DR5 molecules enriched on MDSCs could allow for selective depletion of MDSCs while sparing other myeloid populations. This strategy warrants further exploration to determine its impact on TME reprogramming and CAR-T function.
Oncolytic viruses (OVs) represent another innovative approach for tackling the immunosuppressive TME. OVs can selectively infect and lyse tumor cells, releasing tumor-associated antigens and inflammatory cytokines that recruit immune cells. Importantly, certain OVs are known to selectively modulate myeloid populations, reducing MDSC infiltration and polarization [149]. Engineering OVs to deliver genes that inhibit MDSC recruitment (e.g., blocking CCL2 or CXCL12 signaling) or reprogram their function (e.g., promoting differentiation into proinflammatory macrophages) could synergize with CAR-T therapy to enhance antitumor efficacy. The use of immune checkpoint inhibitors in conjunction with CAR-T therapy and MDSC targeting also holds significant potential. While PD-L1 expression on MDSCs directly suppresses T cells, TIM-3 and LAG-3 are increasingly recognized as additional checkpoint molecules contributing to T cell exhaustion. Combining CAR-T therapy with inhibitors targeting these pathways could reverse T cell dysfunction within the TME [150]. Moreover, preclinical models suggest that checkpoint blockade can indirectly reduce MDSC-mediated suppression by altering their recruitment and differentiation, further amplifying the therapeutic effect.
Critical to the success of these strategies is improving our understanding of MDSC biology. Advances in single-cell genomics and metabolomics provide unique opportunities to dissect the heterogeneity of MDSCs and identify specific molecular markers that distinguish them from other myeloid cells. For instance, characterizing MDSC subsets that dominate in different tumor types and TME conditions could lead to the development of targeted therapies tailored to specific cancers. Similarly, elucidating metabolic vulnerabilities in MDSCs, such as their reliance on fatty acid oxidation or glycolysis, could inform the design of metabolic inhibitors to selectively disrupt their function. Another emerging area involves genetically engineering CAR-T cells to withstand or counteract MDSC-mediated suppression. Last but not the least, CAR-T cells could be modified to express dominant-negative cytokine receptors to resist TGF-β or IL-10 signaling or to secrete factors that deplete or repolarize MDSCs within the TME. Furthermore, incorporating combinatorial antigen recognition to simultaneously target both tumor cells and MDSCs could enhance therapeutic precision and efficacy.
Conclusion
In recent years, our understanding of the pivotal roles played by MDSCs in cancer progression and immunosuppression has significantly advanced. MDSCs are key contributors to tumor proliferation, migration, invasion, angiogenesis, and the extravasation of CTCs, driving tumor progression and metastasis. At the same time, their immunosuppressive activities within the TME profoundly inhibit T cell-mediated anti-tumor responses, creating a major barrier to effective immunotherapy.
With the progress in MDSC biology research and the advancement of CAR-T immunotherapy technologies, preclinical studies have begun to explore the combination of MDSC-targeted strategies with CAR-T cell therapy to enhance efficacy against solid tumors. These combinatorial approaches have shown promising early results, but significant challenges remain. Chief among these is the difficulty of selectively targeting MDSCs due to the lack of specific markers that distinguish them from neutrophils and monocytes. However, the absence of MDSCs in steady-state conditions presents a unique opportunity for selective intervention, particularly when combined with cutting-edge single-cell genomics and metabolomics. These tools enable a deeper understanding of the genomic, metabolic, and functional distinctions between MDSCs and other myeloid populations, paving the way for highly specific therapeutic strategies with minimal off-target effects. Looking ahead, the integration of MDSC-targeting strategies with next-generation immunotherapy approaches such as BiTEs, OVs, and immune checkpoint inhibitors represents a particularly exciting frontier. BiTEs can redirect immune cells to target MDSCs or tumor cells, while OVs have the potential to disrupt the TME and diminish MDSC-mediated immunosuppression. Checkpoint inhibitors, by reversing T cell exhaustion, may further amplify the therapeutic benefits of CAR-T cell therapy. By combining these emerging approaches with advanced genomic and metabolomic profiling, the challenges posed by MDSCs within the TME may be overcomed. Continued research and innovation in this area hold the potential to transform CAR-T therapy into a more effective and durable treatment for solid tumors, offering new hope for patients and clinicians alike.
Abbreviations
- CAR-T cells
Chimeric Antigen Receptor T-cells
- MDSCs
Myeloid-derived Suppressor Cells
- NK cells
Natural Killer cells
- CTLs
Cytotoxic CD8+ T-cells
- Tregs
Regulatory T Cells
- TAMs
Tumor-associated Macrophages
- DCs
Dendritic Cells
- APCs
Antigen-presenting Cells
- PMN-MDSCs
Polymorphonuclear myeloid-derived suppressor cells
- M-MDSCs
Monocytic myeloid-derived suppressor cells
- CAFs
Cancer-associated fibroblasts
- IL-10
Interleukin-10
- TGF-β
Transforming Growth Factor Beta
- PGE2
Prostaglandin E2
- IFN-γ
Interferon Gamma
- VEGF
Vascular Endothelial Growth Factor
- FGF-2
Fibroblast growth factor-2
- ANG-2
Angiopoietin-2
- VCAM-1
Vascular cell adhesion molecule-1
- PPARγ
Peroxisome proliferator-activated receptor-gamma
- IL-1β
Interleukin-1 Beta
- SDF-1
Stromal Cell-Derived Factor 1
- PDGF-BB
Platelet-Derived Growth Factor-BB
- IL-2R
Interleukin-2 receptor
- CCR2
C-C Motif Chemokine Receptor 2
- CXCR2
C-X-C Motif Chemokine Receptor 2
- EGFRvIII
Epidermal growth factor receptor variant III
- IL13Rα2
Interleukin-13 receptor alpha2
- IL-13Rα1
Interleukin-13 receptor alpha 1
- IL-4Rα
Interleukin 4 receptor alpha
- IL15Rα
Interleukin-15 receptor alpha
- Bv8
Bombina Variegata Peptide 8
- TRAIL-R
Tumor necrosis factor-related apoptosis-induced ligand-receptor
- DR5
Death receptor 5
- LILRB2
Leukocyte immunoglobulin-like receptor subfamily B member 2
- NE
Neutrophil elastase
- MMP-9
Matrix metalloprotease-9
- IRS-1
Insulin receptor substrate-1
- PI3K
Phosphatidylinositol 3-kinase
- AKT
Ser and Thr kinase
- SPARC
Secreted Protein Acidic and Rich in Cysteine
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- IDO
Indole amine 2, 3-dioxygenase
- L-Arg
L-Arginine
- Arg-1
Arginase 1
- CAT-2B
Cationic Amino Acid Transporter 2B
- NOS2
Inducible Nitric Oxide Synthase
- NOX2
NADPH Oxidase 2
- O2−
Superoxide Anions
- O2
Oxygen
- NO
Nitric Oxide
- MPO
Myeloperoxidase
- HOCl
Hypochlorous Acid
- H2O2
Hydrogen Peroxide
- TCA
Tricarboxylic acid
- PTPs
Protein tyrosine phosphatases
- ONOO−
Peroxynitrite
- N2O3
Dinitrogen trioxide
- LCK
Lymphocyte-specific protein tyrosine kinase
- PDE5
Phosphodiesterase-5
- CPT1
Carnitine palmitoyltransferase 1
- FAO
Fatty acid oxidation
- COX-2
Cyclooxygenase-2
- PERK
PKR-like endoplasmic reticulum kinase
- PARP
Poly (ADP-ribose) polymerase
- ERK
Extracellular signal-related kinase
- LXR
Liver X receptor
- NRF2
NF erythroid 2-related factor 2
- ER
Endoplasmic reticulum
- CHOP
C/EBP-homologous protein
- TIPE2
Tumor necrosis factor-α–induced protein 8-like 2
- TOLLIP
Toll interacting protein
- HIF-1α
Hypoxia-Inducible Factor 1 Alpha
- STAT3
Signal Transducer and Activator of Transcription 3
- CAIX
Carbonic Anhydrase IX
- AP-1
Activator protein 1
- cFLIP
Cellular FLICE-like inhibitory protein
- TCR
T cell receptor
- BiTEs
Bispecific T-cell Engagers
- OVs
Oncolytic Viruses
- ICB
Immune Checkpoint Blockade
- PD-1
Programmed Cell Death Protein 1
- PD-L1
Programmed Death Ligand 1
- CTLA-4
Cytotoxic T-Lymphocyte Antigen 4
- LAG-3
Lymphocyte Activation Gene 3
- TIM-3
T-cell Immunoglobulin and Mucin Domain-containing Protein 3
- PFS
Progression-free survival
- OS
Overall survival
- mAb
Monoclonal Antibody
- NETs
Neutrophil extracellular traps
- HFRT
Hypo fractionated radiotherapy
- scFv
Single chain variable fragment
- IARC
International Agency for Research on Cancer
- TME
Tumor Microenvironment
- CTCs
Circulating tumor cells
- EMT
Epithelial-Mesenchymal Transition
- MET
Mesenchymal-Epithelial Transition
- PMN
Pre-metastasis niche
- PSMA
Prostate-specific membrane antigen
- GD2
Disialoganglioside
- EpCAM
Epithelial Cell Adhesion Molecule
- FAP
Fibroblast Activation Protein
- PDAC
Pancreatic Ductal Adenocarcinoma
- CLDN18.2
Claudin18.2
- BC
Breast Cancer
- GBM
Glioblastoma
- MUC1
Mucin 1
- TAA
Tumor-associated antigen
- mCRPC
Metastatic castration-resistant prostate cancer
- HNSCC
Head and neck squamous cell carcinoma
- GO
Gemtuzumab ozogamicin
- 5-Aza
5-Azacytidine
- VPA
Valproic acid
- Nor-NOHA
N-hydroxylnor-l-Arg
- L-NMMA
L-NG-monomethyl-l-arginine
- NAC
N-acetylcysteine
- C-PTIO
Carboxy-PTIO
- ATRA
All-trans retinoic acid
- FOLFIRINOX
5-Fluorouracil, leucovorin, irinotecan, and oxaliplatin
Authors’ contributions
DY conceptualized the review, wrote and revised the original and revised manuscript. NMF Abdalsalam completed the literature search, diagrams and figures preparation, original and revised manuscript writing. AI contributed to partial revised manuscript writing. MA Saliu contributed to partial original manuscript writing. TL and XW provided advices and critical review in the original and revised manuscript. All authors reviewed and approved the final manuscript.
Funding
This work was supported by National Key R&D Program of China (2019YFA0906100 and 2021YFC3300101), National Natural Science Foundation of China (82071772), Guangdong Basic and Applied Basic Research Foundation (2022A1515010070), Shenzhen Basic Science Research Project (JCYJ20220818102018038 and JCYJ20220531095612029), Shenzhen Medical Research Fund (A2301047), and Futian Healthcare Research Project (FTWS2023066).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The review did not involve any primary data collection or human subjects research, thus eliminating the need for ethical approval and participant consent.
Consent for publication
All authors have agreed to publish this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tzu-Ming Liu, Email: tmliu@umac.mo.
Xiaochun Wan, Email: xc.wan@siat.ac.cn.
Dehong Yan, Email: dh.yan@siat.ac.cn.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. [DOI] [PubMed] [Google Scholar]
- 2.Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21:509–28. [DOI] [PubMed] [Google Scholar]
- 3.Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184:5309–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry ST, Gabrilovich DI, Sansom OJ, Campbell AD, Morton JP. Therapeutic targeting of tumour myeloid cells. Nat Rev Cancer. 2023;23:216–37. [DOI] [PubMed] [Google Scholar]
- 6.Singleton DC, Macann A, Wilson WR. Therapeutic targeting of the hypoxic tumour microenvironment. Nat Rev Clin Oncol. 2021;18:751–72. [DOI] [PubMed] [Google Scholar]
- 7.Dey P, Kimmelman AC, DePinho RA. Metabolic codependencies in the tumor microenvironment. Cancer Discov. 2021;11:1067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goenka A, Khan F, Verma B, Sinha P, Dmello CC, Jogalekar MP, et al. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun (Lond). 2023;43:525–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huynh D, Winter P, Märkl F, Endres S, Kobold S. Beyond direct killing-novel cellular immunotherapeutic strategies to reshape the tumor microenvironment. Semin Immunopathol. 2023;45:215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labanieh L, Mackall CL. CAR immune cells: design principles, resistance and the next generation. Nature. 2023;614:635–48. [DOI] [PubMed] [Google Scholar]
- 11.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Rui W, Zhao X, Lin X. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell Mol Immunol. 2021;18:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayala Ceja M, Khericha M, Harris CM, Puig-Saus C, Chen YY. CAR-T cell manufacturing: major process parameters and next-generation strategies. J Exp Med. 2024;221:e20230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol Cancer. 2022;21:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afolabi LO, Afolabi MO, Sani MM, Okunowo WO, Yan D, Chen L, et al. Exploiting the CRISPR-Cas9 gene-editing system for human cancers and immunotherapy. Clin Transl Immunology. 2021;10:e1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Johnson KCC, Gatti-Mays ME, Li Z. Emerging strategies in targeting tumor-resident myeloid cells for cancer immunotherapy. J Hematol Oncol. 2022;15:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan D, Adeshakin AO, Xu M, Afolabi LO, Zhang G, Chen YH, et al. Lipid metabolic pathways confer the immunosuppressive function of myeloid-derived suppressor cells in tumor. Front Immunol. 2019;10:1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeshakin AO, Adeshakin FO, Yan D, Wan X. Regulating histone deacetylase signaling pathways of myeloid-derived suppressor cells enhanced T Cell-Based Immunotherapy. Front Immunol. 2022;13:781660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Condamine T, Ramachandran I, Youn J-I, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albelda SM. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat Rev Clin Oncol. 2024;21:47–66. [DOI] [PubMed] [Google Scholar]
- 23.Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn J-I, Cheng P, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Sanctis F, Adamo A, Canè S, Ugel S. Targeting tumour-reprogrammed myeloid cells: the new battleground in cancer immunotherapy. Semin Immunopathol. 2023;45:163–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yui S, Osawa Y, Ichisugi T, Morimoto-Kamata R. Neutrophil cathepsin G, but not elastase, induces aggregation of MCF-7 mammary carcinoma cells by a protease activity-dependent cell-oriented mechanism. Mediators Inflamm. 2014;2014:971409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sangaletti S, Talarico G, Chiodoni C, Cappetti B, Botti L, Portararo P, et al. SPARC is a new myeloid-derived suppressor cell marker licensing suppressive activities. Front Immunol. 2019;10:1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sangaletti S, Tripodo C, Santangelo A, Castioni N, Portararo P, Gulino A, et al. Mesenchymal transition of high-grade breast carcinomas depends on extracellular matrix control of myeloid suppressor cell activity. Cell Rep. 2016;17:233–48. [DOI] [PubMed] [Google Scholar]
- 30.Gao D, Joshi N, Choi H, Ryu S, Hahn M, Catena R, et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012;72:1384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sprouse ML, Welte T, Boral D, Liu HN, Yin W, Vishnoi M, et al. PMN-MDSCs Enhance CTC Metastatic Properties through Reciprocal Interactions via ROS/Notch/Nodal Signaling. Int J Mol Sci. 2019;20:1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortiz-Espinosa S, Morales X, Senent Y, Alignani D, Tavira B, Macaya I, et al. Complement C5a induces the formation of neutrophil extracellular traps by myeloid-derived suppressor cells to promote metastasis. Cancer Lett. 2022;529:70–84. [DOI] [PubMed] [Google Scholar]
- 34.Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–7. [DOI] [PubMed] [Google Scholar]
- 35.Adrover JM, McDowell SAC, He X-Y, Quail DF, Egeblad M. NETworking with cancer: the bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell. 2023;41:505–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurt B, Schulick R, Edil B, El Kasmi KC, Barnett C. Cancer-promoting mechanisms of tumor-associated neutrophils. Am J Surg. 2017;214:938–44. [DOI] [PubMed] [Google Scholar]
- 37.Gordon-Weeks AN, Lim SY, Yuzhalin AE, Jones K, Markelc B, Kim KJ, et al. Neutrophils promote hepatic metastasis growth through fibroblast growth factor 2-dependent angiogenesis in mice. Hepatology. 2017;65:1920–35. [DOI] [PubMed] [Google Scholar]
- 38.He Q, Liu M, Huang W, Chen X, Zhang B, Zhang T, et al. IL-1β-Induced elevation of solute carrier family 7 Member 11 promotes hepatocellular carcinoma metastasis through up-regulating programmed death ligand 1 and colony-stimulating factor 1. Hepatology. 2021;74:3174–93. [DOI] [PubMed] [Google Scholar]
- 39.Shojaei F, Wu X, Zhong C, Yu L, Liang X-H, Yao J, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–31. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi R, Amano H, Ito Y, Eshima K, Satoh T, Iwamura M, et al. Microsomal prostaglandin E synthase-1 promotes lung metastasis via SDF-1/CXCR4-mediated recruitment of CD11b+Gr1+MDSCs from bone marrow. Biomed Pharmacother. 2020;121:109581. [DOI] [PubMed] [Google Scholar]
- 41.Hsu Y-L, Yen M-C, Chang W-A, Tsai P-H, Pan Y-C, Liao S-H, et al. CXCL17-derived CD11b+Gr-1+ myeloid-derived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB. Breast Cancer Res. 2019;21:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng Z, Rong Y, Teng Y, Zhuang X, Samykutty A, Mu J, et al. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;36:639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noman MZ, Janji B, Hu S, Wu JC, Martelli F, Bronte V, et al. Tumor-promoting effects of myeloid-derived suppressor cells are potentiated by hypoxia-induced expression of miR-210. Cancer Res. 2015;75:3771–87. [DOI] [PubMed] [Google Scholar]
- 44.Raber P, Ochoa AC, Rodríguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest. 2012;41:614–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao Y, Feng Y, Zhang Y, Zhu X, Jin F. L-Arginine supplementation inhibits the growth of breast cancer by enhancing innate and adaptive immune responses mediated by suppression of MDSCs in vivo. BMC Cancer. 2016;16:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang B, Wang X, Ren X. Amino acid metabolism related to immune tolerance by MDSCs. Int Rev Immunol. 2012;31:177–83. [DOI] [PubMed] [Google Scholar]
- 48.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adeshakin AO, Liu W, Adeshakin FO, Afolabi LO, Zhang M, Zhang G, et al. Regulation of ROS in myeloid-derived suppressor cells through targeting fatty acid transport protein 2 enhanced anti-PD-L1 tumor immunotherapy. Cell Immunol. 2021;362:104286. [DOI] [PubMed] [Google Scholar]
- 50.Moghadam ZM, Henneke P, Kolter J. From flies to men: ROS and the NADPH oxidase in phagocytes. Front Cell Dev Biol. 2021;9:628991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ostrand-Rosenberg S, Beury DW, Parker KH, Horn LA. Survival of the fittest: how myeloid-derived suppressor cells survive in the inhospitable tumor microenvironment. Cancer Immunol Immunother. 2020;69:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gülow K, Tümen D, Heumann P, Schmid S, Kandulski A, Müller M, et al. Unraveling the role of reactive oxygen species in T lymphocyte signaling. Int J Mol Sci. 2024;25:6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welsh CL, Madan LK. Protein tyrosine phosphatase regulation by reactive oxygen species. Adv Cancer Res. 2024;162:45–74. [DOI] [PubMed] [Google Scholar]
- 54.Siret C, Collignon A, Silvy F, Robert S, Cheyrol T, André P, et al. Deciphering the crosstalk between myeloid-derived suppressor cells and regulatory T Cells in pancreatic ductal adenocarcinoma. Front Immunol. 2019;10:3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glorieux C, Xia X, Huang P. The Role of Oncogenes and Redox Signaling in the Regulation of PD-L1 in Cancer. Cancers (Basel). 2021;13:4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Sanctis F, Sandri S, Ferrarini G, Pagliarello I, Sartoris S, Ugel S, et al. The emerging immunological role of post-translational modifications by reactive nitrogen species in cancer microenvironment. Front Immunol. 2014;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feng S, Cheng X, Zhang L, Lu X, Chaudhary S, Teng R, et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc Natl Acad Sci U S A. 2018;115:10094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang A, Qin Y, Springer TA. Loss of LRRC33-Dependent TGFβ1 activation enhances antitumor immunity and checkpoint blockade therapy. Cancer Immunol Res. 2022;10:453–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tauriello DVF, Sancho E, Batlle E. Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22:25–44. [DOI] [PubMed] [Google Scholar]
- 61.Wu AA, Drake V, Huang H-S, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4:e1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber R, Groth C, Lasser S, Arkhypov I, Petrova V, Altevogt P, et al. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol. 2021;359:104254. [DOI] [PubMed] [Google Scholar]
- 63.Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P. PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Invest. 2012;41:635–57. [DOI] [PubMed] [Google Scholar]
- 64.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol. 2020;5:eaay1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol. 2010;185:1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C-X, Huang D-J, Baloche V, Zhang L, Xu J-X, Li B-W, et al. Galectin-9 promotes a suppressive microenvironment in human cancer by enhancing STING degradation. Oncogenesis. 2020;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qi H, Li Y, Liu X, Jiang Y, Li Z, Xu X, et al. Tim-3 regulates the immunosuppressive function of decidual MDSCs via the Fyn-STAT3-C/EBPβ pathway during Toxoplasma gondii infection. PLoS Pathog. 2023;19:e1011329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang B, Pan P-Y, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. [DOI] [PubMed] [Google Scholar]
- 70.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. [DOI] [PubMed] [Google Scholar]
- 72.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. [DOI] [PubMed] [Google Scholar]
- 73.Welters MJ, van der Sluis TC, van Meir H, Loof NM, van Ham VJ, van Duikeren S, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med. 2016;8:334ra52. [DOI] [PubMed] [Google Scholar]
- 74.Huang X, Cui S, Shu Y. Cisplatin selectively downregulated the frequency and immunoinhibitory function of myeloid-derived suppressor cells in a murine B16 melanoma model. Immunol Res. 2016;64:160–70. [DOI] [PubMed] [Google Scholar]
- 75.Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang M, Wang L, Liu W, Wang T, De Sanctis F, Zhu L, et al. Targeting Inhibition of accumulation and function of myeloid-derived suppressor cells by artemisinin via PI3K/AKT, mTOR, and MAPK Pathways Enhances Anti-PD-L1 immunotherapy in melanoma and liver tumors. J Immunol Res. 2022;2022:2253436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, et al. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an Agonistic TRAIL-R2 Antibody. Clin Cancer Res. 2017;23:2942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Q, Yan D, Zhang Q, Zhang G, Xia M, Li J, et al. Treatment of acetaminophen-induced liver failure by blocking the death checkpoint protein TRAIL. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165583. [DOI] [PubMed] [Google Scholar]
- 79.Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med. 2014;20:676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fultang L, Panetti S, Ng M, Collins P, Graef S, Rizkalla N, et al. MDSC targeting with Gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers. EBioMedicine. 2019;47:235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarhan D, Brandt L, Felices M, Guldevall K, Lenvik T, Hinderlie P, et al. 161533 TriKE stimulates NK-cell function to overcome myeloid-derived suppressor cells in MDS. Blood Adv. 2018;2:1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tavazoie MF, Pollack I, Tanqueco R, Ostendorf BN, Reis BS, Gonsalves FC, et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell. 2018;172:825-840.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dang Y, Yu J, Zhao S, Jin L, Cao X, Wang Q. GOLM1 drives colorectal cancer metastasis by regulating myeloid-derived suppressor cells. J Cancer. 2021;12:7158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flores-Toro JA, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Charo IF, et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc Natl Acad Sci U S A. 2020;117:1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang L, Wang B, Qin J, Zhou H, Majumdar APN, Peng F. Blockade of CCR5-mediated myeloid derived suppressor cell accumulation enhances anti-PD1 efficacy in gastric cancer. Immunopharmacol Immunotoxicol. 2018;40:91–7. [DOI] [PubMed] [Google Scholar]
- 86.Pant A, Hwa-Lin Bergsneider B, Srivastava S, Kim T, Jain A, Bom S, et al. CCR2 and CCR5 co-inhibition modulates immunosuppressive myeloid milieu in glioma and synergizes with anti-PD-1 therapy. Oncoimmunology. 2024;13:2338965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deshpande NU, Bianchi A, Amirian H, De Castro Silva I, Rafie CI, Surnar B, Rajkumar K, Ogobuiro IC, Patel M, Mehra S, Nagathihalli NS, Merchant NB, Dhar S, Datta J. Cell-specific nanoengineering strategy disrupts tolerogenic signaling from myeloid-derived suppressor cells to invigorate antitumor immunity in pancreatic cancer. bioRxiv [Preprint]. 2024 May 30:2024.05.25.594901. 10.1101/2024.05.25.594901. PMID: 38854120; PMCID: PMC11160608.
- 89.Lu Z, Zou J, Li S, Topper MJ, Tao Y, Zhang H, et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature. 2020;579:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adeshakin AO, Yan D, Zhang M, Wang L, Adeshakin FO, Liu W, et al. Blockade of myeloid-derived suppressor cell function by valproic acid enhanced anti-PD-L1 tumor immunotherapy. Biochem Biophys Res Commun. 2020;522:604–11. [DOI] [PubMed] [Google Scholar]
- 91.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–49. [DOI] [PubMed] [Google Scholar]
- 92.Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A. 2005;102:4185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pedre B, Barayeu U, Ezeriņa D, Dick TP. The mechanism of action of N-acetylcysteine (NAC): the emerging role of H2S and sulfane sulfur species. Pharmacol Ther. 2021;228:107916. [DOI] [PubMed] [Google Scholar]
- 95.Glorieux C, Calderon PB. Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol Chem. 2017;398:1095–108. [DOI] [PubMed] [Google Scholar]
- 96.Nagaraj S, Youn J-I, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hirano K, Hosoi A, Matsushita H, Iino T, Ueha S, Matsushima K, et al. The nitric oxide radical scavenger carboxy-PTIO reduces the immunosuppressive activity of myeloid-derived suppressor cells and potentiates the antitumor activity of adoptive cytotoxic T lymphocyte immunotherapy. Oncoimmunology. 2015;4:e1019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams CMD, Noll JE, Bradey AL, Duggan J, Wilczek VJ, Masavuli MG, et al. Myeloperoxidase creates a permissive microenvironmental niche for the progression of multiple myeloma. Br J Haematol. 2023;203:614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67:11021–8. [DOI] [PubMed] [Google Scholar]
- 100.Fleet JC, Burcham GN, Calvert RD, Elzey BD, Ratliff TL. 1α, 25 Dihydroxyvitamin D (1,25(OH)2D) inhibits the T cell suppressive function of myeloid derived suppressor cells (MDSC). J Steroid Biochem Mol Biol. 2020;198:105557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, et al. Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol. 2013;43:2943–55. [DOI] [PubMed] [Google Scholar]
- 102.Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res. 2015;3:1236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mohamady Farouk Abdalsalam N, Liang Z, Kashaf Tariq H, Ibrahim A, Li R, Wan X, et al. Etomoxir sodium salt promotes imidazole ketone erastin-induced myeloid-derived suppressor cell ferroptosis and enhances cancer therapy. Biology (Basel). 2024;13:949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adeshakin AO, Adeshakin FO, Liu W, Li H, Yan D, Wan X. Lipidomics data showing the effect of lipofermata on myeloid-derived suppressor cells in the spleens of tumor-bearing mice. Data Brief. 2021;35:106882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen H-M, van der Touw W, Wang YS, Kang K, Mai S, Zhang J, et al. Blocking immunoinhibitory receptor LILRB2 reprograms tumor-associated myeloid cells and promotes antitumor immunity. J Clin Invest. 2018;128:5647–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, et al. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mohamed E, Sierra RA, Trillo-Tinoco J, Cao Y, Innamarato P, Payne KK, et al. The unfolded protein response mediator PERK governs myeloid cell-driven immunosuppression in tumors through Inhibition of STING Signaling. Immunity. 2020;52:668-682.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan D, Wang J, Sun H, Zamani A, Zhang H, Chen W, et al. TIPE2 specifies the functional polarization of myeloid-derived suppressor cells during tumorigenesis. J Exp Med. 2020;217:e20182005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y, Lee C, Geng S, Li L. Enhanced tumor immune surveillance through neutrophil reprogramming due to Tollip deficiency. JCI Insight. 2019;4:e122939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li H, Ding J, Lu M, Liu H, Miao Y, Li L, et al. CAIX-specific CAR-T cells and sunitinib show synergistic effects against metastatic renal cancer models. J Immunother. 2020;43:16–28. [DOI] [PubMed] [Google Scholar]
- 114.Lu M, Zhang X, Gao X, Sun S, Wei X, Hu X, et al. Lenvatinib enhances T cell immunity and the efficacy of adoptive chimeric antigen receptor-modified T cells by decreasing myeloid-derived suppressor cells in cancer. Pharmacol Res. 2021;174:105829. [DOI] [PubMed] [Google Scholar]
- 115.Zhang X, Sun S, Miao Y, Yuan Y, Zhao W, Li H, et al. Docetaxel enhances the therapeutic efficacy of PSMA-specific CAR-T cells against prostate cancer models by suppressing MDSCs. J Cancer Res Clin Oncol. 2022;148:3511–20. [DOI] [PubMed] [Google Scholar]
- 116.Alzubi J, Dettmer-Monaco V, Kuehle J, Thorausch N, Seidl M, Taromi S, et al. PSMA-directed CAR T cells combined with low-dose docetaxel treatment induce tumor regression in a prostate cancer xenograft model. Mol Ther Oncolytics. 2020;18:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang B, Hu M, Ma Q, Li K, Li X, He X, et al. Optimized CAR-T therapy based on spatiotemporal changes and chemotactic mechanisms of MDSCs induced by hypofractionated radiotherapy. Mol Ther. 2023;31:2105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun R, Luo H, Su J, Di S, Zhou M, Shi B, et al. Olaparib suppresses MDSC recruitment via SDF1α/CXCR4 axis to improve the anti-tumor efficacy of CAR-T cells on breast cancer in mice. Mol Ther. 2021;29:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y, Sun Y, Wang P, Li S, Dong Y, Zhou M, et al. FAP-targeted CAR-T suppresses MDSCs recruitment to improve the antitumor efficacy of claudin18.2-targeted CAR-T against pancreatic cancer. J Transl Med. 2023;21:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun R, Sun Y, Wu C, Liu Y, Zhou M, Dong Y, et al. CXCR4-modified CAR-T cells suppresses MDSCs recruitment via STAT3/NF-κB/SDF-1α axis to enhance efficacy against pancreatic cancer. Mol Ther. 2023;31:3193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Long AH, Highfill SL, Cui Y, Smith JP, Walker AJ, Ramakrishna S, et al. Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunol Res. 2016;4:869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rahaman SO, Sharma P, Harbor PC, Aman MJ, Vogelbaum MA, Haque SJ. IL-13R(alpha)2, a decoy receptor for IL-13 acts as an inhibitor of IL-4-dependent signal transduction in glioblastoma cells. Cancer Res. 2002;62:1103–9. [PubMed] [Google Scholar]
- 123.Balyasnikova IV, Wainwright DA, Solomaha E, Lee G, Han Y, Thaci B, et al. Characterization and immunotherapeutic implications for a novel antibody targeting interleukin (IL)-13 receptor α2. J Biol Chem. 2012;287:30215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krenciute G, Krebs S, Torres D, Wu M-F, Liu H, Dotti G, et al. Characterization and functional analysis of scFv-based chimeric antigen receptors to redirect T Cells to IL13Rα2-positive glioma. Mol Ther. 2016;24:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brown CE, Aguilar B, Starr R, Yang X, Chang W-C, Weng L, et al. Optimization of IL13Rα2-targeted chimeric antigen Receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther. 2018;26:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pituch KC, Miska J, Krenciute G, Panek WK, Li G, Rodriguez-Cruz T, et al. Adoptive transfer of IL13Rα2-specific chimeric antigen receptor T Cells creates a pro-inflammatory environment in glioblastoma. Mol Ther. 2018;26:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Krenciute G, Prinzing BL, Yi Z, Wu M-F, Liu H, Dotti G, et al. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol Res. 2017;5:571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zannikou M, Duffy JT, Levine RN, Seblani M, Liu Q, Presser A, et al. IL15 modification enables CAR T cells to act as a dual targeting agent against tumor cells and myeloid-derived suppressor cells in GBM. J Immunother Cancer. 2023;11:e006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dasgupta S, Bhattacharya-Chatterjee M, O’Malley BW, Chatterjee SK. Inhibition of NK cell activity through TGF-beta 1 by down-regulation of NKG2D in a murine model of head and neck cancer. J Immunol. 2005;175:5541–50. [DOI] [PubMed] [Google Scholar]
- 130.Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183:6939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parihar R, Rivas C, Huynh M, Omer B, Lapteva N, Metelitsa LS, et al. NK Cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol Res. 2019;7:363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Condamine T, Kumar V, Ramachandran IR, Youn J-I, Celis E, Finnberg N, et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin Invest. 2014;124:2626–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Piggott L, Silva A, Robinson T, Santiago-Gómez A, Simões BM, Becker M, et al. Acquired resistance of ER-positive breast cancer to endocrine treatment confers an adaptive sensitivity to TRAIL through posttranslational downregulation of c-FLIP. Clin Cancer Res. 2018;24:2452–63. [DOI] [PubMed] [Google Scholar]
- 134.He M-X, He Y-W. c-FLIP protects T lymphocytes from apoptosis in the intrinsic pathway. J Immunol. 2015;194:3444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Davidovich P, Higgins CA, Najda Z, Longley DB, Martin SJ. cFLIPL acts as a suppressor of TRAIL- and Fas-initiated inflammation by inhibiting assembly of caspase-8/FADD/RIPK1 NF-κB-activating complexes. Cell Rep. 2023;42:113476. [DOI] [PubMed] [Google Scholar]
- 136.Tschumi BO, Dumauthioz N, Marti B, Zhang L, Lanitis E, Irving M, et al. CART cells are prone to Fas- and DR5-mediated cell death. J Immunother Cancer. 2018;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bajgain P, Tawinwung S, D’Elia L, Sukumaran S, Watanabe N, Hoyos V, et al. CAR T cell therapy for breast cancer: harnessing the tumor milieu to drive T cell activation. J Immunother Cancer. 2018;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nalawade SA, Shafer P, Bajgain P, McKenna MK, Ali A, Kelly L, et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J Immunother Cancer. 2021;9:e003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sternberg C, Armstrong A, Pili R, Ng S, Huddart R, Agarwal N, et al. Randomized, Double-blind, Placebo-controlled Phase III study of tasquinimod in men with metastatic castration-resistant prostate cancer. J Clin Oncol. 2016;34:2636–43. [DOI] [PubMed] [Google Scholar]
- 141.Tobin RP, Cogswell DT, Cates VM, Davis DM, Borgers JSW, Van Gulick RJ, et al. Targeting MDSC differentiation using ATRA: a Phase I/II clinical trial combining Pembrolizumab and all-trans retinoic acid for metastatic melanoma. Clin Cancer Res. 2023;29:1209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Limagne E, Euvrard R, Thibaudin M, Rébé C, Derangère V, Chevriaux A, et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the Efficacy of a FOLFOX-Bevacizumab drug treatment regimen. Cancer Res. 2016;76:5241–52. [DOI] [PubMed] [Google Scholar]
- 143.Luginbuhl AJ, Johnson JM, Harshyne LA, Linnenbach AJ, Shukla SK, Alnemri A, et al. Tadalafil enhances immune signatures in response to neoadjuvant nivolumab in resectable head and neck squamous cell carcinoma. Clin Cancer Res. 2022;28:915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of glioblastoma after chimeric antigen receptor T-Cell therapy. N Engl J Med. 2016;375:2561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603:934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qi C, Liu C, Gong J, Liu D, Wang X, Zhang P, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial final results. Nat Med. 2024;30:2224–34. [DOI] [PubMed] [Google Scholar]
- 147.Del Bufalo F, De Angelis B, Caruana I, Del Baldo G, De Ioris MA, Serra A, et al. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N Engl J Med. 2023;388:1284–95. [DOI] [PubMed] [Google Scholar]
- 148.Jitschin R, Saul D, Braun M, Tohumeken S, Völkl S, Kischel R, et al. CD33/CD3-bispecific T-cell engaging (BiTE®) antibody construct targets monocytic AML myeloid-derived suppressor cells. J Immunother Cancer. 2018;6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Z, Yang N, Lu H, Chen Y, Xu L, Wang Z, et al. Improved antitumor effects elicited by an oncolytic HSV-1 expressing a novel B7H3nb/CD3 BsAb. Cancer Lett. 2024;588:216760. [DOI] [PubMed] [Google Scholar]
- 150.Cianciotti BC, Magnani ZI, Ugolini A, Camisa B, Merelli I, Vavassori V, et al. TIM-3, LAG-3, or 2B4 gene disruptions increase the anti-tumor response of engineered T cells. Front Immunol. 2024;15:1315283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.