Abstract
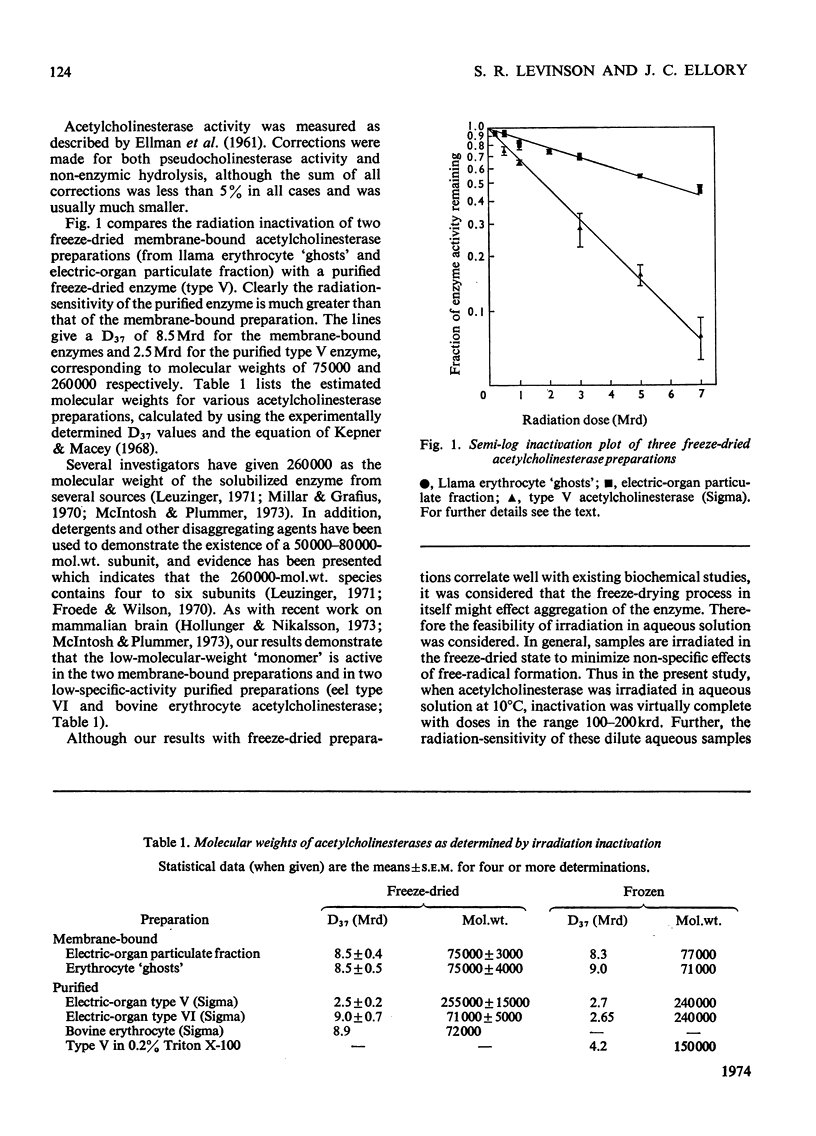
The molecular size of acetylcholinesterase (EC 3.1.1.7) from the electric organ of Electrophorus electricus and erythrocyte `ghosts' was estimated in both membrane-bound and purified preparations by irradiation inactivation. Results suggest that the form of the enzyme in the membrane is a monomer of molecular weight approx. 75000 and that multiple forms of the enzyme observed in solubilized preparations are aggregates of this monomer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- FLUKE D. J., SERLIN I. The size and shape of the radiosensitive acetylcholinesterase unit. J Biol Chem. 1956 Dec;223(2):727–736. [PubMed] [Google Scholar]
- Fortes P. A., Ellory J. C., Lew V. L. Suramin: a potent ATPase inhibitor which acts on the inside surface of the sodium pump. Biochim Biophys Acta. 1973 Aug 22;318(2):262–272. doi: 10.1016/0005-2736(73)90119-3. [DOI] [PubMed] [Google Scholar]
- Froede H. C., Wilson I. B. On the subunit structure of acetylcholinesterase. Isr J Med Sci. 1970 Mar-Apr;6(2):179–184. [PubMed] [Google Scholar]
- Hollunger E. G., Niklasson B. H. The release and molecular state of mammalian brain acetylcholinesterase. J Neurochem. 1973 Mar;20(3):821–836. doi: 10.1111/j.1471-4159.1973.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Aprison M. H. Mammalian brain acetylcholinesterase. Purification and properties. J Neurochem. 1966 Dec;13(12):1351–1365. doi: 10.1111/j.1471-4159.1966.tb04298.x. [DOI] [PubMed] [Google Scholar]
- Kepner G. R., Macey R. I. Membrane enzyme systems. Molecular size determinations by radiation inactivation. Biochim Biophys Acta. 1968 Sep 17;163(2):188–203. doi: 10.1016/0005-2736(68)90097-7. [DOI] [PubMed] [Google Scholar]
- LAWLER H. C. A simplified procedure for for the partial purification of acetylcholinesterase from electric tissue. J Biol Chem. 1959 Apr;234(4):799–801. [PubMed] [Google Scholar]
- Levinson S. R., Ellory J. C. Molecular size of the tetrodotoxin binding site estimated by irradiation inactivation. Nat New Biol. 1973 Sep 26;245(143):122–123. doi: 10.1038/newbio245122a0. [DOI] [PubMed] [Google Scholar]
- McIntosh C. H., Plummer D. T. Multiple forms of acetylcholinesterase from pig brain. Biochem J. 1973 Aug;133(4):655–665. doi: 10.1042/bj1330655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar D. B., Grafius M. A., Palmer D. A. Enzymatically active half-monomers of acetylcholinesterase. Eur J Biochem. 1973 Sep 3;37(3):425–433. doi: 10.1111/j.1432-1033.1973.tb03002.x. [DOI] [PubMed] [Google Scholar]
- Millar David B., Grafius Melba A. The subunit molecular weight of acetylcholinesterase. FEBS Lett. 1970 Dec 23;12(1):61–64. doi: 10.1016/0014-5793(70)80596-8. [DOI] [PubMed] [Google Scholar]


