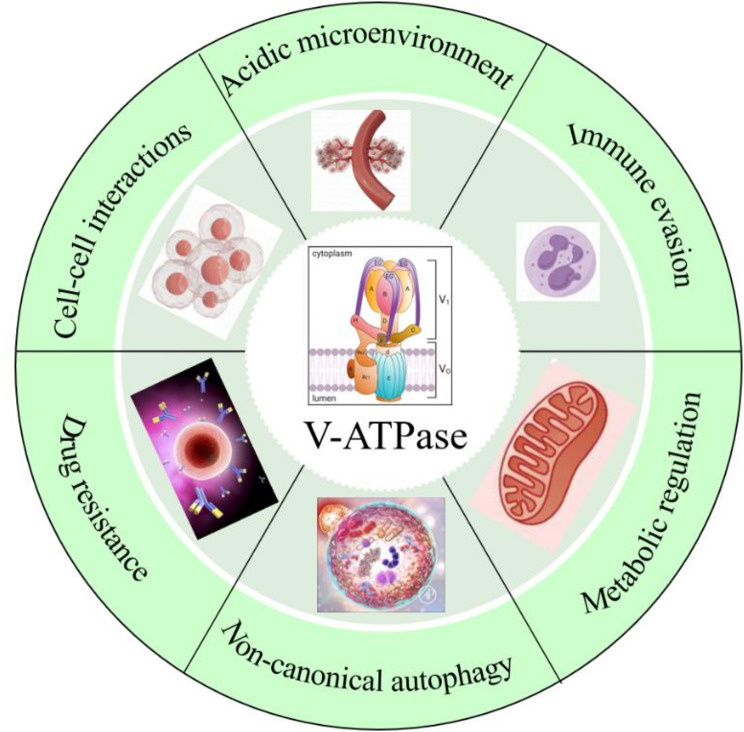
Abstract
Vacuolar-type H+-ATPase (V-ATPase) is a crucial proton pump that plays an essential role in maintaining intracellular pH homeostasis and a variety of physiological processes. This review provides an in-depth exploration of the structural components, functional mechanisms, and regulatory modes of V-ATPase in cancer cells. Comprising two main domains, V1 and V0, V-ATPase drives the proton pump through ATP hydrolysis, sustaining the pH balance within the cell and organelles. In cancer cells, the enhanced activity of V-ATPase is closely associated with the proliferation and metastasis of tumor cells, and it promotes the growth and invasion of tumor cells by regulating pH values in the tumor microenvironment. Moreover, the interaction between V-ATPase and key metabolic regulatory factors, the mechanistic target of rapamycin complex 1 (mTORC1) and AMP-activated protein kinase (AMPK), impacts the metabolic state of cancer cells. The role of V-ATPase in tumor drug resistance and its regulatory mechanism in non-canonical autophagy offer new perspectives and potential targets for cancer therapy. Future research directions will focus on the specific mechanisms of action of V-ATPase in the tumor microenvironment and how to translate its inhibitors into clinical applications, providing significant scientific evidence for the development of new therapeutic strategies.
Graphical Abstract
Keywords: V-ATPase, Proton pump, pH homeostasis, Therapeutic targets
Introduction
In the microscopic world of the cell, pH balance is the foundation of vital activities, and Vacuolar-type H+-ATPase (V-ATPase) is a key regulator of this balance [1]. This multifunctional proton pump not only plays an essential role in cellular physiological processes but also exerts complex and critical functions in the abnormal behavior of cancer cells.
V-ATPase is composed of two main structural domains, V1 and V0, which work in concert to achieve transmembrane transport of protons. The V1 domain is located in the cytoplasm and is responsible for the hydrolysis of ATP, providing energy for the operation of the proton pump; the Vo domain is embedded in the cell membrane and directly participates in the transport of protons. This exquisite structural design enables V-ATPase to effectively regulate the pH environment inside and outside the cell, influencing cellular metabolic activities and signal transduction [2].
In cancer cells, the role of V-ATPase is particularly pronounced. A growing body of evidence indicates that the expression levels of V-ATPase subunits are abnormally elevated in various cancer cells and are closely related to the invasiveness and metastatic potential of cancer cells [3]. V-ATPase creates a microenvironment conducive to the growth and spread of tumor cells by regulating the acidity of the extracellular environment. Moreover, V-ATPase is also involved in the formation of drug resistance in cancer cells; its enhanced activity helps cancer cells survive under the pressure of chemotherapeutic drugs.
The dysfunction of V-ATPase not only affects the biological behavior of cancer cells but may also disrupt intracellular signaling pathways, such as the activity of key metabolic regulatory factors like mTORC1 and AMPK, thereby affecting the metabolic state and survival capacity of cancer cells [4]. These findings reveal the multifaceted role of V-ATPase in tumor development, making it a highly potential target in cancer therapy.
The purpose of this review is to synthesize current research progress on the role of V-ATPase in cancer cells, explore its mechanisms of action in tumor development, metastasis, and drug resistance formation, and discuss its potential and prospects as a target for cancer treatment. We will evaluate the application of V-ATPase inhibitors in cancer therapy and discuss how to develop new anti-cancer strategies by regulating the activity of V-ATPase. By deeply analyzing the role of V-ATPase in the tumor microenvironment, we hope to provide new perspectives and methods for cancer treatment, bring new hope to patients, and promote the development of this field, laying a solid foundation for future clinical applications.
A new perspective on the structure and function of V-ATPase
To better illustrate the structural components of V - ATPase, the following diagram is presented (Fig. 1). This diagram clearly shows the composition of the cytosolic V1 complex and the membrane-bound V0 complex, along with the arrangement of their subunits. Such a complex is crucial for maintaining intracellular pH balance and a variety of physiological processes [5].
Fig. 1.
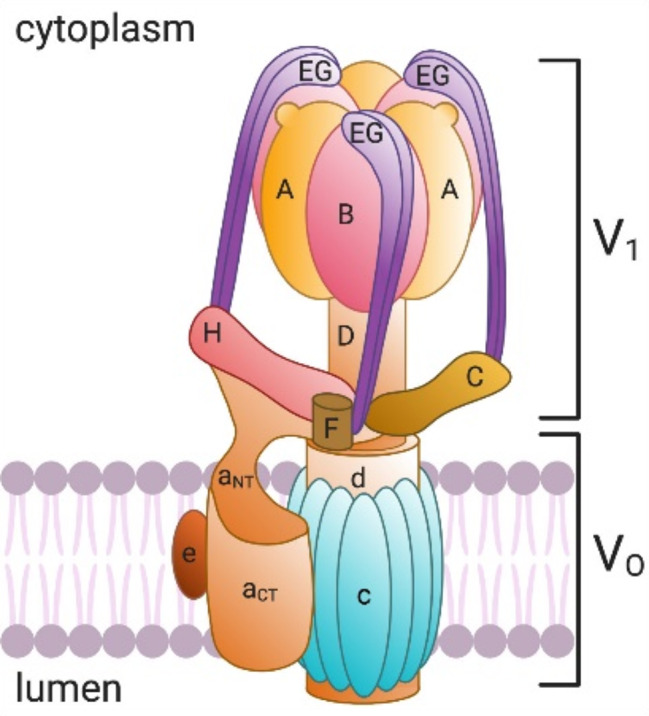
V-ATPase structure and subunit composition. Copyright 2021 American Journal of Physiology-Cell Physiology
The V1 complex is responsible for ATP hydrolysis and is made up of eight subunits: A, B, C, D, E, F, G, and H. Here, subunits A and B form the catalytic hexamer, subunit C forms a collar-like structure, subunits D and F form the central shaft, subunits E and G form the peripheral shaft, and subunit H forms the connecting arm. The V0 complex is responsible for the transmembrane transport of protons and consists of four subunits: a, c, d, and e. Subunit a has multiple isoforms, including a1, a2, a3, a4, etc., each with different localization and function within the cell [6]. For instance, the a1 subunit is primarily located in the presynaptic membrane and secretory vesicles [7, 8], the a2 subunit is located in early endosomes [9], the a3 subunit is located in lysosomes and osteoclasts [10], and the a4 subunit is mainly found in the kidney and efferent duct cells [11]. Subunit c forms a ring structure that participates in the transmembrane transport of protons, while subunits d and e form connecting arms [12].
In the catalytic process of V-ATPase, the hydrolysis of ATP leads to conformational changes in the A3B3 hexamer, which drives the rotation of the central shaft and protein lipid ring. Protons enter through the hydrophilic semi-channel of subunit a in the V0 domain and protonate the glutamate residues in each protein lipid subunit. As the protein lipid ring rotates, the protonated glutamate sequentially encounters key arginine residues in subunit a, allowing the protons to be released and expelled through the luminal-facing semi-channel. This rotational mechanism enables V-ATPase to efficiently transport protons from the cytoplasm to the interior of organelles or extracellular space, thereby maintaining the pH balance of the cell and organelles [13]. For example, in lysosomes, V-ATPase maintains an acidic environment that activates hydrolytic enzymes within lysosomes, which is crucial for the degradation of large molecules [14]. In addition, V-ATPase is also involved in the fusion of autophagosomes with lysosomes during the autophagy process, as well as the coupled transport of small molecules within the cell [15].
On the plasma membrane, specific V-ATPases participate in special cellular functions such as bone resorption [16, 17], sperm maturation [18], and urine acidification [19] by pumping protons into the extracellular space. However, the activity of V-ATPase is strictly regulated to ensure that necessary physiological processes occur within a specific pH range. For instance, the reversible assembly and disassembly of V-ATPase, which involves the separation and reassembly of the V1 and Vo domains, is an important form of its regulation in response to various signals and environmental conditions.
Recent research advances have significantly enhanced our understanding of the structure and function of human V-ATPase. Through advanced cryo-electron microscopy technology, researchers have revealed the detailed structure of V-ATPase in different functional states, where the luminal domain of the ATP6AP1 subunit exhibits a β-prism structure similar to that of LAMP family members. This finding breaks through traditional sequence homology boundaries and provides a new perspective on the assembly mechanism of V-ATPase. Moreover, research has identified glycolipids such as Dol-pp-glycan and lipids as key components of V-ATPase assembly and function, forming a complex glycoprotein-lipid complex with the protein subunits of V-ATPase. Mass spectrometry analysis further reveals that subunits of V-ATPase, especially ATP6AP1, a, and e, undergo N-linked glycosylation, playing a key role in protein stability, intracellular localization, and folding [20]. These details of structure and function not only provide new insights into the role of V-ATPase in cellular physiology but are also crucial for revealing its role in tumor biology. The increased activity and relocalization on the plasma membrane of V-ATPase are associated with the proliferation and metastasis of tumor cells, making it a potential target for cancer therapy. A deep understanding of the assembly mechanism, functional regulation, and the role of V-ATPase in tumor development is significant for developing new therapeutic strategies.
The interaction between V-ATPase, mTORC1 and AMPK
mTORC1 and AMPK are two key metabolic regulatory factors within the cell, working together to maintain cellular growth, metabolism, and energy balance. mTORC1 serves as the primary sensor for the cell’s response to nutrients and growth factors, promoting anabolic activities of the cell by facilitating processes such as protein synthesis, lipid synthesis, and DNA replication [21]. On the other hand, AMPK is activated when the cell faces energy deficiency, helping to restore energy balance by promoting energy-generating pathways like fatty acid oxidation, while simultaneously inhibiting energy-intensive synthetic processes, such as the synthesis of fatty acids and cholesterol [22].
V-ATPase plays an essential role in the cell’s metabolic state, not only participating in the regulation of intracellular pH and ion balance but also acting as a common regulatory hub for mTORC1 and AMPK, two key metabolic regulatory factors, through its critical role in amino acid sensing and mTORC1 activation [23].
In an environment with abundant nutrients, V-ATPase enhances the activity of mTORC1 by activating the GTPases RagA/B on the Ragulator complex, thereby promoting the cell’s anabolic metabolism. This process is crucial for the rapid proliferation of tumor cells and the synthesis of large biological molecules. However, when nutrients are scarce or the cell is under energy stress, the role of V-ATPase changes. Its interaction with the AXIN/LKB1-AMPK complex is enhanced, leading to the activation of AMPK and the inhibition of mTORC1. This shift prompts the cell to transition from anabolic to catabolic metabolism, conserving energy and adapting to adverse conditions. V-ATPase acts as a key regulatory point in this process, responding to the cell’s nutritional status through changes in its activity, thereby affecting the metabolism and survival of tumor cells [24].
Furthermore, the activity of V-ATPase on lysosomes may indirectly affect the perception of amino acids and the activity of mTORC1 by regulating the pH environment of the lysosomes. Amino acids are direct activators of mTORC1, so V-ATPase may have a significant impact on the activation of mTORC1 in this way [25, 26].
In tumor cells, the role of V-ATPase is more complex. Studies have shown that the regulatory response of AMPK induced by the inhibition of V-ATPase differs between tumor and non-tumor cells. The possible reasons or underlying mechanisms for the differential response of AMPK to V-ATPase inhibition in tumor cells compared to non-tumor cells have not been fully elucidated, but some potential explanations and unique properties of tumor cells may contribute to this specific regulatory response. Firstly, compared to non-tumor cells, tumor cells exhibit significant differences in energy metabolism and pH regulation mechanisms. They typically display higher metabolic activity and adaptability to acidic environments, which is related to their rapid proliferation needs and resistance to chemotherapeutic drugs. V-ATPase plays a crucial role in maintaining the pH balance inside and outside the cell, especially in the tumor microenvironment, where this balance is vital for the survival and invasiveness of tumor cells. Therefore, the inhibition of V-ATPase may have a more direct impact on the metabolic state of tumor cells, leading to changes in the activation status and responsiveness of AMPK. Secondly, AMPK in tumor cells may be decoupled from the activity of V-ATPase, possibly due to the abnormal upregulation of V-ATPase expression and activity in these cells. In non-tumor cells, the inhibition of V-ATPase leads to increased phosphorylation and lysosomal localization of AMPK, while in tumor cells, AMPK is already constitutively activated, and the inhibition of V-ATPases does not affect its phosphorylation and localization. This suggests that the regulation of AMPK in tumor cells may involve other signaling pathways that are more active in tumor cells or insensitive to V-ATPase inhibition [27]. Additionally, tumor cells may possess unique metabolic pathways and signaling networks that respond differently to the inhibition of V-ATPase. For example, tumor cells may rely on specific metabolic intermediates or stress signals to maintain AMPK activity, which may not exist or be less important in non-tumor cells. This difference may lead to a differential response of AMPK to V-ATPase inhibition in tumor and non-tumor cells. In summary, there are significant differences between tumor and non-tumor cells in terms of energy metabolism, pH regulation, the regulatory state of AMPK, and sensitivity to V-ATPase inhibition, which may explain the differential response of AMPK to the inhibition of V-ATPases.
In summary, V-ATPase plays a multifaceted role in the metabolism of tumor cells. It is not only directly involved in the regulation of cellular pH and ion balance but also affects the response and adaptation of tumor cells to changes in nutritional status through its interactive effects with mTORC1 and AMPK, two key metabolic regulatory factors. These findings emphasize the importance of V-ATPase in the metabolic regulation of tumor cells and provide potential targets for the development of new therapies targeting tumor metabolism.
The role of V-ATPase in the tumor microenvironment
There are known connections between V-ATPase activity, the increased energy requirements of cancer cells, and hypoxic conditions. V-ATPases are proton pumps that acidify intracellular compartments and the extracellular microenvironment in cancer cells. In cancer cells, V-ATPase activity is upregulated due to metabolic reprogramming to sustain rapid growth and proliferation by maintaining the acidic pH necessary for certain enzymes in glycolysis and other metabolic pathways, as well as being associated with enhanced endosomal trafficking for internalizing growth factors and nutrients for increased energy and biomass production. Additionally, V-ATPases contribute to extracellular acidification promoting tumor invasion, angiogenesis, and metastasis. Cancer cells have a high demand for energy and building blocks, meeting this through glycolysis even in the presence of oxygen (the Warburg effect) and lactate production that contributes to the acidic tumor microenvironment [28]. Hypoxia, a common feature of the tumor microenvironment due to rapid growth and inadequate blood supply, can induce the expression and activity of V-ATPases in cancer cells as a cellular adaptation to maintain ion homeostasis and cope with metabolic stress [29]. Hypoxic cancer cells may further upregulate glycolysis and other metabolic pathways dependent on V-ATPase-mediated acidification to sustain energy production and biomass synthesis. Hypoxia and the resulting activation of V-ATPases contribute to the aggressive behavior of cancer cells. So V-ATPase activity is connected to the increased energy requirements of cancer cells and hypoxic conditions through its role in maintaining the acidic pH for cancer cell metabolism, endosomal trafficking, and adaptation to the hypoxic tumor microenvironment, making it a potential target for anti-cancer therapies.
V-ATPase plays a pivotal role in tumor cells’ evasion of immune surveillance. This multi-subunit protein complex functions by pumping protons (H+) from the inside of the cell to the outside, thereby maintaining a pH gradient across the cell membrane. In tumor cells, the enhanced activity of V-ATPase leads to the acidification of the extracellular environment, creating an acidic tumor microenvironment (TME), which provides favorable conditions for the proliferation, survival, metastasis, and signal transduction of tumor cells [30].
V-ATPase plays a pivotal role in the polarization of macrophages towards an M2-like phenotype, with its mechanisms of action including the influence on intracellular pH and lysosomal function. For instance, overexpression of V-ATPase can increase lysosomal acidification, thereby enhancing the phagocytic capacity of macrophages. Moreover, V-ATPase promotes the polarization towards the M2-like phenotype by modulating the ERK/MAPK signaling pathway [31], as well as impacting the Lamtor1 and mTORC1 signaling pathways [32]. Specific signaling pathways and factors, such as the cytokines IL-4, IL-10, and IL-13, also play a crucial role in V-ATPase-mediated M2-like polarization [33]. The role of V-ATPase is not limited to creating an acidic environment conducive to tumor cells; it also directly inhibits the phagocytic ability of macrophages by maintaining an acidic extracellular pH and tends to recruit macrophages with weaker phagocytic capabilities. Moreover, this acidic environment may be involved in polarizing tumor-associated macrophages (TAMs) to an M2-like phenotype, which impairs their ability to detect and recognize cancer cells. Pharmacological or gene-editing techniques targeting V-ATPase can restore the sensitivity of cancer cells to macrophage-mediated programmed cell clearance (PrCR), helping to inhibit tumor development. Specifically, the blocking of V-ATPase can enhance the expression of the ferritin receptor CD71 on the surface of cancer cells, making them more susceptible to antibody targeting and phagocytosis [34, 35].
Additionally, V-ATPase affects the interaction between tumor cells and immune cells by regulating the pH of the endoplasmic reticulum and influencing cholesterol metabolism within tumor cells. For example, the ATP6V0A1 subunit promotes cholesterol absorption and accumulation, leading to increased cholesterol levels in the endoplasmic reticulum and increased production of 24-hydroxycholesterol (24-OHC). As a typical liver X receptor (LXR) agonist, 24-OHC promotes tumor cell immune evasion by activating the LXR signaling pathway. At the same time, 24-OHC induced by the ATP6V0A1 subunit can also suppress the activity of memory CD8+ T cells by activating the TGF-β1 signaling pathway, thereby weakening their anti-tumor activity [36].
On the other hand, the expression of ATP6V1B1 is associated with the resistance of tumor cells to antibody-dependent cellular cytotoxicity (ADCC). The reduced activity of V-ATPase leads to acidification of the intracellular environment of tumor cells, which is not conducive to the biological activity of granzymes released by natural killer cells (NK), as granzymes are most active at neutral pH. Therefore, the downregulation of ATP6V1B1 changes the cytoplasmic pH of tumor cells, creating an environment unfavorable to the biological activity of granzymes, thus affecting the ability of NK cells to induce apoptosis in tumor cells [37].
In summary, V-ATPase plays a key role in tumor cells’ evasion of immune surveillance by regulating intracellular pH and cholesterol metabolism. Targeting specific subunits of V-ATPase, such as ATP6V0A1 and ATP6V1B1, may provide potential targets for the development of new immunotherapy strategies.
The role of V-ATPase in tumor drug resistance
In tumor cells, the abnormal activity of V-ATPase is closely related to the formation of multidrug resistance (MDR). MDR is one of the main reasons for the failure of chemotherapy, involving the tumor cells’ resistance to one anticancer drug and then cross-resistance to other drugs that are structurally and pharmacologically unrelated. V-ATPase provides a defense mechanism for tumor cells by maintaining the alkalization of intracellular pH and the acidification of the extracellular environment, which helps them survive under the action of chemotherapy drugs.
The presence of V-ATPase isoforms leads to functional diversity of V-ATPase, which in turn is related to multidrug resistance in tumor cells. On the one hand, different subunit isoforms may affect the proton transport efficiency of V-ATPase, thereby affecting the pH values inside and outside the cells [38]. Tumor cells often escape apoptosis by reducing the extracellular pH value and maintaining the intracellular alkalinity, thereby promoting the emergence of multidrug resistance [39]. On the other hand, V-ATPase isoforms may be involved in pumping weakly basic drugs out of cells, reducing the concentration of drugs in cells, thereby reducing drug efficacy and leading to drug resistance [40]. In addition, V-ATPase isoforms may also affect the function of lysosomes, including the degradation of drugs and endocytic bodies [41]. Changes in lysosomal function may lead to accelerated drug degradation or endocytic body dysfunction, thereby affecting drug uptake and action and promoting drug resistance. Although some studies have shown that ATP6L (the c subunit of the V0 domain) and ATP6V0A1 (the a subunit of the V0 domain) may be involved in the resistance mechanisms of specific types of cancer [42], the specific relationship between V-ATPase isoforms and the resistance mechanisms of different types of cancer still needs further research to find more effective strategies for overcoming drug resistance targeted at V-ATPase subunits.
In tumor cells, the overexpression or enhanced activity of V-ATPase can lead to an increase in intracellular pH, and this reversal of the pH gradient is associated with the development of MDR. For example, in non-small cell lung cancer (NSCLC), the combination of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) with V-ATPase inhibitors has been found to enhance cell death, as V-ATPase inhibitors (such as bafilomycin A1 or concanamycin A) can induce the expression of BCL2/adenovirus E1B 19 kDa interacting protein 3 (BNIP3), and the activation of hypoxia inducible factor 1α (HIF-1α) depends on this expression. The induction of HIF-1α and BNIP3 is thought to promote resistance to cell death, while EGFR TKIs can inhibit the expression of Hif-1α and BNIP3 induced by V-ATPase inhibitors, thereby enhancing the sensitivity of cells to these inhibitors [43].
Furthermore, the activity of V-ATPase in tumor cells also involves the uptake and accumulation of chemotherapy drugs by cells. Since V-ATPase can regulate the pH of acidic vesicles within the cell, chemotherapy drugs may be sequestered and inactivated in these vesicles, thereby reducing the toxic effect of the drugs on tumor cells. This mechanism is a way for tumor cells to evade the attack of chemotherapy drugs [44]. Therefore, V-ATPase not only participates in the formation of MDR by regulating the pH gradient but may also promote the development of drug resistance by affecting the distribution and accumulation of drugs within cells.
In some cases, inhibitors of V-ATPase can reverse the drug resistance of tumor cells to certain chemotherapy drugs, indicating that V-ATPase is a potential therapeutic target [45]. Intervention targeting V-ATPase may help to improve the efficacy of chemotherapy drugs and overcome the drug resistance of tumor cells. However, the exact mechanism of action of V-ATPase in tumor cells still needs further research to develop more effective cancer treatment strategies.
The regulatory role of V-ATPase in atypical autophagy and its potential for cancer therapy
The regulatory mechanism of V-ATPase in atypical autophagy involves its direct recruitment and activity modulation on the single-membrane structures of endosomes and lysosomes. Specifically, V-ATPase, as a multi-subunit proton pump, regulates the transmembrane transport of protons through the reversible association of its V0-V1 complex, a process crucial for maintaining the acidic environment of endosomes and lysosomes. In non-canonical autophagy, the V0-V1 association of V-ATPase is enhanced, promoting the recruitment of ATG16L1, which is achieved through the interaction of the C-terminal WD40 domain of ATG16L1 with V-ATPase, and this interaction depends on specific amino acid residues on ATG16L1, such as K490. This recruitment and binding result in the atypical lipidation of ATG8 family proteins (such as LC3) on the single-membrane structures of endosomes and lysosomes, known as CASM (Conjugation of ATG8 to Single Membranes), a hallmark event of non-canonical autophagy [46, 47].
Furthermore, the activity of V-ATPase is regulated by various factors, including ion concentration, pH balance, and interaction with ATG16L1. For example, certain pathogen effector proteins (such as the SopF protein of Salmonella) can inhibit the interaction of V-ATPase with ATG16L1 by ADP-ribosylation specific subunits of V-ATPase, thereby blocking the non-canonical autophagy process [47–49]. This regulatory mechanism plays a role not only in the internal autophagy process of the cell but also has an important role in the cell’s immune response to foreign pathogens.
In the context of cancer therapy, V-ATPase has become a potential therapeutic target due to its key role in non-canonical autophagy. By using specific V-ATPase inhibitors, such as 249 C, the non-canonical autophagy process can be effectively blocked, thereby inhibiting the growth of tumor cells that depend on the non-canonical autophagy pathway. 249 C binds to the ATP6V1H subunit of V-ATPase, inhibiting its activity and causing the acidification of lysosomes to be hindered, which in turn affects autophagy and macropinocytosis. This is selectively toxic to certain KRAS mutant cancer cells [50]. These cells are particularly sensitive to the inhibition of V-ATPase because they overly rely on V-ATPase for energy metabolism and nutrient supply. This provides new ideas for the development of precision treatment strategies for specific KRAS mutant cancers.
Therapeutic potential of V-ATPase in cancer treatment
An increasing number of studies suggest a correlation between the upregulation of V-ATPase and shorter patient survival or cancer resistance. One study demonstrated that the upregulation of V-ATPase promotes resistance to anoikis (a form of cell death associated with detachment from the extracellular matrix) and tumor metastasis through the activation of Signal Transducer and Activator of Transcription 3 (STAT3) [51]. The study indicated that V-ATPase is upregulated in cancer cells upon detachment from the ECM, which is crucial for cancer cells to evade anoikis. In this study, the inhibition of V-ATPase sensitized human cervical cancer, breast cancer, and mouse melanoma cells to anoikis by increasing the production of reactive oxygen species (ROS), the accumulation of misfolded proteins, and impairing lung metastasis in vivo. Furthermore, the resistance to anoikis and the clearance of misfolded protein aggregates in tumor cells can be restored by scavenging ROS. Mechanistically, STAT3 upregulates the expression of V-ATPase, while the blockade of STAT3 activity inhibits the expression of V-ATPase, sensitizing cells to anoikis, increasing ROS production, and the accumulation of misfolded proteins. These data reveal an unreported role of STAT3 in mediating the upregulation of V-ATPase to promote resistance to anoikis, thus providing an alternative target for cancer metastasis. This study supports the correlation between the upregulation of V-ATPase and cancer resistance and tumor metastasis, which may be associated with reduced patient survival.
The V-ATPase exhibits complex specific expression and functions in different types of tumors, with its subunit isoforms closely related to the invasiveness, metastatic potential, and resistance to chemotherapy drugs of tumors. These findings provide a scientific basis for the subunits or isoforms of V-ATPase as potential molecular markers for tumor diagnosis and prognosis. In particular, recent studies have identified a specific transmembrane protein TM9SF4, which is closely related to the regulation of V-ATPase activity. TM9SF4 interacts with the ATP6V1H subunit of V-ATPase, and this interaction is crucial for the assembly and activity of V-ATPase. In colorectal cancer, the inhibition of TM9SF4 reverses the tumor cell pH gradient by reducing the assembly of the V-ATPase V0/V1 subunits, lowering the cytoplasmic pH, and increasing the pH of intracellular vesicles and the extracellular environment. This reversal of the pH gradient is associated with a significant reduction in the invasive behavior of tumor cells and an increased sensitivity to the chemotherapy drug 5-fluorouracil. This suggests that TM9SF4 not only plays a key role in the regulation of V-ATPase activity but may also play an important role in the formation of tumor cell invasiveness and drug resistance [52]. In breast cancer, the expression levels of the a3 and a4 subunit isoforms are significantly higher in cells with low invasiveness, suggesting that they may serve as markers to distinguish tumors from normal tissues. In particular, the knockdown of the a3 subunit in MDA-MB-231 breast cancer cells significantly reduced their invasiveness in vitro, while overexpression of a3 in MCF10a cells increased invasiveness and the localization of V-ATPase on the plasma membrane, highlighting its key role in breast cancer invasiveness [53]. In addition, in esophageal squamous cell carcinoma (ESCC), studies have found differences in the expression patterns of the V-ATPase C subunit isoforms, especially the high C1 and low C2 expression pattern, which can accurately distinguish ESCC from normal tissues. This suggests that the expression ratio of V-ATPase subunits or isoforms may form a conformation code that controls the regulation of the H+ pump and interactions related to tumor occurrence [54].
In hepatocellular carcinoma (HCC), the high expression of the V-ATPase subunit ATP6V1F indicates its role in the progression of HCC [55]. In gastric cancer, the V1A subunit of V-ATPase is positively correlated with tumor grade, pathological type, and grade, and is associated with drug resistance in tumor samples, further emphasizing the multifaceted role of V-ATPase in tumor development [56].
Further studies have also found that in prostate cancer, the inhibition of V-ATPase can reduce the activity of the mutated androgen receptor (AR), indicating that the regulation of V-ATPase may be related to the hormone sensitivity and castration resistance of prostate cancer [57]. In breast cancer, the silencing of ATP6V1C1 can inhibit tumor growth and bone metastasis, suggesting that ATP6V1C1 may promote cancer growth and metastasis by activating V-ATPase activity [58]. In lung cancer, FBXO9 inhibits the migration, tumor sphere growth, and metastasis of lung cancer cells by promoting the ubiquitination of the V-ATPase catalytic subunit A (ATP6V1A) [59].
The specific expression and dysfunction of V-ATPase subunits or isoforms in various types of tumors provide a scientific basis for their potential as tumor diagnostic and prognostic molecular markers. These findings emphasize the multifaceted role of V-ATPase subunits or isoforms in tumor development, including promoting the invasion, metastasis, and chemoresistance of tumor cells. Therefore, specific subunits or isoforms of V-ATPase may become important biomarkers for early cancer diagnosis, disease progression monitoring, and prognostic assessment. However, to realize the potential of these subunits or isoforms as clinical markers, prospective studies in a broader patient population are needed to verify their sensitivity, specificity, and predictive value. In addition, future research also needs to explore the specific mechanisms of V-ATPase subunits or isoforms in the tumor microenvironment and how they interact with other biomarkers to provide more comprehensive molecular information for precision cancer treatment.
Future research directions
In recent years, V-ATPase has garnered widespread attention as an emerging target for cancer therapy, mainly due to its critical role in the invasiveness and drug resistance of tumor cells. Studies have confirmed that V-ATPase inhibitors such as Concana-mycin A and Bafilomycin A1 can inhibit growth and induce cell death in various tumor cells [60]. In addition, new V-ATPase inhibitors like Salicylihalamide and NIK-12,192 have also shown significant anti-tumor activity in preclinical studies. These inhibitors effectively prevent the invasion and metastasis of tumor cells by disrupting their metabolism and endocytic pathways [61].
The following table compiles key data from a series of clinical studies, offering a snapshot of the current research landscape. It outlines the phase of the study, the names of the drugs involved, the types of cancer targeted, the cell lines used in the research, and any additional pertinent notes. This compilation serves to illustrate the breadth of ongoing investigations and the specific focuses of each study, providing a framework for understanding the practical applications and experimental designs shaping the future of V-ATPase inhibitor research in cancer therapy (Table 1).
Table 1.
Overview of clinical studies involving V-ATPase inhibitors
| Study Phase | Drug Name | Cancer Type | Cell Lines | Additional Notes |
|---|---|---|---|---|
| Phase I | Bafilomycin A1 | Non-Small Cell Lung Cancer | H1975, A549 | Dose escalation study [62] |
| Phase II | Concanamycin A | Metastatic Breast Cancer | Hs 578T, SK-BR-3 | Efficacy and safety [63] |
| Phase I/II | Saliphenylhalamide | Multiple tumors | Many tumor cell lines | antiproliferative activity [64] |
| Phase I | NIK-12,192 | Multiple tumors | Many tumor cell lines | Single-agent study [65] |
| Phase II | TM9SF4 inhibitor | Colorectal Cancer | HCT116, SW480 | Targeting V-ATPase assembly [52] |
| Phase I | V-ATPase shRNA | Hepatocellular Carcinoma | HCCLM3 | Gene silencing approach [66] |
| Phase II | Archazolid B | Bladder Cancer | T24 | Resistance modulation [67] |
As our understanding of the role of V-ATPase in tumor biology deepens, future research will focus more on elucidating its specific mechanisms of action in the tumor microenvironment, including how it regulates immune responses, affects intracellular pH balance, and participates in the metabolic reprogramming of cancer cells. This knowledge will provide a theoretical basis for the development of more specific, less toxic V-ATPase inhibitors and help assess their potential in the treatment of different types of cancer.
Clinical application research will concentrate on improving existing inhibitors and discovering new compounds that target V-ATPase in cancer cells more effectively while reducing the impact on normal cells. Additionally, research will explore the combined use of V-ATPase inhibitors with other treatment methods (such as chemotherapy, radiotherapy, and immunotherapy) to enhance therapeutic effects and overcome multidrug resistance in tumors. This may involve studying the expression patterns of V-ATPase in different tumor microenvironments and its role at various stages of tumor development.
Ultimately, preclinical studies and clinical trials will assess the safety, efficacy, and optimal dosing strategies of V-ATPase inhibitors, providing key scientific evidence for their application in cancer therapy. These studies will help develop future treatment strategies, potentially making V-ATPase inhibitors a single drug or in combination with other anti-cancer drugs to improve therapeutic outcomes and overcome drug resistance in tumors. Despite challenges, including ensuring the specificity of the drug, reducing potential side effects, and developing effective drug delivery systems, the prospects for the clinical application of V-ATPase inhibitors remain broad and promising, offering new treatment options for cancer patients.
Conclusions
As highlighted in this review, V-ATPase is a multifaceted enzyme with significant implications in cancer biology. It underscores the enzyme’s critical role in maintaining intracellular pH homeostasis and its profound impact on the physiological processes within cancer cells. The enhanced activity of V-ATPase is intimately linked to the proliferation, metastasis, and invasion capabilities of tumor cells, as it modulates the tumor microenvironment’s pH. Moreover, V-ATPase’s interaction with metabolic regulators like mTORC1 and AMPK influences the metabolic state of cancer cells, highlighting its potential as a target for therapeutic intervention. The review also elucidates V-ATPase’s involvement in tumor drug resistance and its regulatory mechanism in non-canonical autophagy, presenting new avenues for cancer therapy. By targeting V-ATPase or its specific subunits, it may be possible to develop novel strategies to combat cancer progression and overcome drug resistance. Future research is poised to focus on unraveling the intricate mechanisms of V-ATPase in the tumor microenvironment and translating these insights into clinical applications, offering a promising horizon for the development of precision cancer treatments.
Acknowledgements
Not applicable.
Author contributions
Tingting Chen wrote the main maunuscript text and all authors reviewed the manuscript.
Funding
This work was supported by Doctoral Research Startup Fund (4SG24298G), Youth Talent Program of Jinzhou city and Liaoning Province (JXYC230106, XLYC2203096) and Natural Science Foundation of Science and Technology Department of Liaoning Province (2022-MS-384).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Banerjee S, Kane PM. Regulation of V-ATPase activity and organelle pH by Phosphatidylinositol Phosphate Lipids. Front Cell Dev Biol. 2020;8:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayek SR, Rane HS, Parra KJ. Reciprocal regulation of V-ATPase and glycolytic pathway elements in Health and Disease. Front Physiol. 2019;10:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuire C, Cotter K, Stransky L, Forgac M. Regulation of V-ATPase assembly and function of V-ATPases in tumor cell invasiveness. Biochim Biophys Acta. 2016;1857:1213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang CS, Hawley SA, Zong Y, Li M, Wang Z, Gray A, Ma T, Cui J, Feng JW, Zhu M, et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. 2017;548:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins MP, Forgac M. Regulation and function of V-ATPases in physiology and disease. Biochim Biophys Acta Biomembr. 2020;1862:183341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshansky V, Rubinstein JL, Gruber G. Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta. 2014;1837:857–79. [DOI] [PubMed] [Google Scholar]
- 7.Peri F, Nusslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–27. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, et al. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol. 2006;8:124–36. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Kou H, Demy DL, Liu W, Li J, Wen Z, Herbomel P, Huang Z, Zhang W, Xu J. The different roles of V-ATPase a subunits in phagocytosis/endocytosis and autophagy. Autophagy 2024:1–17. [DOI] [PMC free article] [PubMed]
- 11.Gleize V, Boisselier B, Marie Y, Poea-Guyon S, Sanson M, Morel N. The renal v-ATPase a4 subunit is expressed in specific subtypes of human gliomas. Glia. 2012;60:1004–12. [DOI] [PubMed] [Google Scholar]
- 12.Cotter K, Stransky L, McGuire C, Forgac M. Recent insights into the structure, regulation, and function of the V-ATPases. Trends Biochem Sci. 2015;40:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oot RA, Couoh-Cardel S, Sharma S, Stam NJ, Wilkens S. Breaking up and making up: the secret life of the vacuolar H(+) -ATPase. Protein Sci. 2017;26:896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Sha Z, Tang Y, Jin C, Gao W, Chen C, Yu L, Lv N, Liu S, Xu F, et al. Defective Lamtor5 leads to autoimmunity by deregulating v-ATPase and lysosomal acidification. Adv Sci (Weinh). 2024;11:e2400446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timimi L, Wrobel AG, Chiduza GN, Maslen SL, Torres-Mendez A, Montaner B, Davis C, Minckley T, Hole KL, Serio A, et al. The V-ATPase/ATG16L1 axis is controlled by the V(1)H subunit. Mol Cell. 2024;84:2966–e29832969. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Z, Wang X, Ma Y, Duan X. Atp6v1h Deficiency blocks Bone loss in simulated microgravity mice through the Fos-Jun-Src-Integrin Pathway. Int J Mol Sci. 2024;25:637. [DOI] [PMC free article] [PubMed]
- 17.Chu A, Zirngibl RA, Manolson MF. The V-ATPase a3 subunit: structure, function and therapeutic potential of an essential Biomolecule in Osteoclastic Bone Resorption. Int J Mol Sci. 2021;22:6934. [DOI] [PMC free article] [PubMed]
- 18.Battistone MA, Merkulova M, Park YJ, Peralta MA, Gombar F, Brown D, Breton S. Unravelling purinergic regulation in the epididymis: activation of V-ATPase-dependent acidification by luminal ATP and adenosine. J Physiol. 2019;597:1957–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourgeois S, Bounoure L, Mouro-Chanteloup I, Colin Y, Brown D, Wagner CA. The ammonia transporter RhCG modulates urinary acidification by interacting with the vacuolar proton-ATPases in renal intercalated cells. Kidney Int. 2018;93:390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Wu D, Robinson CV, Wu H, Fu TM. Structures of a complete human V-ATPase reveal mechanisms of its Assembly. Mol Cell. 2020;80:501–e511503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang C, Tan X, Liu N, Yan P, Hou T, Wei W. Nutrient sensing of mTORC1 signaling in cancer and aging. Semin Cancer Biol. 2024;106–107:1–12. [DOI] [PubMed]
- 22.Langer HT, Rohm M, Goncalves MD, Sylow L. AMPK as a mediator of tissue preservation: time for a shift in dogma? Nat Rev Endocrinol. 2024;20:526–40. [DOI] [PubMed] [Google Scholar]
- 23.Lin SC, Hardie DG. AMPK: sensing glucose as well as Cellular Energy Status. Cell Metab. 2018;27:299–313. [DOI] [PubMed] [Google Scholar]
- 24.Zhang CS, Jiang B, Li M, Zhu M, Peng Y, Zhang YL, Wu YQ, Li TY, Liang Y, Lu Z, et al. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 2014;20:526–40. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012;18:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartel K, Muller R, von Schwarzenberg K. Differential regulation of AMP-activated protein kinase in healthy and cancer cells explains why V-ATPase inhibition selectively kills cancer cells. J Biol Chem. 2019;294:17239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barba I, Carrillo-Bosch L, Seoane J. Targeting the Warburg Effect in Cancer: where do we stand? Int J Mol Sci. 2024;25:3142. [DOI] [PMC free article] [PubMed]
- 29.Chen M, Lu J, Wei W, Lv Y, Zhang X, Yao Y, Wang L, Ling T, Zou X. Effects of Proton pump inhibitors on reversing multidrug resistance via downregulating V-ATPases/PI3K/Akt/mTOR/HIF-1alpha signaling pathway through TSC1/2 complex and Rheb in human gastric adenocarcinoma cells in vitro and in vivo. Onco Targets Ther. 2018;11:6705–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barar J, Omidi Y. Dysregulated pH in Tumor Microenvironment checkmates Cancer Therapy. Bioimpacts. 2013;3:149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Dai Y, Chu L. V-ATPase B2 promotes microglial phagocytosis of myelin debris by inactivating the MAPK signaling pathway. Neuropeptides. 2024;106:102436. [DOI] [PubMed] [Google Scholar]
- 32.Kimura T, Nada S, Takegahara N, Okuno T, Nojima S, Kang S, Ito D, Morimoto K, Hosokawa T, Hayama Y, et al. Polarization of M2 macrophages requires Lamtor1 that integrates cytokine and amino-acid signals. Nat Commun. 2016;7:13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Wu Q, Geller DA, Yan Y. Macrophage metabolism, phenotype, function, and therapy in hepatocellular carcinoma (HCC). J Transl Med. 2023;21:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Y, Li X, Dong D, Zhang B, Xue Y, Shang P. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am J Cancer Res. 2018;8:916–31. [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Cao X, Li B, Zhao Z, Chen S, Lai SWT, Muend SA, Nossa GK, Wang L, Guo W, et al. Warburg Effect is a Cancer Immune Evasion mechanism against macrophage immunosurveillance. Front Immunol. 2020;11:621757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang TX, Huang HS, Dong SW, Chen JY, Zhang B, Li HH, Zhang TT, Xie Q, Long QY, Yang Y, et al. ATP6V0A1-dependent cholesterol absorption in colorectal cancer cells triggers immunosuppressive signaling to inactivate memory CD8(+) T cells. Nat Commun. 2024;15:5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishie M, Suzuki E, Hattori M, Kawaguch K, Kataoka TR, Hirata M, Pu F, Kotake T, Tsuda M, Yamaguchi A, et al. Downregulated ATP6V1B1 expression acidifies the intracellular environment of cancer cells leading to resistance to antibody-dependent cellular cytotoxicity. Cancer Immunol Immunother. 2021;70:817–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasanthakumar T, Rubinstein JL. Structure and roles of V-type ATPases. Trends Biochem Sci. 2020;45:295–307. [DOI] [PubMed] [Google Scholar]
- 39.Jahan S, Mukherjee S, Ali S, Bhardwaj U, Choudhary RK, Balakrishnan S, Naseem A, Mir SA, Banawas S, Alaidarous M et al. Pioneer Role of Extracellular Vesicles as Modulators of Cancer Initiation in Progression, Drug Therapy, and Vaccine Prospects. Cells 2022, 11. [DOI] [PMC free article] [PubMed]
- 40.Shaikh S, Nandy SK, Canti C, Lavandero S. Bafilomycin-A1 and ML9 exert different lysosomal actions to Induce Cell Death. Curr Mol Pharmacol. 2019;12:261–71. [DOI] [PubMed] [Google Scholar]
- 41.Xia Y, Liu N, Xie X, Bi G, Ba H, Li L, Zhang J, Deng X, Yao Y, Tang Z, et al. The macrophage-specific V-ATPase subunit ATP6V0D2 restricts inflammasome activation and bacterial infection by facilitating autophagosome-lysosome fusion. Autophagy. 2019;15:960–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You H, Jin J, Shu H, Yu B, De Milito A, Lozupone F, Deng Y, Tang N, Yao G, Fais S, et al. Small interfering RNA targeting the subunit ATP6L of proton pump V-ATPase overcomes chemoresistance of breast cancer cells. Cancer Lett. 2009;280:110–9. [DOI] [PubMed] [Google Scholar]
- 43.Jin HO, Hong SE, Kim CS, Park JA, Kim JH, Kim JY, Kim B, Chang YH, Hong SI, Hong YJ, et al. Combined effects of EGFR tyrosine kinase inhibitors and vATPase inhibitors in NSCLC cells. Toxicol Appl Pharmacol. 2015;287:17–25. [DOI] [PubMed] [Google Scholar]
- 44.Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y, Yasui N, Yoneda T. The a3 isoform vacuolar type H(+)-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res. 2011;9:845–55. [DOI] [PubMed] [Google Scholar]
- 45.Daniel C, Bell C, Burton C, Harguindey S, Reshkin SJ, Rauch C. The role of proton dynamics in the development and maintenance of multidrug resistance in cancer. Biochim Biophys Acta. 2013;1832:606–17. [DOI] [PubMed] [Google Scholar]
- 46.Cross J, Durgan J, McEwan DG, Tayler M, Ryan KM, Florey O. Lysosome damage triggers direct ATG8 conjugation and ATG2 engagement via non-canonical autophagy. J Cell Biol. 2023;222:e202303078. [DOI] [PMC free article] [PubMed]
- 47.Hooper KM, Jacquin E, Li T, Goodwin JM, Brumell JH, Durgan J, et al. V-ATPase is a universal regulator of LC3-associated phagocytosis and non-canonical autophagy. J Cell Biol. 2022;221:e202105112. [DOI] [PMC free article] [PubMed]
- 48.Xu Y, Zhou P, Cheng S, Lu Q, Nowak K, Hopp AK, Li L, Shi X, Zhou Z, Gao W, et al. A bacterial effector reveals the V-ATPase-ATG16L1 Axis that initiates Xenophagy. Cell. 2019;178:552–e566520. [DOI] [PubMed] [Google Scholar]
- 49.Durgan J, Florey O. Many roads lead to CASM: diverse stimuli of noncanonical autophagy share a unifying molecular mechanism. Sci Adv. 2022;8:eabo1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tolani B, Celli A, Yao Y, Tan YZ, Fetter R, Liem CR, de Smith AJ, Vasanthakumar T, Bisignano P, Cotton AD, et al. Ras-mutant cancers are sensitive to small molecule inhibition of V-type ATPases in mice. Nat Biotechnol. 2022;40:1834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adeshakin FO, Adeshakin AO, Liu Z, Lu X, Cheng J, Zhang P, Yan D, Zhang G, Wan X. Upregulation of V-ATPase by STAT3 activation promotes Anoikis Resistance and Tumor Metastasis. J Cancer. 2021;12:4819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lozupone F, Borghi M, Marzoli F, Azzarito T, Matarrese P, Iessi E, Venturi G, Meschini S, Canitano A, Bona R, et al. TM9SF4 is a novel V-ATPase-interacting protein that modulates tumor pH alterations associated with drug resistance and invasiveness of colon cancer cells. Oncogene. 2015;34:5163–74. [DOI] [PubMed] [Google Scholar]
- 53.Su K, Collins MP, McGuire CM, Alshagawi MA, Alamoudi MK, Li Z, Forgac M. Isoform a4 of the vacuolar ATPase a subunit promotes 4T1-12B breast cancer cell-dependent tumor growth and metastasis in vivo. J Biol Chem. 2022;298:102395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Couto-Vieira J, Nicolau-Neto P, Costa EP, Figueira FF, Simao TA, Okorokova-Facanha AL, Ribeiro Pinto LF, Facanha AR. Multi-cancer V-ATPase molecular signatures: a distinctive balance of subunit C isoforms in esophageal carcinoma. EBioMedicine. 2020;51:102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu X, Li D, Zhu H, Yu T, Xiong X, Xu X. ATP6V1F is a novel prognostic biomarker and potential immunotherapy target for hepatocellular carcinoma. BMC Med Genomics. 2023;16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu P, Chen H, Han L, Zou X, Shen W. Expression and role of V1A subunit of V-ATPases in gastric cancer cells. Int J Clin Oncol. 2015;20:725–35. [DOI] [PubMed] [Google Scholar]
- 57.Whitton B, Okamoto H, Rose-Zerilli M, Packham G, Crabb SJ. V-ATPase inhibition decreases mutant androgen receptor activity in castrate-resistant prostate Cancer. Mol Cancer Ther. 2021;20:739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feng S, Zhu G, McConnell M, Deng L, Zhao Q, Wu M, Zhou Q, Wang J, Qi J, Li YP, Chen W. Silencing of atp6v1c1 prevents breast cancer growth and bone metastasis. Int J Biol Sci. 2013;9:853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu L, Chen X, Wu L, Huang K, Wang Z, Zheng Y, Zheng C, Zhang Z, Chen J, Wei J, et al. Ubiquitin ligase subunit FBXO9 inhibits V-ATPase assembly and impedes lung cancer metastasis. Exp Hematol Oncol. 2024;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cotter K, Capecci J, Sennoune S, Huss M, Maier M, Martinez-Zaguilan R, Forgac M. Activity of plasma membrane V-ATPases is critical for the invasion of MDA-MB231 breast cancer cells. J Biol Chem. 2015;290:3680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asgharzadeh MR, Barar J, Pourseif MM, Eskandani M, Jafari Niya M, Mashayekhi MR, Omidi Y. Molecular machineries of pH dysregulation in tumor microenvironment: potential targets for cancer therapy. Bioimpacts. 2017;7:115–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alizadeh J, Glogowska A, Thliveris J, Kalantari F, Shojaei S, Hombach-Klonisch S, Klonisch T, Ghavami S. Autophagy modulates transforming growth factor beta 1 induced epithelial to mesenchymal transition in non-small cell lung cancer cells. Biochim Biophys Acta Mol Cell Res. 2018;1865:749–68. [DOI] [PubMed] [Google Scholar]
- 63.Pereira CS, Guedes JP, Goncalves M, Loureiro L, Castro L, Geros H, Rodrigues LR, Corte-Real M. Lactoferrin selectively triggers apoptosis in highly metastatic breast cancer cells through inhibition of plasmalemmal V-H+-ATPase. Oncotarget. 2016;7:62144–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lebreton S, Jaunbergs J, Roth MG, Ferguson DA, De Brabander JK. Evaluating the potential of vacuolar ATPase inhibitors as anticancer agents and multigram synthesis of the potent salicylihalamide analog saliphenylhalamide. Bioorg Med Chem Lett. 2008;18:5879–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Supino R, Scovassi AI, Croce AC, Dal Bo L, Favini E, Corbelli A, Farina C, Misiano P, Zunino F. Biological effects of a new vacuolar-H,-ATPase inhibitor in colon carcinoma cell lines. Ann N Y Acad Sci. 2009;1171:606–16. [DOI] [PubMed] [Google Scholar]
- 66.Lu X, Qin W, Li J, Tan N, Pan D, Zhang H, Xie L, Yao G, Shu H, Yao M, et al. The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res. 2005;65:6843–9. [DOI] [PubMed] [Google Scholar]
- 67.Hamm R, Chen YR, Seo EJ, Zeino M, Wu CF, Muller R, Yang NS, Efferth T. Induction of cholesterol biosynthesis by archazolid B in T24 bladder cancer cells. Biochem Pharmacol. 2014;91:18–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.