Abstract
Objectives
The aim of the present study is to evaluate the accuracy of a digital template on the three-dimensional accuracy of edentulous implantation through a retrospective study to provide more clinical evidence for the use of digital templates in edentulous patient.
Materials and methods
This study evaluates the efficacy of a digital surgical template in edentulous jaws, comparing preoperative plans with postoperative outcomes across four metrics: platform, apex, depth, and angular deviations. Utilizing a patient with an edentulous maxilla as a case study, this research employs CBCT for preoperative and postoperative assessments, with deviations analyzed via 3-Shape software. Comparing these deviations with average deviations in lierature.
Results
The average platform deviations at positions 12, 14, 16, 22, 24, 26 were 0.98 ± 0.03 mm, 1.43 ± 0.02 mm, 1.27 ± 0.04 mm, 1.35 ± 0.03 mm, 1.34 ± 0.02 mm, and 1.42 ± 0.03 mm, respectively. The average apex deviations were 1.28 ± 0.02 mm, 1.39 ± 0.03 mm, 1.47 ± 0.04 mm, 1.26 ± 0.04 mm, 1.40 ± 0.04 mm, and 1.48 ± 0.03 mm, respectively, the average angular deviations were 3.50°± 0.08°, 2.87°± 0.07°, 3.49°± 0.06°, 3.36°± 0.10°, 3.41°± 0.13°, and 3.69°± 0.11°, and average depth deviations were 0.29 ± 0.03 mm, 0.26 ± 0.05 mm, 0.59 ± 0.05 mm, 0.28 ± 0.04 mm, 0.47 ± 0.02 mm, 0.53 ± 0.03 mm. Compared with a total mean deviation of 1.2 mm (1.04 mm to 1.44 mm) of platform deviation, 1.4 mm (1.28 mm to 1.58 mm) of apex deviation, angular deviation of 3.5°(3.0° to 3.96°) and depth deviation of 0.2 mm (-0.25 mm to 0.57 mm) reported in literature. While all measured deviations fell within clinically acceptable limits, certain parameters exceeded the benchmarks, suggesting areas for improvement in digital surgical planning and execution.
Conclusions
This study indicates that while all measured deviations fell within clinically acceptable limits, certain parameters exceeded the benchmarks, suggesting areas for improvement in digital surgical planning and execution. Based on these data, the potential of digital guide plates to fulfill precision requirements in edentulous jaw implantation can be proved, contributing valuable insights into the optimization of implant surgery protocols.
Clinical relevance
Now, the digital template is accepted by many doctors. However, clinical research has not thoroughly verified whether the new digital technology is more accurate than traditional technology. So, this study aims to explore the effect of a whole-process digital template on edentulous implantation and provide more clinical evidence.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-05265-8.
Keywords: Implant restoration, Digital surgical guide plate, Edentulous jaws, Postoperative, Precision requirement
Introduction
Implant restoration represents a cornerstone in addressing dental defects or tooth loss, with the integration of digital and 3D printing technologies marking a revolution in dental practices. The advent of such technologies has broadened their application within dentistry, notably in the domain of implant surgery. Numerous studies have shown that implant surgery with the help of digital technology not only contributes to accurate surgical execution but also bridges the gap between surgical planning and actual procedure [1–3], including digital templates, dynamic navigation, augmented reality, and surgical robots. Among them, a critical component for the enduring success of dental implants is the digital template [2].
The workflow of implant surgery using a digital guide fabricated by all implant planning software encompasses four stages: digital data acquisition, implant planning, surgical template fabrication, and the guided implantation itself [4, 5]. This approach ensures the translation of planned surgical interventions into the surgical setting with heightened accuracy and shorter surgery time, which means less surgical trauma [6]. Importantly, plate-guided surgery demonstrates superior precision over traditional freehand techniques, markedly minimizing the risk of implant misalignment [7]. Moreover, flapless implant procedures enable immediate loading, catering promptly to the patient’s functional requirements [8]. Additionally, in the aesthetic enhancement of the anterior dental region, the implant plate is a key tool for precise bone augmentation [9].
Despite these advances, questions remain regarding the potential increase in digital template deviation with the number of implants and the impact of digital template support on surgical accuracy [10]. Notably, tooth-supported templates exhibit the highest precision, whereas mucosa-supported variants are prone to greater mobility [11, 12]. Additionally, since the development of dental resin and the limitation of interdental space, digital guides without metal sleeves have been widely used, and they have been proven to be more accurate than guides with main metal sleeves [13, 14]. Furthermore, the superiority of digital over traditional techniques in terms of accuracy awaits further empirical validation through clinical studies, especially in fully edentulous Implant Placement [15].
Thus, to evaluate the efficacy of digital templates in edentulous implant placement and provide further clinical evidence, this retrospective investigation seeks to assess the three-dimensional accuracy impact of a comprehensive digital template process on edentulous jaw implantation. By comparing preoperative designs with postoperative implant positions, this study aims to analyze deviations at various levels: the platform, apex, depth, and angular orientation. Our hypothesis posits that a full-process digital template will satisfy the precision requirements for edentulous jaw implantations, offering valuable clinical insights into digital template research and application.
Materials and methods
Patient selection
Our study included a patient with an edentulous maxilla who underwent an all-on-6 implant procedure guided by a digital template in the West China Hospital of Stomatology. We evaluated the accuracy of an all-on-6 implant procedure in a patient with an edentulous maxilla, guided by a digitally fabricated digital template. This research was conducted at the West China Hospital of Stomatology, with ethical approval details provided in the annex. Eligibility criteria excluded patients with systemic conditions contraindicated for implant surgery, such as diabetes, Sjogren’s syndrome, or cardiovascular diseases. Since it is a retrospective study, the inclusion criteria require patients to have good preoperative and postoperative CT data quality and complete data and information records.
Initially, the patient underwent preoperative maxillofacial Cone Beam Computed Tomography (CBCT). Subsequent scans involved the patient wearing a radiopaque denture to enhance the maxillofacial region’s imaging. These scans, alongside the denture’s CBCT images, were integrated using 3-Shape software by aligning developmental points. The intraoral scanner used for impression taking was the (TRIOS 3; 3Shape). Oral scan data were then incorporated to facilitate a three-dimensional reconstruction of the maxilla. A virtual three-dimensional implantation plan was designed for positions 16, 14, 12, 22, 24, and 26 using Computer-Aided Design/Computer-Aided Manufacturing (CAD/CAM) technologies using 3-shape software. The guide plate was then fabricated from a biocompatible resin using a 3D printer (Formlabs, USA). For stabilization during surgery, the template was secured with 6 bone fixation pins. These pins were strategically placed to ensure maximum stability and accuracy during the drilling and implant placement phases. The prosthetic process involved immediate loading of the implants. After implant placement, a provisional prosthesis was fabricated and fitted to the patient on the same day. The provisional phase was carefully managed by adjusting the occlusion and ensuring that the temporary prosthesis did not place excessive load on the implants during the healing period. This approach allowed for both functional and aesthetic restoration while the implants integrated with the bone.
Data measurement
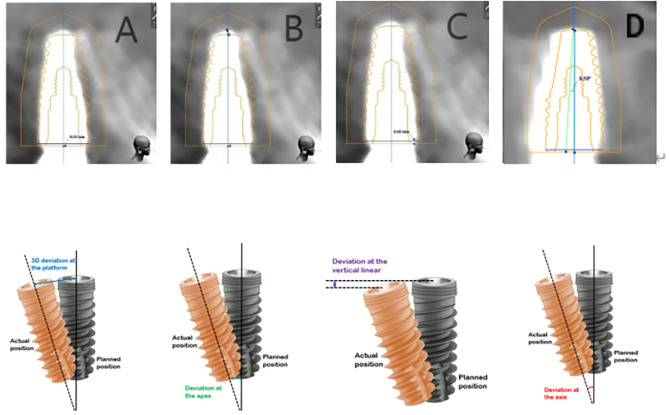
Postoperative evaluation entailed another CBCT scan to ascertain implant positions within the maxilla. The implant position was reconstructed using a digital template and matched with postoperative CBCT in 3-shape software to restore the preoperative implant position and finally evaluate the surgical deviation. Following the lieratures [12, 16]. We measured four key parameters for each implant: deviations at the platform and apex, depth deviation, and angular deviation, using the software’s measurement tools (Fig. 1). These measurements were conducted thrice by three independent observers to ensure accuracy, rounding off to two decimal places.
Fig. 1.
The method of measuring the deviation between planned implant and actual implant. (A) The method of measuring the deviation at the platform. (BC) The method of measuring the deviation at the apex. (C) The method of measuring the depth deviation. (D) The method of measuring the angular deviation
Data analysis
Our analysis referenced a consensus paper by Tahmaseb et al. (2018), which provided a meta-analysis on the precision of digitally guided implant placements [17]. This paper reported an average error at the implant platform of 1.2 mm, an apex deviation of 1.4 mm, a depth deviation of 0.2 mm, and an angular deviation of 3.5°. We employed SPSS22.0 for statistical analysis, comparing our findings against these benchmarks. Perform a single-sample t-test on the part larger than the average deviation after comparing each part’s deviation with the average deviation reported in the literature. A P-value of < 0.05 was deemed statistically significant, aiming to contribute to the body of evidence on the efficacy of digital templates in dental implantology, mean and 95% CI are shown.
Results
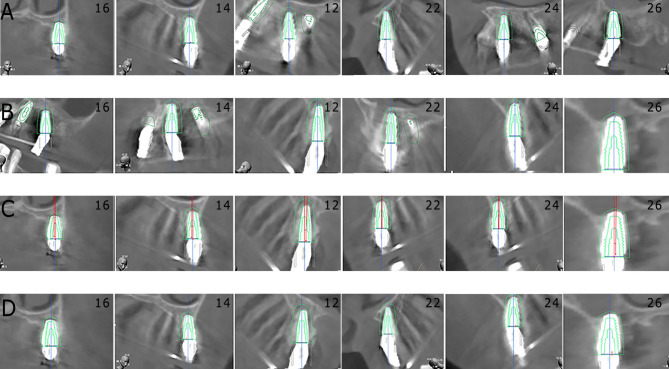
Our study aligned postoperative CBCT images with their preoperative counterparts to assess the discrepancies in implant positioning. Figure 2 illustrates this comparison, focusing on deviations across four critical dimensions: platform, apex, angular, and depth for each implant.
Fig. 2.
The match between the actual and planned implants. (A) The platform deviations of all implants. (B) The apex deviations of all implants. (C) The angular deviations of all implants. (D) The depth deviations of all implants
Platform deviations
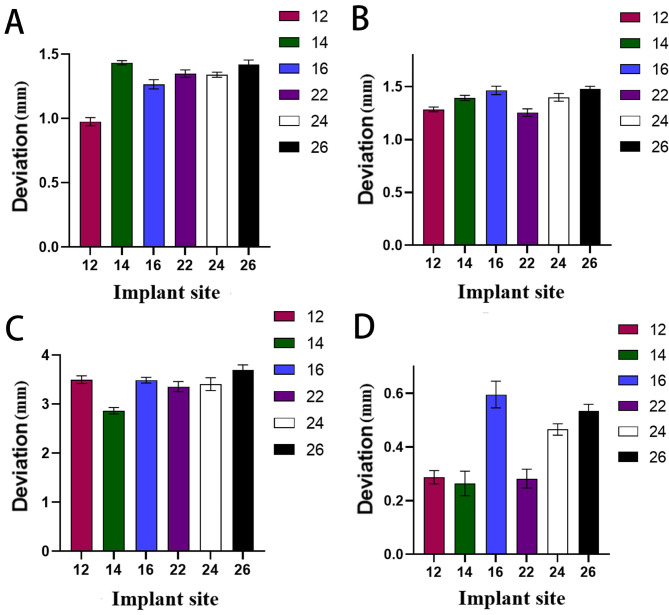
Illustrated in Figs. 3A and 4A, platform deviations were analyzed for implants at positions 12, 14, 16, 22, 24, and 26. The observed mean deviations were as follows: 0.98 ± 0.03 mm, 1.43 ± 0.02 mm, 1.27 ± 0.04 mm, 1.35 ± 0.03 mm, 1.34 ± 0.02 mm, and 1.42 ± 0.03 mm, respectively. At position 12, the platform deviation was much lower than at other positions, which are relatively concentrated. Although these values align with the consensus paper’s reported range, except for position 12, the averages exceeded the consensus paper’s mean platform deviation, indicating a statistically significant difference.
Fig. 3.
The deviations of all implants were expressed as the means ± SD of triplicate measurements from three independent experiments. The (A) platform deviations, (B) apex deviations, (C) angular deviations and (D) depth deviations of all implants were measured
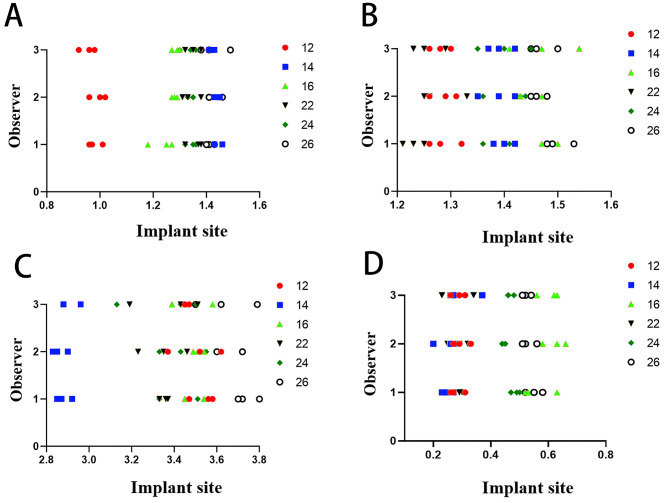
Fig. 4.
The details of all deviations. (A) The platform deviations of all implants. (B) The apex deviations of all implants. (C) The angular deviations of all implants. (D) The depth deviations of all implants
Apex deviations
As depicted in Figs. 3B and 4B, we quantified the apex deviations for the same implant positions. The averages were 1.28 ± 0.02 mm, 1.39 ± 0.03 mm, 1.47 ± 0.04 mm, 1.26 ± 0.04 mm, 1.40 ± 0.04 mm, and 1.48 ± 0.03 mm, respectively. At positions 12 and 22, the apex deviation was minimal, and the deviation was more significant in the farther position. All measurements fell within the consensus paper’s deviation range, yet for positions 16 and 26, the deviations notably surpassed the consensus paper’s average apex deviation, marking a significant discrepancy.
Angular deviations
The angular deviations, detailed in Figs. 3C and 4C, showed average deviations of 3.50°± 0.08°, 2.87°± 0.07°, 3.49°± 0.06°, 3.36°± 0.10°, 3.41°± 0.13°, and 3.69°± 0.11° for each respective implant position. At position 14, the angular deviation was minimal, and the deviations of other positions were relatively concentrated. These deviations remained within the consensus paper’s expected range; however, position 26’s average angular deviation was significantly higher than the consensus average, suggesting a notable variance.
Depth deviations
Explored in Figs. 3D and 4D, depth deviations were presented as follows: 0.29 ± 0.03 mm, 0.26 ± 0.05 mm, 0.59 ± 0.05 mm, 0.28 ± 0.04 mm, 0.47 ± 0.02 mm, 0.53 ± 0.03 mm for each implant position. The depth deviation of positions 16, 26 and 24 was slightly larger than those of other positions. Although these deviations complied with the consensus paper’s deviation range, the mean depth deviations exceeded the consensus average, indicating a statistically significant distinction.
Our study assessed the deviations between actual and planned implant positions across four dimensions (Table 1). The results underscore the precision achievable with digital guided implant surgery, even though certain deviations—specifically in depth and at certain positions—exceeded established benchmarks. This analysis not only reaffirms the reliability of digital guidance but also highlights areas for potential refinement in surgical planning and execution.
Table 1.
Statistical test for the deviation between the planned and actual implant positions
| Group | Implant sites | |||||
|---|---|---|---|---|---|---|
| 12 | 14 | 16 | 22 | 24 | 26 | |
| Platform deviation | 0.98 ± 0.03 | 1.43 ± 0.02 | 1.27 ± 0.04 | 1.35 ± 0.03 | 1.34 ± 0.02 | 1.42 ± 0.03 |
| Apex deviation | 1.28 ± 0.02 | 1.39 ± 0.03 | 1.47 ± 0.04 | 1.26 ± 0.04 | 1.40 ± 0.04 | 1.48 ± 0.03 |
| Angular deviation | 3.50 ± 0.08 | 2.87 ± 0.07 | 3.49 ± 0.06 | 3.36 ± 0.10 | 3.41 ± 0.13 | 3.69 ± 0.11 |
| Depth deviation | 0.29 ± 0.03 | 0.26 ± 0.05 | 0.59 ± 0.05 | 0.28 ± 0.04 | 0.47 ± 0.02 | 0.53 ± 0.03 |
Discussion
The landscape of implant restoration has evolved significantly, with improved clinical outcomes fostering acceptance among healthcare professionals and patients alike [17]. Among the methodologies for implant placement—free-handed surgery, static computer-assisted surgery, and dynamic navigation—each presents unique advantages and challenges. Traditional free-handed approaches, while benefiting from the surgeon’s experience, often lack precision in implant orientation [18, 19]. In the absence of digital technology, the precision of freehand implant surgery can be improved by the plastic sleeves [19]. In contrast, digital templates and navigation technologies offer enhanced three-dimensional placement accuracy and reduced risk of compromising critical anatomical structures. Despite their merits, dynamic navigation systems are marked by high costs and complex operation, particularly for patients with edentulous mandibles, where jaw movement poses additional challenges [20]. Thus, digital templates emerge as a preferred solution for these cases due to their accessibility and effectiveness.
Our study meticulously assessed the deviations between actual and planned implant positions across four dimensions (Table 1). Analysis revealed all deviations fell within clinically acceptable ranges, though depth and platform deviations for most implants slightly exceeded consensus averages. It shows that the average deviation of the mucosa-supported digital templates are slightly larger than that of all digital templates, which is consistent with other literature [12, 21]. Notably, posterior implants demonstrated more significant deviations than their anterior counterparts, attributed to operational constraints in tighter spaces, the looser alveolar ridge, and potential digital template movement due to mucosal elasticity and pin fixation depth [16, 22]. However, it is worth noting that the limitation of this retrospective study is that the number of patients included is small, and more clinical evidence is needed.
Several variables contribute to inaccuracies, including the surgeon’s expertise, jaw density, mucosal thickness, guide design, and implant dimensions. Despite these challenges, digital templates enhance surgical precision but are not without limitations [23–25]. The method of support and stabilization, visibility during surgery, and drill type accessibility are influenced by digital templates use [3]. However, these technologies do not supplant the surgeon’s critical role, instead serving as tools to facilitate complex procedures [26].
To mitigate deviations and enhance accuracy, strategies include diversifying the anchor points of radio-digital templates, ensuring patient stability during imaging, validating digital templates fit on patient models, and leveraging remaining stable teeth to support tooth-guided implantation [27, 28]. In summary, digital templates stand as a pivotal tool in implant surgery, enhancing accuracy and offering a less invasive approach. Despite the absence of distinct anatomical markers in edentulous cases, digital guides significantly reduce surgical complexity and trauma, meriting broader adoption in clinical practice.
However, some things could be improved in digital templates, including compromised site irrigation, inability to modify the plan, and requirement of interdental space, etc. The robot-assisted implant surgery and dynamic navigation do not need to use the digital templates, which can fill these defects and can achieve similar or exceed the accuracy of the digital template planting, especially in edentulous jaws [1, 29, 30], and probably are prospects regarding guided surgery. At the same time, artificial intelligence has identified and segmented specific structures such as bones, nerves, teeth, maxillary sinus, etc. In addition, there have been many studies on AI to develop implant planning and drilling protocol [31, 32], but more evidence is still needed, which will be the future of implant restoration.
Conclusion
Our investigation into the three-dimensional accuracy of implant placement in an edentulous maxilla, guided by digital fabrication of a surgical plate, highlights the pivotal role of advanced technologies in dental implantology. Despite the overall deviations remaining within acceptable clinical thresholds, our analysis revealed specific instances where precision could be further optimized. Notably, the study confirmed the superiority of digital templates over traditional freehand techniques in terms of accuracy, while also identifying the influence of various factors on implant placement precision, such as the digital template’s support mechanism and the surgical environment. The findings advocate for a balanced approach that leverages the benefits of digital technologies while acknowledging the indispensable role of the surgeon’s expertise. This study has far-reaching implications for clinical practice. It provides a clinical basis for doctors to use digital technology and suggestions for improving the digital template’s accuracy. Future efforts should focus on delivering more clinical evidence, refining digital template design, integrating AI assistance, analyzing preoperative situations, enhancing surgical planning, and embracing a multidisciplinary approach to minimize deviations and improve patient outcomes. Ultimately, digital templates represent a significant advancement in implant surgery, promising more predictable, precise, and minimally invasive treatments for patients with dental deficiencies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
X. F. contributed to the conception and data analysis and drafted the manuscript; M. L. contributed to the data analysis of the manuscript; W. S. and Y. J. contributed to the conception and design; F. L. contributed to the conception, design, and data analysis and revised the manuscript.
Funding
No funding was obtained for this study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The West China Hospital of Stomatology Institutional Review Board (WCSHIRB) has formally APPROVED this projects (WCHS-IRB-CT-2024-177).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dibart S, Kernitsky-Barnatan J, Di Battista M, Montesani L. Robot assisted implant surgery: hype or hope? J Stomatology Oral Maxillofacial Surg. 2023;124(6, Supplement):101612. [DOI] [PubMed] [Google Scholar]
- 2.Al Yafi F, Camenisch B, Al-Sabbagh M. Is Digital guided Implant surgery Accurate and Reliable? Dental Clin N Am. 2019;63(3):381–97. [DOI] [PubMed] [Google Scholar]
- 3.Pellegrino G, Mangano C, Mangano R, Ferri A, Taraschi V, Marchetti C. Augmented reality for dental implantology: a pilot clinical report of two cases. BMC Oral Health. 2019;19(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YW, Hanak BW, Yang TC, Wilson TA, Hsia JM, Walsh HE, et al. Computer-assisted surgery in medical and dental applications. Expert Rev Med Devices. 2021;18(7):669–96. [DOI] [PubMed] [Google Scholar]
- 5.Kernen F, Kramer J, Wanner L, Wismeijer D, Nelson K, Flügge T. A review of virtual planning software for guided implant surgery - data import and visualization, drill guide design and manufacturing. BMC Oral Health. 2020;20(1):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zadrożny Ł, Czajkowska M, Tallarico M, Wagner L, Markowski J, Mijiritsky E, et al. Prosthetic Surgical templates and Dental Implant Site Time Preparation: an in Vitro Study. Prosthesis. 2022;4(1):25–37. [Google Scholar]
- 7.Kivovics M, Takács A, Pénzes D, Németh O, Mijiritsky E. Accuracy of dental implant placement using augmented reality-based navigation, static computer assisted implant surgery, and the free-hand method: an in vitro study. J Dent. 2022;119:104070. [DOI] [PubMed] [Google Scholar]
- 8.Turkyilmaz I, Gavras JN. Fabrication of immediately loaded implant-retained maxillary overdenture with flapless surgery using a CAD/CAM surgical guide: A technical report. Prim Dent J. 2022;11(4):61–5. [DOI] [PubMed]
- 9.Liu X, Fang Z, Feng J, Yang SF, Ren YP. Application of computer-aided design and 3D-printed template for accurate bone augmentation in the aesthetic region of anterior teeth. BMC Oral Health. 2023;23(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yimarj P, Subbalekha K, Dhanesuan K, Siriwatana K, Mattheos N, Pimkhaokham A. Comparison of the accuracy of implant position for two-implants supported fixed dental prosthesis using static and dynamic computer-assisted implant surgery: a randomized controlled clinical trial. Clin Implant Dent Relat Res. 2020;22(6):672–8. [DOI] [PubMed] [Google Scholar]
- 11.Naeini EN, Atashkadeh M, De Bruyn H, D’Haese J. Narrative review regarding the applicability, accuracy, and clinical outcome of flapless implant surgery with or without computer guidance. Clin Implant Dent Rel Res. 2020;22(4):454–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Putra RH, Yoda N, Astuti ER, Sasaki K. The accuracy of implant placement with computer-guided surgery in partially edentulous patients and possible influencing factors: a systematic review and meta-analysis. J Prosthodont Res. 2022;66(1):29–39. [DOI] [PubMed] [Google Scholar]
- 13.Park J, Song YW, Park S, Kim J, Park J, Lee J. Clinical factors influencing implant positioning by guided surgery using a nonmetal sleeve template in the partially edentulous ridge: multiple regression analysis of a prospective cohort. Clin Oral Implants Res. 2020;31(12):1187–98. [DOI] [PubMed] [Google Scholar]
- 14.Tallarico M, Czajkowska M, Cicciù M, Giardina F, Minciarelli A, Zadrożny Ł, et al. Accuracy of surgical templates with and without metallic sleeves in case of partial arch restorations: a systematic review. J Dent. 2021;115:103852. [DOI] [PubMed] [Google Scholar]
- 15.Yang JW, Liu Q, Yue ZG, Hou JX, Afrashtehfar KI. Digital Workflow for full-Arch Immediate Implant Placement using a Stackable Surgical Guide fabricated using SLM technology. J Prosthodont. 2021;30(8):645–50. [DOI] [PubMed] [Google Scholar]
- 16.D’haese R, Vrombaut T, Hommez G, De Bruyn H, Vandeweghe S. Accuracy of guided Implant surgery in the Edentulous Jaw using Desktop 3D-Printed Mucosal supported guides. J Clin Med. 2021;10(3):391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tahmaseb A, Wu V, Wismeijer D, Coucke W, Evans C. The accuracy of static computer-aided implant surgery: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(Suppl 16):416–35. [DOI] [PubMed] [Google Scholar]
- 18.Yazdani J, Ahmadian E, Sharifi S, Shahi S, Maleki Dizaj S. A short view on nanohydroxyapatite as coating of dental implants. Biomed Pharmacother. 2018;105:553–7. [DOI] [PubMed] [Google Scholar]
- 19.Wei SM, Zhu Y, Wei JX, Zhang CN, Shi JY, Lai HC. Accuracy of dynamic navigation in implant surgery: a systematic review and meta-analysis. Clin Oral Implants Res. 2021;32(4):383–93. [DOI] [PubMed] [Google Scholar]
- 20.Zadrożny Ł, Czajkowska M, Mijiritsky E, Wagner L. Repeatability of Freehand implantations supported with Universal Plastic Sleeves—In Vitro Study. IJERPH. 2020;17(12):4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain S, Sayed ME, Ibraheem WI, Ageeli AA, Gandhi S, Jokhadar HF, et al. Accuracy comparison between Robot-assisted Dental Implant Placement and Static/Dynamic Computer-assisted Implant surgery: a systematic review and Meta-analysis of in Vitro studies. Med (Kaunas). 2023;60(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marlière DAA, Demètrio MS, Picinini LS, Oliveira RGD, Netto HDDMC. Accuracy of computer-guided surgery for dental implant placement in fully edentulous patients: a systematic review. Eur J Dent. 2018;12(01):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mai HN, Lee DH. Effects of supporting conditions and anchor microscrew on the stabilization of the implant guide template during the drilling process: an in vitro study. J Prosthet Dent. 2020;124(6):727.e1-727.e8 [DOI] [PubMed] [Google Scholar]
- 24.Kivovics M, Pénzes D, Németh O, Mijiritsky E. The influence of Surgical Experience and Bone Density on the Accuracy of Static Computer-assisted Implant surgery in Edentulous Jaws using a mucosa-supported Surgical Template with a half-guided Implant Placement Protocol—A Randomized Clinical Study. Materials. 2020;13(24):5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atay E, Hey J, Beuer F, Böse MWH, Schweyen R. Evaluation of the accuracy of fully guided implant placement by undergraduate students and postgraduate dentists: a comparative prospective clinical study. Int J Implant Dent. 2024;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo C, Juodzbalys G. Accuracy of guided surgery via Stereolithographic Mucosa-supported Surgical Guide in Implant surgery for Edentulous patient: a systematic review. J Oral Maxillofac Res. 2018;9(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sukegawa S, Kanno T, Furuki Y. Application of computer-assisted navigation systems in oral and maxillofacial surgery. Jpn Dent Sci Rev. 2018;54(3):139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessler A, Le V, Folwaczny M. Influence of the tooth position, guided sleeve height, supporting length, manufacturing methods, and resin E-modulus on the in vitro accuracy of surgical implant guides in a free-end situation. Clin Oral Implants Res. 2021;32(9):1097–104. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura A, Nakano T, Ono S, Kaminaka A, Yatani H, Kabata D. Multivariate analysis of causal factors influencing accuracy of guided implant surgery for partial edentulism: a retrospective clinical study. Int J Implant Dent. 2021;7(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawal S. Guided innovations: Robot-assisted dental implant surgery. J Prosthet Dent. 2022;127(5):673–4. [DOI] [PubMed] [Google Scholar]
- 31.Panchal N, Mahmood L, Retana A, Emery R. Dynamic Navigation for Dental Implant surgery. Oral Maxillofac Surg Clin N Am. 2019;31(4):539–47. [DOI] [PubMed] [Google Scholar]
- 32.Macrì M, D’Albis V, D’Albis G, Forte M, Capodiferro S, Favia G, et al. The role and applications of Artificial Intelligence in Dental Implant Planning: a systematic review. Bioengineering. 2024;11(8):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.