Abstract
Electronic cigarette (EC) is widely advertised as a safe alternative to traditional cigarette (TC). We aimed to investigate the cardiovascular effect of EC with/without nicotine compared with TC. We systematically searched PubMed/MEDLINE, EMBASE, and Cochrane CENTRAL for randomized controlled trials that compared the effect of different smoking modalities on cardiovascular function up to 1 October 2024. Analysis used the weighted mean difference (WMD) with a 95% confidence interval (CI) via Comprehensive Meta-Analysis software, version 3.0. The study evaluated key cardiovascular parameters, including pulse wave velocity (PWV), augmentation index at 75 beats/min (AIx75), flow-mediated dilation (FMD), heart rate (HR), systolic blood pressure, and diastolic blood pressure. We analysed 9 trials involving 370 participants. Acute exposure to EC with nicotine (ECN) compared with nicotine-free EC (EC0) increased PWV (WMD = 0.26; 95% CI: 0.14–0.38, P < 0.001), AIx75 (WMD = 4.29; 95% CI: 2.07–6.51, P < 0.001), and HR (WMD = 5.06; 95% CI: 2.13–7.98, P = 0.001), significantly. In contrast, comparison between ECN and TC revealed no significant differences in FMD (WMD = 0.80; 95% CI: −0.09–1.70, P = 0.08). Our meta-analysis suggests that ECN acutely increases arterial stiffness more than EC0 does. Additionally, we found that the acute effect of ECN on endothelial dysfunction is not different from TC. Therefore, our study suggests that vaping cannot be considered as a safe substitute for TC. Further investigation is needed to explore the long-term cardiovascular effects of vaping and its modalities.
Keywords: E-cigarettes, Cardiovascular function, Pulse wave velocity, Blood pressure, Heart rate, Meta-analysis
Introduction
Electronic nicotine delivery systems, commonly known as electronic cigarette (EC) or vapes, are designed to deliver nicotine as a substitute for traditional cigarette (TC). Although advertisement for EC has claims about safety, cessation-related benefits, and the absence of second-hand smoke, these assertions lack scientific support.1 Of particular concern, the aerosols produced by EC, commonly referred to as vapour, contain ∼47 compounds, several of them are recognized by the Food and Drug Administration as harmful to human health2 and some are similar to those found in TC. Cigarette smoke contains more than 4000 chemicals, including oxidizing chemicals, carbon monoxide, volatile organic compound, particulates, heavy metals, and nicotine many of them contribute to cardiovascular diseases (CVDs).3
Smoking is a potent risk factor for cardiovascular events, CVDs, and cerebrovascular diseases, including coronary heart disease, myocardial infarction (MI), stroke, and heart failure.4 Endothelial has a critical role in cardiovascular health through regulating vascular tone and smoking is recognized as a classic risk factor of endothelial dysfunction. The pathophysiological pathways of smoking involve inflammation, oxidative stress, and atherosclerosis,5 leading to impaired production of vasoactive compounds. This results in a state of vasoconstriction, pro-inflammatory, and pro-atherothrombotic condition, ultimately impairing blood circulation and disrupting vascular tone regulation.6 Additionally, atherosclerosis, which is associated with CVD, in its early stages marked by endothelial dysfunction, while in later stages, it is associated with arterial stiffness.7,8 Increased arterial stiffness and impaired wave reflection is fundamental to decreased aortic velocity and the development of systolic hypertension.9 Moreover, smoking stimulates sympathetic nervous system, which in turn increases blood pressure (BP) and heart rate (HR).10,11
Apart from HR and BP, which are indicators of cardiac haemodynamic state, various markers, including pulse wave velocity (PWV), augmentation index (AIx75), and flow-mediated dilation (FMD), are utilized to assess arterial stiffness, endothelial function, and subclinical atherosclerosis.12–14 PWV is widely recognized as a simple, non-invasive, and reliable method for assessing arterial stiffness, with higher PWV indicating a more severe atherosclerotic state. It is an established technique with a well-documented association with cardiovascular outcomes.15,16 Another marker for the assessment of arterial stiffness is AIx75, which is an independent predictor of cardiovascular events and all-cause mortality. AIx75 reflects the interaction between the incident and the reflected pulse wave.17 On the other hand, quantifying FMD is a non-invasive technique for measuring endothelial function and is an independent predictor of CVD outcomes.18,19
The health effects of EC, both the short-term and long-term, remain uncertain, given their relatively recent emergence in the consumer market. Despite these uncertainties, which necessitate caution regarding their safety, it is attracting youth and even former smokers. From 2013 to 2021, the prevalence of current established smokers decreased from 19.6% to 6.1%, while through the same time period, the prevalence of current established vapers increased from 3.8% to 14.5%.20 Although an epidemiologic study showed that daily vaping is associated with increased risk of MI,21 a meta-analysis of 20 observational studies found no significant association between EC use and CVD.22 This inconsistency highlights the need for further research to clarify the effect of vaping on the cardiovascular system. In this study, we investigated the acute effect of vaping on arterial stiffness, endothelial dysfunction, and cardiac physiology by comparing (i) nicotine containing e-cigarettes (ECN) to nicotine-free e-cigarettes (EC0) and (ii) ECN to TC. Additionally, we compared the effect of different smoking modalities on cardiovascular indices at various time points.
Methods
This systematic review and meta-analysis adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement guideline.23 The protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42023489557) on 15 December 2023.
Data source and search strategy
We thoroughly searched PubMed/MEDLINE, EMBASE, and the Cochrane CENTRAL databases up to 1 October 2024, to identify randomized controlled trials (RCTs) examining the effect of different smoking modalities on cardiovascular functionality markers. The search terms used included ‘Electronic Cigarette Vapour’, ‘E-Cigarette Vapour’, ‘Electronic Nicotine Delivery System’, ‘Electronic Cigarettes’, ‘E-Cig’, ‘E-Cigarette’, ‘Electronic Cigarette’, ‘Vaping’, ‘Vape’, ‘Smoking Cessation’, ‘Electronic tobacco’, ‘brachial artery’, ‘vasodilation’, ‘endothelium’, ‘vascular’, ‘endothelial function’, ‘flow-mediated dilation’, ‘Vascular Stiffness’, ‘arterial stiffness’, ‘arterial compliance’, ‘arterial distensibility, ‘PWV’, ‘Endothelial progenitor cell’, and ‘randomized controlled trial’. The complete search strategy is provided in the Supplementary file. Only studies published in English were included.
Study selection
The collected records from the database searches were merged, and duplicates were removed through the utilization of EndNote X7 (Thomson Reuters, Toronto, ON, Canada). Two authors (M.A. and M.C.) conducted a thorough assessment of the records independently, utilizing the title/abstract and full-text screening process to exclude any studies that did not align with the study’s eligibility criteria. In case of any discrepancies, a third reviewer (M.J.N.) was involved.
The studies included in the analysis met the following criteria:
Participants: The studies included healthy smokers and non-smokers without a history of CVD and excluded pregnant women.
Intervention: The intervention investigated was the use of ECN.
Comparison: The comparison included the use of EC0 or TC.
Outcome: The primary outcome was the assessment of cardiovascular risk by measurement of PWV and AIx75 as indicators of arterial stiffness, FMD as an indicator of endothelial dysfunction, and HR, systolic blood pressure (SBP), and diastolic blood pressure (DBP) as markers of cardiac physiology.
Data extraction
Two authors (M.A. and M.C.) collaboratively used a structured data extraction form and proceeded to extract information from all included studies. The extracted data encompassed the primary author’s name, publication year, study duration, study type, baseline participants’ characteristics (e.g. age, sex, and nationality), geographical location(s) of the study, sample size, type of intervention, duration of intervention, concentration of nicotine, and outcomes. Discrepancies were addressed through mutual agreement.
Quality assessment
The assessment of study quality was carried out by two authors (M.A. and M.C.) utilizing the Cochrane Collaboration tool for assessing the risk of bias in RCTs,24 with any discrepancies resolved by a third reviewer (M.J.N.). This tool covers various domains, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, completeness of outcome data, as well as additional considerations such as selective reporting and potential biases.
Data analysis
Three separate analyses were performed. First, we compared the acute effect (immediately to 30 min after exposure) of ECN to EC0 on cardiovascular indices. Second, we compared the acute effect of ECN to TC on cardiovascular indices. Third, we conducted a subgroup analysis, considering several time points including immediately 10, 30, 60, and 120 min after exposure. The statistical analysis was performed using the Comprehensive Meta-Analysis software, version 3.0 (Biostat Inc., Englewood, NJ, USA). The pooled statistic was represented by the weighted mean difference (WMD) accompanied by a corresponding 95% confidence interval (CI). Heterogeneity among the studies was evaluated using the I2 value and P-value. In instances of low statistical heterogeneity (I2 ≤ 50% or P ≥ 0.1), the fixed-effect model was applied. Conversely, when a substantial level of inter-study heterogeneity was observed (I2 > 50% or P < 0.1), the random-effects model was employed. Between-study heterogeneity was assessed using Cochran's Q-test and the I2 statistic. Begg’s test was used to evaluate publication bias, with a P-value <0.05 considered indicative of statistically significant publication bias.
Results
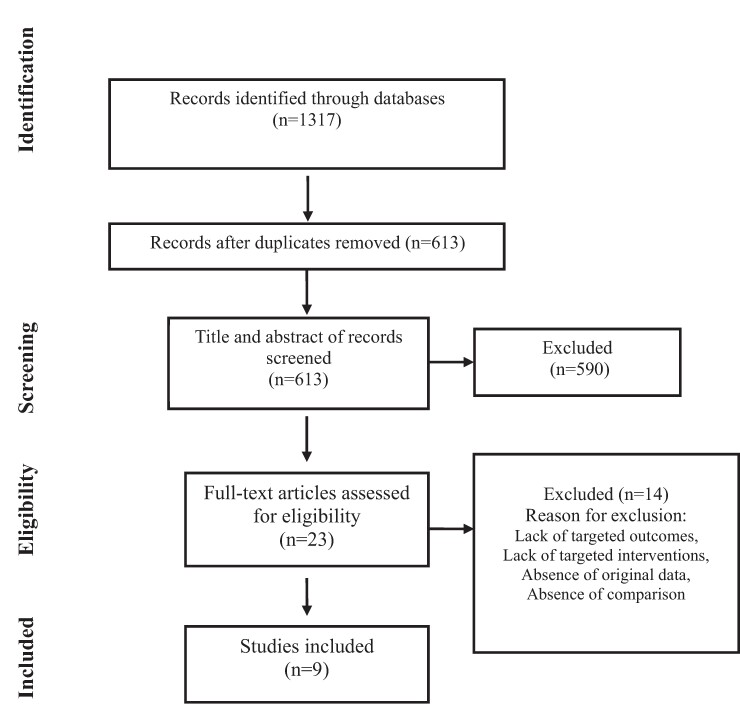
Figure 1 illustrates the flow diagram of the systematic review process. This thorough review yielded a total of 9 records involving 370 participants who were eligible for our study.25–33
Figure 1.
Flow diagram of study selection for inclusion in the systematic review and meta-analysis.
As shown in Table 1, the included studies cover diverse populations and study designs aimed at comparing the effect of EC0, ECN, and TC on vascular endothelium function. The studies were conducted in various countries, including the USA, Italy, Greece, Germany, Belgium, and Sweden. The mean ages varied across the studies. Interventions included a range of ECs such as EC fluid with nicotine, EC fluid without nicotine, tobacco-flavoured EC, and second-generation ‘pen-like’ EC devices, heat-not-burn cigarettes, and third-generation EC. The number of puffs that were consumed varied from 9 to 30 per vaping session. The outcomes focused on comparing the effect of these interventions on endothelial function and arterial stiffness indices including PWV, AIx75, FMD, HR, DBP, and SBP with a time point of assessment ranging from immediately to 120 min after exposure.
Table 1.
Characteristics of included studies
| Author | Year | Country | Population | Sample size | Age (year) Mean ± SD | Control | Time point of assessment | Design | Wash out period | Nicotine mg/mL |
|---|---|---|---|---|---|---|---|---|---|---|
| Carnevale et al.25 | 2016 | Italy | Healthy smoker | 20/20 | 28.0 ± 5.3 | TC | 30 min | RCD | 1 W | 16 |
| Carnevale et al.25 | 2016 | Italy | Non-smoker | 20/20 | 28.0 ± 5.3 | TC | 30 min | RCD | 1 W | 16 |
| Chaumont et al.26 | 2018 | Belgium | Healthy tobacco smokers | 25/25 | 23 ± 0.4 | EC0 + Sham vaping | Immediately, 10 min | RCD | 1 W | 3 |
| Ikonomidis et al.27 | 2018 | Greece | Healthy current smokers | 35/35 | 48 ± 5 | EC0 + Sham vaping | 7 min | RCD | 60 min | 12 |
| Franzen et al.28 | 2018 | Germany | Healthy current smokers | 15/15 | 22.9 ± 3.5 | EC0+ TC | NM | RCD | 24 H | 24 |
| Antoniewicz et al.29 | 2019 | Sweden | Healthy occasional users of tobacco products | 15/15 | 26 ± 3 | EC0 | Immediately, 10 min, 30 min, 2H | RCD | 1 W | 19 |
| Cossio et al.30 | 2019 | USA | Healthy tobacco product users | 16/16 | 24 ± 3 | EC0 | Immediately | RCT | NA | 5.4 |
| Biondi-zoccai et al.31 | 2019 | Italy | Healthy Smokers | 20/20 | 35 ± 13 | EC + TC | Immediately | RCD | 1 W | NM |
| Haptonstall et al.32 | 2020 | USA | Healthy non-smoker | 39/41 | 26.3 ± 5.2 | EC0 | 5 min | RCD | 1 W | 46.8 |
| Haptonstall et al.32 | 2020 | USA | Healthy Smoker | 23/22 | 27.4 ± 5.45 | EC0 | 5 min | RCD | 1 W | 73.5 |
| Lyytinen et al.33 | 2023 | Sweden | Healthy occasional smokers | 22/22 | 18–45 | EC0 | 30 min, 60 min | RCD | 1 W | 19 |
TC, tobacco cigarette; EC, electronic cigarette; EC0, electronic cigarette without nicotine; ECN, electronic cigarette with nicotine; H, hour; mg/mL, milligram per millilitre; min, minute; NA, not applicable; NM, not mentioned; RCD, randomized cross-over design; RCT, randomized clinical trial; TC, traditional cigarette; USA, United States of America; W, week.
Quality assessment
The assessment of the risk of bias is outlined in Table 2. Overall, the evaluation of bias across the included studies indicated a generally acceptable methodological rigor. Notably, the study by Carnevale et al.,25 Ikonomidis et al.,27 Antoniewicz et al.,29 Cossio et al.,30 Haptonstall et al.,32 and Lyytinen et al.33 showed higher risks in allocation concealment.
Table 2.
Quality assessment of the included studies
| Author | Year | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of outcome assessors | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|---|
| Carnevale et al.25 | 2016 | Low | High | Low | Low | Low | Low | Low |
| Chaumont et al.26 | 2018 | Low | Low | Low | Low | Low | Low | Low |
| Ikonomidis et al.27 | 2018 | Low | High | High | High | Low | Low | Low |
| Franzen et al.28 | 2018 | Low | Low | Low | Low | Low | Low | Low |
| Antoniewicz et al.29 | 2019 | Low | High | Low | Low | Low | Low | Low |
| Cossio et al.30 | 2019 | Low | High | Low | Low | Low | Low | Low |
| Biondie-zoccai et al.31 | 2019 | Low | Low | Low | Low | Low | Low | Low |
| Haptonstall et al.32 | 2020 | Low | High | High | High | Low | Low | Low |
| Lyytinen et al.33 | 2023 | Low | High | Low | Low | Low | Low | Low |
Comparison of the acute effect of electronic cigarette with nicotine to nicotine-free electronic cigarette
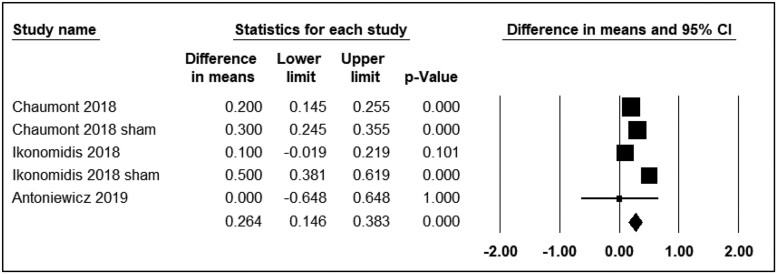
Pulse wave velocity
Four studies reported the differential effects of ECN and EC0 on PWV. The assessment time points varied from immediately to 15 min after exposure. Three studies were included in the analysis (n = 110). The use of ECN significantly affected PWV levels (WMD = 0.26; 95% CI: 0.14 to 0.38, P < 0.001) (Figure 2). Additionally, Franzen et al.28 reported that the consumption of ECN resulted in a significant alteration in PWV after 15 min.
Figure 2.
Acute effects of nicotine containing electronic cigarettes on pulse wave velocity, comparison to nicotine-free electronic cigarettes: a random-effect model.
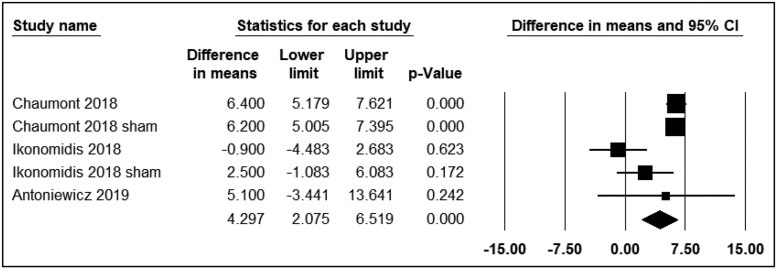
Augmentation index 75
Three studies reported the differential effects of ECN and EC0 on PWV. The time points varied from immediately to 15 min after exposure. All three studies were included in the analysis (n = 110). The use of ECN resulted in significant changes in AIx75 levels (WMD = 4.29; 95% CI: 2.07–6.51, P < 0.001) compared with EC0 (Figure 3).
Figure 3.
Acute effects of nicotine containing electronic cigarettes on augmentation index 75, comparison to nicotine-free electronic cigarettes: a random-effect model.
Flow-mediated dilation
Cossio et al.30 conducted a RCT comparing the effects of ECN to EC0 on FMD. This study suggested no acute effect of ECN compared with EC0 on subclinical vascular function as measured by FMD levels. Haptonstall et al.32 reported no significant difference in FMD before and after exposure to ECN and EC0.
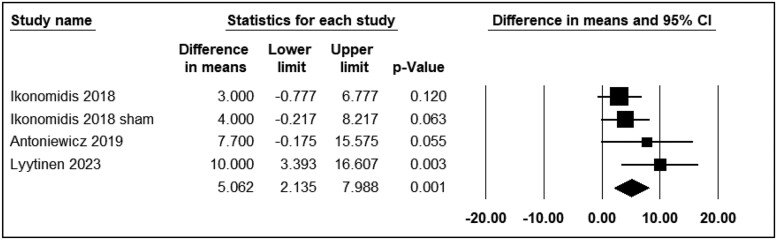
Heart rate
Five studies reported the differential effects of ECN and EC0 on HR. The assessment time points varied from immediately to 30 min after exposure. Three studies were included in the analysis (n = 107). The use of ECN significantly changed HR levels (WMD = 5.06; 95% CI: 2.13 to 7.98, P = 0.001) (Figure 4). Franzen et al.28 and Haptonstall et al.32 reported a notable increase in HR with ECN use.
Figure 4.
Acute effects of nicotine containing electronic cigarettes on heart rate, comparison to nicotine-free electronic cigarettes: a random-effect model.
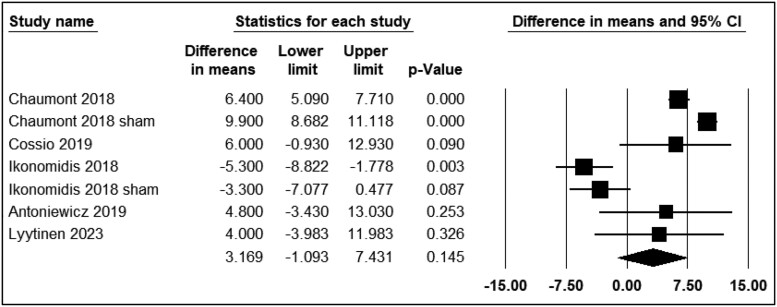
Systolic blood pressure
Seven studies reported the differential effects of ECN and EC0 on SBP. The assessment time points varied from immediately to 30 min after exposure. Five studies were included in the analysis (n = 148). The use of ECN did not result in significant changes in SBP levels (WMD = 3.16; 95% CI: −1.09 to 7.43, P = 0.14) (Figure 5). However, Franzen et al.28 and Haptonstall et al.32 reported a notable increase in SBP with ECN use.
Figure 5.
Acute effects of nicotine containing electronic cigarettes on systolic blood pressure, comparison to nicotine-free electronic cigarettes: a random-effect model.
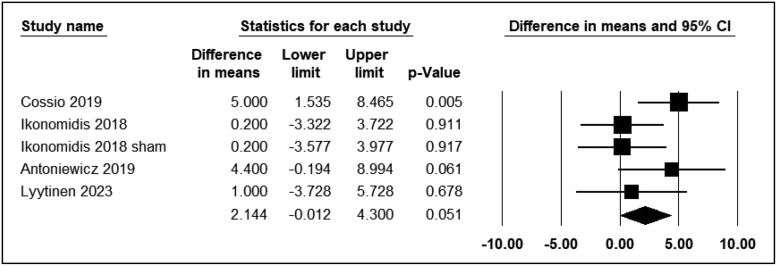
Diastolic blood pressure
Five studies reported the differential effects of ECN and EC0 on DBP. The assessment time points varied from immediately to 30 min after exposure. Four studies were included in the analysis (n = 123). The use of ECN did not result in significant changes in DBP levels (WMD = 2.14; 95% CI: −0.01 to 4.30, P = 0.05) (Figure 6). However, Haptonstall et al.32 reported a notable increase in DBP with ECN use.
Figure 6.
Acute effects of nicotine containing electronic cigarettes on diastolic blood pressure, comparison to nicotine-free electronic cigarettes: a random-effect model.
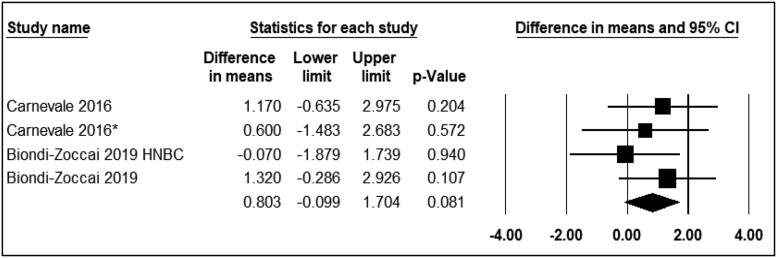
Comparison of the acute effect of electronic cigarette with nicotine and traditional smoking
Two studies compared the effects of ECN and TC on FMD. The assessment time points varied from immediately to 30 min after exposure. The analysis showed that the use of ECN compared with TC did not result in significant changes in FMD levels (WMD = 0.80; 95% CI: −0.09 to 1.70, P = 0.08) (Figure 7).
Figure 7.
Acute effects of nicotine containing electronic cigarettes on flow-mediated dilation, comparison to traditional cigarettes: a random-effect model.
Subgroup analysis
In the subgroup analysis, the study population was divided into two distinct groups: ECN and EC0. These groups were analysed separately based on the variables reported in the included studies at various time points, to assess potential differences in their effects over time. The overall results are presented in Supplementary material online, Table S1.
Comparison of pulse wave velocity, augmentation index at 75 beats/min, and diastolic blood pressure, immediately after exposure to electronic cigarette with nicotine and nicotine-free electronic cigarette
In comparison to EC0, exposure to ECN generally showed a significant increase in PWV (WMD = 0.19; 95% CI: 0.14–0.25, P < 0.001), AIx75 (WMD = 6.37; 95% CI: 5.16–7.58, P < 0.001), and DBP (WMD = 4.78; 95% CI: 2.01–7.54, P = 0.001) immediately after exposure (see Supplementary material online, Figure S1).
Comparison of blood pressure, 10 min after exposure to electronic cigarette with nicotine and nicotine-free electronic cigarette
Comparing the effect of ECN to EC0 on SBP revealed no significant difference 10 min after exposure (WMD = 2.10; 95% CI: −7.15–11.36, P = 0.65) (see Supplementary material online, Figure S2).
Comparison of blood pressure and heart rate, 30 min after exposure to electronic cigarette with nicotine and nicotine-free electronic cigarette
Exposure to ECN compared with EC0 showed no significant difference in SBP (WMD = 4.67; 95% CI: −1.53–10.88, P = 0.14) and DBP (WMD = 1.80; 95% CI: −1.63–5.23, P = 0.30) after 30 min. However, ECN compared with EC0 increased HR (WMD = 6.84; 95% CI: 0.38–13.30, P = 0.03) 30 min after exposure (see Supplementary material online, Figure S3).
Comparison of blood pressure, 60 min after exposure to electronic cigarette with nicotine and nicotine-free electronic cigarette
The effects of ECN and EC0 on SBP (WMD = −0.36; 95% CI: −4.99–4.26, P = 0.87) and DBP (WMD = 0.21; 95% CI: −2.76–3.19, P = 0.88) showed no significant difference 60 min after exposure (see Supplementary material online, Figure S4).
Comparison of systolic blood pressure, 120 min after exposure to electronic cigarette with nicotine and nicotine-free electronic cigarette
The effects of ECN and EC0 on SBP, 120 min after exposure, revealed no significant difference (WMD = 2.04; 95% CI: −2.83–6.92, P = 0.41) (see Supplementary material online, Figure S5).
Comparison of FMD 30 min after exposure to electronic cigarette with nicotine and traditional cigarette
When comparing the effect of ECN to TC, there was no notable difference in FMD 30 min after exposure (WMD = 0.92; 95% CI: −0.43–2.29, P = 0.18) (see Supplementary material online, Figure S6).
Discussion
Our systematic review and meta-analysis includes 9 clinical trials with a total of 370 subjects, aimed at comparing the effect of ECN to EC0 and ECN to TC on cardiovascular indices. Our findings indicate that the acute effect of ECN compared with EC0 is an elevation in PWV, AIx75, and HR, while the acute effect of ECN on endothelial dysfunction is not different from TC. Additionally, vaping ECN may result in an immediate increase in PWV, AIx75, and DBP, which compared with EC0 is significant. The mean differences in other parameters were not statistically significant.
Previous studies reported that tobacco smoking is a determinant of CVDs and increases the risk of cardiovascular events, including acute MI, sudden cardiac death, and stroke.34 The adverse effect of smoking on endothelial dysfunction, throughout all phases of atherosclerosis has been widely studied.35 Exposure to tobacco smoke impacts various components of the haemostatic process, including endothelial cells, platelets, fibrinogen, and coagulation factors.36 This initiates vascular dysfunction by reducing nitric oxide (NO) availability and increasing the expression of adhesion molecules, resulting in endothelial dysfunction. Furthermore, smoking promotes tissue remodelling, pro-thrombotic activity, and systemic inflammation, all of which contribute to atherogenic changes in the vessel wall.37 One common component of both EC and TC is nicotine. Of great notice is that, when interpreting results from experimental studies comparing the effects caused by TC and EC, several elements should be considered. First, differences in nicotine blood level concentration depend on the rate and pattern of consumption. A study by D'Ruiz et al. showed that EC use, compared with TC, delivers the same amount of nicotine but with slower absorption.38 Second, nicotine receptors undergo desensitization and develop tolerance, which is important to consider when evaluating the outcome of acute experimental exposure. The effects of a single, short-term exposure might differ from those seen with prolonged exposure in regular TC or EC users.39
Flow-mediated dilatation, pulse wave velocity, and augmentation Index 75
Endothelial dysfunction is a valuable indicator for cardiovascular risk and is known as a key feature of early stage systemic atherosclerosis.25 Markers such as FMD, PWV, and AIx75 are commonly used to assess vascular function, though each may be best suited to specific conditions or reflect specific abnormalities. Results of a meta-analysis by Witte et al. found that FMD is associated with cardiovascular risk factors only in low risk populations.40 Meanwhile, McEniery et al. suggested that AIx may be more sensitive in younger adults; whereas, PWV serves as a more reliable indicator in elderly.41 Our analysis did not find a significant difference in the FMD impairment after acute exposure to either ECN compared with EC0, or ECN compared with TC. Since FMD is more reflective of chronic smoking exposure,42 this lack of difference may indicate that the acute effects of these smoking modalities on vascular endothelial function are comparable in the short-term. The findings of our study are supported by a meta-analysis conducted by Meng et al.,43 which included four studies and similarly reported no significant difference in FMD when comparing the acute effects of EC to TC. A pathway through which nicotine impacts cardiovascular health is by inactivation and reducing the bioavailability of NO, a critical molecule for vessel dilation. This reduction in NO leads to a decrease in FMD, which has been observed in both TC and EC users.25
Yufu et al. demonstrated that smokers experience a reduction in FMD, which could be anticipated by an increase in PWV.44 In a healthy vascular system, the pulse wave returns to the heart during diastole, supporting coronary blood flow. However, in a stiffened vascular system, PWV is elevated, causing the wave to return to the heart pre-maturely during systole. This early reflection increases cardiac afterload and reduces diastolic augmentation, impairing coronary perfusion.45 Saz-Lara et al. in their systematic review and meta-analysis focused on the effect of smoking, vaping, and smoking cessation on arterial stiffness. They calculated the pooled effect size using the standardized mean difference and showed an increase in PWV after smoking cessation, which was equal to a moderate reduction in arterial stiffness. Moreover, their results demonstrated that both traditional smoking and vaping significantly increased the PWV.46 Similar findings were reported in the clinical trial by Franzen et al.28 which showed an increase in AIx75 and PWV, 15 min after exposure to ECN. Our results further confirm these findings, demonstrating that ECN, compared with EC0, results in a more pronounced immediate increase in PWV and AIx75, although both ECN and EC0 contribute to an elevation in these vascular markers. Additionally, our results showed that exposure to ECN compared with EC0 may result in the acute elevation of PWV and AIx75.
Heart rate, systolic blood pressure, and diastolic blood pressure
Vlachopoulos et al. compared the effects of EC and TC on blood pressure and found that both EC and TC significantly increased SBP and DBP, with no significant difference between the two in the magnitude of these changes.47 Skotsimara et al. in their meta-analysis included three studies and showed that switching from TC to EC did not affect HR; however, it reduced both SBP and DBP.48 Our results showed that vaping ECN compared with EC0 may lead to a greater increase in DBP, immediately after exposure, and an elevation in HR, 30 min after exposure. The difference in findings of the two studies may be related to different time points. Nicotine typically stimulates the sympathetic nervous system, leading to elevated BP and HR. However, nicotine’s effect on coronary blood flow is more complex and can even be contradictory. While nicotine reduces coronary blood flow by constricting coronary arteries, it can also increase cardiac output, which naturally leads to coronary artery dilation and an increase in blood flow. The net effect depends on the balance between these opposing actions, often resulting in a less-than-expected increase in blood flow to the heart muscle.39 Mueller et al. compared the effect of vaping and TC on cardiovascular response and showed that nicotine consumption, regardless of the method of use, has an acute dose-dependent effect on both blood pressure and HR. They argued that since vapers tend to consume nicotine more frequently, they may exhibit an enhanced cardiovascular response,49 which may persist and intensify throughout the day, as vapours have fewer rest periods between nicotine intake.
Our study has several notable strengths. Unlike previous meta-analysis studies, our systematic review and meta-analysis extended the comparison to include EC0, ECN, and TC, while evaluating a broader range of cardiovascular parameters including PWV, AIx75, and FMD, HR, SBP, and DBP. Furthermore, our study incorporated data from multiple time points (immediately, 10 min, 30 min, 60 min, and 120 min after exposure), providing a comprehensive understanding of the temporal dynamics of the observed effects. However, the number of studies assessing outcomes at longer time intervals remains limited, highlighting the need for further investigation into the long-term cardiovascular effects of ECs and their impact on cardiovascular event risk over extended periods. On the other hand, our study faced several limitations. First, variability in participant characteristics across the included studies may contribute to heterogeneity, potentially affecting the generalizability of our findings. Second, lack of long-term follow-up data limits our ability to evaluate the sustained effects of EC use on vascular parameters, necessitating caution when extrapolating our results to chronic exposure scenarios. Third, the wide variety of EC products introduces complexity in assessing their collective impact, as the specific constituents of ECN and EC0 may vary between brands, potentially influencing the observed outcomes.
In conclusion, based on the results of our comprehensive systematic review and meta-analysis, e-cigarette with nicotine may cause arterial stiffness immediately after exposure, suggesting potential cardiovascular event risks, which is greater than the effect of e-cigarette without nicotine. The results of our study found that the effect of e-cigarette with nicotine on FMD is comparable with TC. Given the evidence of acute cardiovascular effects associated with e-cigarette use, promoting vaping as a safe alternative to traditional smoking is not supported by current data and warrants serious reconsideration.
Supplementary Material
Contributor Information
Mahdis Cheraghi, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran Province, Tehran, District 1, Daneshjou Blvd, Q9XV+XG7, 19839 69411, Iran.
Mehrnaz Amiri, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran Province, Tehran, District 1, Daneshjou Blvd, Q9XV+XG7, 19839 69411, Iran.
Fatemeh Omidi, Department of Cardiology, Imam Hossein Hospital, Shahid Beheshti University of Medical Sciences, Tehran Province, Tehran, District 1, Daneshjou Blvd, Q9XV+XG7, 19839 69411, Iran.
Amir Hashem Shahidi Bonjar, Clinician Scientist of Dental Materials and Restorative Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran Province, Tehran, District 1, Daneshjou Blvd, Q9XV+XG7, 19839 69411, Iran.
Hooman Bakhshi, Department of Medicine, Division of Cardiology, Johns Hopkins University School of Medicine, Sheikh Zayed Tower, 1800 Orleans Street, Baltimore, MD 21287, USA.
Atefeh Vaezi, Division of Pulmonary, Critical Care, and Sleep Medicine, College of Medicine-Jacksonville, University of Florida, 653-1 West 8th Street, L20 Jacksonville, FL 32209, USA.
Mohammad Javad Nasiri, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran Province, Tehran, District 1, Daneshjou Blvd, Q9XV+XG7, 19839 69411, Iran.
Mehdi Mirsaeidi, Division of Pulmonary, Critical Care, and Sleep Medicine, College of Medicine-Jacksonville, University of Florida, 653-1 West 8th Street, L20 Jacksonville, FL 32209, USA.
Lead author biography

Dr. Mehdi Mirsaeidi, M.D., M.P.H., is a Professor of Medicine and Chief of Pulmonary, Critical Care, and Sleep Medicine at the University of Florida College of Medicine in Jacksonville. His research centres on respiratory diseases and the health impacts of environmental exposures, including tobacco and vaping. Dr. Mirsaeidi’s lab investigates the acute and chronic effects of vaping on respiratory and cardiovascular health, providing critical insights into its role in disease exacerbation. His team has demonstrated that vaping increases the risk of pulmonary infections and induces inflammation in bronchial epithelial cells, advancing understanding of its harmful effects.
Data availability
The data used to support the findings of this study are included within the article.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Authors’ contribution
All authors contributed equally to this work.
Funding
The Research Department of the School of Medicine (M.J.N.) and Shahid Beheshti University of Medical Sciences, Tehran, Iran (43008522).
References
- 1. Grana RA, Ling PM. “Smoking revolution”: a content analysis of electronic cigarette retail websites. Am J Prev Med 2014;46:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eshraghian EA, Al-Delaimy WK. A review of constituents identified in e-cigarette liquids and aerosols. Tob Prev Cessat 2021;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol 2013;10:219–230. [DOI] [PubMed] [Google Scholar]
- 4. Udo H, Services H. The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta, GA: US Department of Health and Human Services, Centers for Disease; 2014. [Google Scholar]
- 5. McEvoy JW, Nasir K, DeFilippis AP, Lima JAC, Bluemke DA, Hundley WG, Barr RG, Budoff MJ, Szklo M, Navas-Acien A, Polak JF, Blumenthal RS, Post WS, Blaha MJ. Relationship of cigarette smoking with inflammation and subclinical vascular disease. Arterioscler Thromb Vasc Biol 2015;35:1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, Li H, Lamas S, Münzel T. Targeting vascular (endothelial) dysfunction. Br J Pharmacol 2017;174:1591–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis. Stroke 2001;32:454–460. [DOI] [PubMed] [Google Scholar]
- 8. Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol 2016;77:1–7. [DOI] [PubMed] [Google Scholar]
- 9. Blacher J, Safar ME. Large-artery stiffness, hypertension and cardiovascular risk in older patients. Nat Clin Pract Cardiovasc Med 2005;2:450–455. [DOI] [PubMed] [Google Scholar]
- 10. Hering D, Somers VK, Kara T, Kucharska W, Jurak P, Bieniaszewski L, Narkiewicz K. Sympathetic neural responses to smoking are age dependent. J Hypertens 2006;24:691–695. [DOI] [PubMed] [Google Scholar]
- 11. Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des 2010;16:2518–2525. [DOI] [PubMed] [Google Scholar]
- 12. Fiori G, Fuiano F, Scorza A, Conforto S, Sciuto SA. Non-invasive methods for PWV measurement in blood vessel stiffness assessment. IEEE Rev Biomed Eng 2022;15:169–183. [DOI] [PubMed] [Google Scholar]
- 13. Janner JH, Godtfredsen NS, Ladelund S, Vestbo J, Prescott E. The association between aortic augmentation index and cardiovascular risk factors in a large unselected population. J Hum Hypertens 2012;26:476–484. [DOI] [PubMed] [Google Scholar]
- 14. Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 2013;168:344–351. [DOI] [PubMed] [Google Scholar]
- 15. Collaboration TRVfAS . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 2010;31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A; Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005;111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 17. Tomiyama H, Yamashina A. Non-invasive vascular function tests: their pathophysiological background and clinical application. Circ J 2010;74:24–33. [DOI] [PubMed] [Google Scholar]
- 18. Thijssen DHJ, Bruno RM, van Mil ACCM, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ, Ghiadoni L. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 2019;40:2534–2547. [DOI] [PubMed] [Google Scholar]
- 19. Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Noma K, Nakashima A, Goto C, Tomiyama H, Takase B, Yamashina A, Higashi Y. Relationship between flow-mediated vasodilation and cardiovascular risk factors in a large community-based study. Heart 2013;99:1837–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanford BT, Brownstein NC, Baker NL, Palmer AM, Smith TT, Rojewski AM, Toll BA. Shift from smoking cigarettes to vaping nicotine in young adults. JAMA Intern Med 2024;184:106–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alzahrani T, Pena I, Temesgen N, Glantz SA. Association between electronic cigarette use and myocardial infarction. Am J Prev Med 2018;55:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen C, Huo C, Mattey-Mora PP, Bidulescu A, Parker MA. Assessing the association between e-cigarette use and cardiovascular disease: a meta-analysis of exclusive and dual use with combustible cigarettes. Addict Behav 2024;157:108086. [DOI] [PubMed] [Google Scholar]
- 23. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. In: Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd; 2019. p205–228. [Google Scholar]
- 25. Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AGM, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest 2016;150:606–612. [DOI] [PubMed] [Google Scholar]
- 26. Chaumont M, de Becker B, Zaher W, Culié A, Deprez G, Mélot C, Reyé F, Van Antwerpen P, Delporte C, Debbas N, Boudjeltia KZ, van de Borne P. Differential effects of e-cigarette on microvascular endothelial function, arterial stiffness and oxidative stress: a randomized crossover trial. Sci Rep 2018;8:10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ikonomidis I, Vlastos D, Kourea K, Kostelli G, Varoudi M, Pavlidis G, Efentakis P, Triantafyllidi H, Parissis J, Andreadou I, Iliodromitis E, Lekakis J. Electronic cigarette smoking increases arterial stiffness and oxidative stress to a lesser extent than a single conventional cigarette: an acute and chronic study. Circulation 2018;137:303–306. [DOI] [PubMed] [Google Scholar]
- 28. Franzen KF, Willig J, Talavera SC, Meusel M, Sayk F, Reppel M, Dalhoff K, Mortensen K, Droemann D. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: a randomized, double-blinded pilot study. Vasc Med 2018;23:419–425. [DOI] [PubMed] [Google Scholar]
- 29. Antoniewicz L, Brynedal A, Hedman L, Lundbäck M, Bosson JA. Acute effects of electronic cigarette inhalation on the vasculature and the conducting airways. Cardiovasc Toxicol 2019;19:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cossio R, Cerra ZA, Tanaka H. Vascular effects of a single bout of electronic cigarette use. Clin Exp Pharmacol Physiol 2020;47:3–6. [DOI] [PubMed] [Google Scholar]
- 31. Biondi-Zoccai G, Sciarretta S, Bullen C, Nocella C, Violi F, Loffredo L, Pignatelli P, Perri L, Peruzzi M, Marullo AGM, De Falco E, Chimenti I, Cammisotto V, Valenti V, Coluzzi F, Cavarretta E, Carrizzo A, Prati F, Carnevale R, Frati G. Acute effects of heat-not-burn, electronic vaping, and traditional tobacco combustion cigarettes: the Sapienza University of Rome-vascular assessment of proatherosclerotic effects of smoking (SUR - VAPES) 2 randomized trial. J Am Heart Assoc 2019;8:e010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haptonstall KP, Choroomi Y, Moheimani R, Nguyen K, Tran E, Lakhani K, Ruedisueli I, Gornbein J, Middlekauff HR. Differential effects of tobacco cigarettes and electronic cigarettes on endothelial function in healthy young people. Am J Physiol Heart Circ Physiol 2020;319:H547–H556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lyytinen G, Brynedal A, Anesäter E, Antoniewicz L, Blomberg A, Wallén H, Bosson JA, Hedman L, Mobarrez F, Tehrani S, Lundbäck M. Electronic cigarette vaping with nicotine causes increased thrombogenicity and impaired microvascular function in healthy volunteers: a randomised clinical trial. Cardiovasc Toxicol 2023;23:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bullen C. Impact of tobacco smoking and smoking cessation on cardiovascular risk and disease. Expert Rev Cardiovasc Ther 2008;6:883–895. [DOI] [PubMed] [Google Scholar]
- 35. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004;43:1731–1737. [DOI] [PubMed] [Google Scholar]
- 36. Barua RS, Ambrose JA. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler Thromb Vasc Biol 2013;33:1460–1467. [DOI] [PubMed] [Google Scholar]
- 37. Messner B, Bernhard D. Smoking and cardiovascular disease. Arterioscler Thromb Vasc Biol 2014;34:509–515. [DOI] [PubMed] [Google Scholar]
- 38. D’Ruiz CD, Graff DW, Yan XS. Nicotine delivery, tolerability and reduction of smoking urge in smokers following short-term use of one brand of electronic cigarettes. BMC Public Health 2015;15:991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med 2016;26:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol 2005;45:1987–1993. [DOI] [PubMed] [Google Scholar]
- 41. McEniery CM, Yasmin HIR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the anglo-cardiff collaborative trial (ACCT). J Am Coll Cardiol 2005;46:1753–1760. [DOI] [PubMed] [Google Scholar]
- 42. Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield J. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993;88:2149–2155. [DOI] [PubMed] [Google Scholar]
- 43. Meng XC, Guo XX, Peng ZY, Wang C, Liu R. Acute effects of electronic cigarettes on vascular endothelial function: a systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol 2023;30:425–435. [DOI] [PubMed] [Google Scholar]
- 44. Yufu K, Takahashi N, Hara M, Saikawa T, Yoshimatsu H. Measurement of the brachial-ankle pulse wave velocity and flow-mediated dilatation in young, healthy smokers. Hypertens Res 2007;30:607–612. [DOI] [PubMed] [Google Scholar]
- 45. Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 1998;16:2079–2084. [DOI] [PubMed] [Google Scholar]
- 46. Saz-Lara A, Martínez-Vizcaíno V, Sequí-Domínguez I, Álvarez-Bueno C, Notario-Pacheco B, Cavero-Redondo I. The effect of smoking and smoking cessation on arterial stiffness: a systematic review and meta-analysis. Eur J Cardiovasc Nurs 2022;21:297–306. [DOI] [PubMed] [Google Scholar]
- 47. Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, Terentes-Printzios D, Georgakopoulos C, Pietri P, Stefanadis C, Tousoulis D. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J Am Coll Cardiol 2016;67:2802–2803. [DOI] [PubMed] [Google Scholar]
- 48. Skotsimara G, Antonopoulos AS, Oikonomou E, Siasos G, Ioakeimidis N, Tsalamandris S, Charalambous G, Galiatsatos N, Vlachopoulos C, Tousoulis D. Cardiovascular effects of electronic cigarettes: a systematic review and meta-analysis. Eur J Prev Cardiol 2019;26:1219–1228. [DOI] [PubMed] [Google Scholar]
- 49. Mueller SD, Britton GR, James GD, Fahs PS. Vaping behaviour patterns and daily blood pressure and heart rate variation: a brief report. Ann Hum Biol 2021;48:535–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included within the article.