Abstract
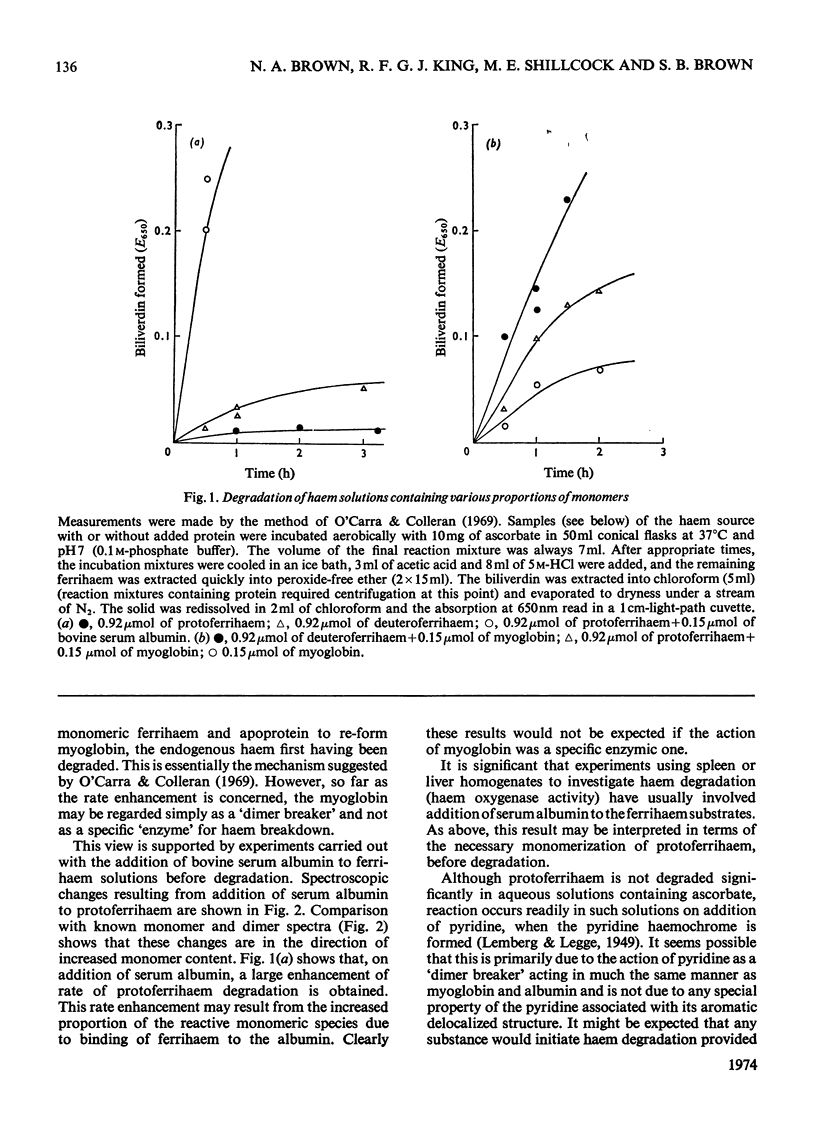
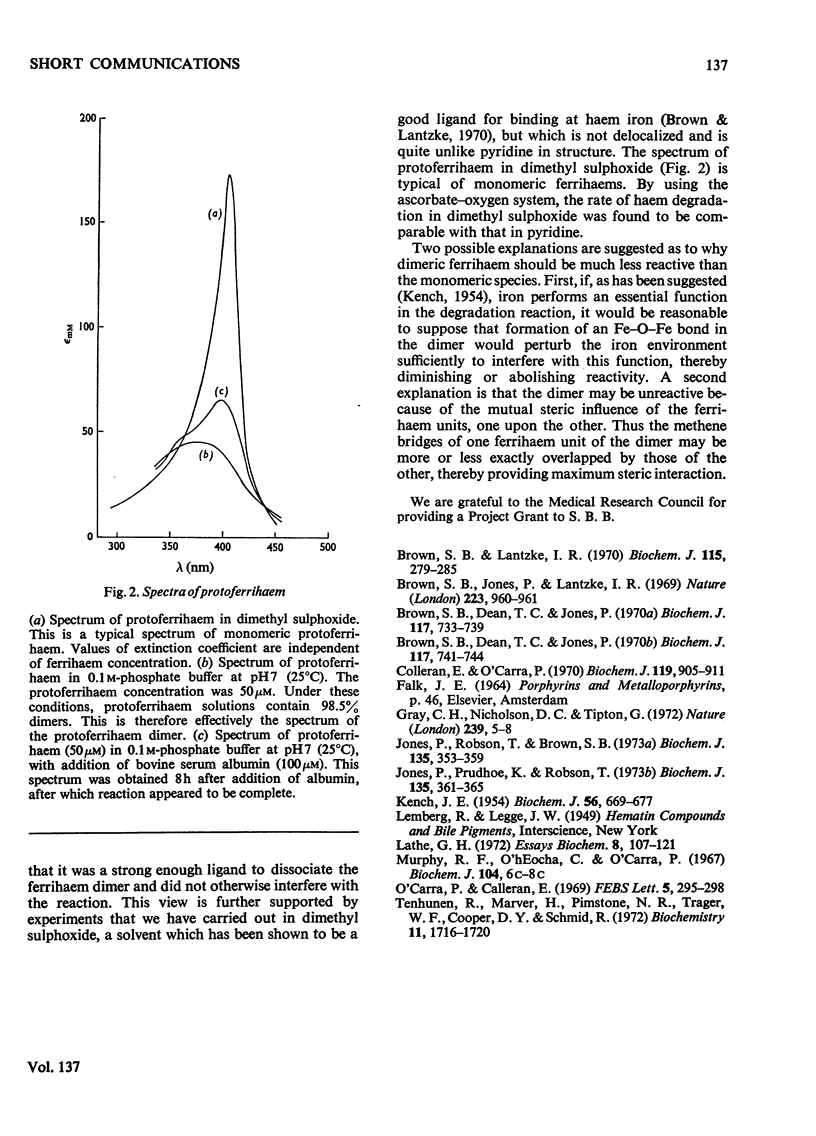
The importance of ferrihaem aggregation in studies of haemoglobin catabolism is assessed in the light of recent work. Experimental evidence is put forward suggesting that monomeric ferrihaems are degraded much more readily than dimeric species. This may offer an alternative explanation for the apparent `apoprotein catalysis' recently observed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 'Carra P. O., Colleran E. HAEM catabolism and coupled oxidation of haemproteins. FEBS Lett. 1969 Nov 29;5(4):295–298. doi: 10.1016/0014-5793(69)80372-8. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Dean T. C., Jones P. Aggregation of ferrihaems. Dimerization and protolytic equilibria of protoferrihaem and deuteroferrihaem in aqueous solution. Biochem J. 1970 May;117(4):733–739. doi: 10.1042/bj1170733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Dean T. C., Jones P. Catalatic activity of iron(3)-centred catalysts. Role of dimerization in the catalytic action of ferrihaems. Biochem J. 1970 May;117(4):741–744. doi: 10.1042/bj1170741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Jones P., Lantzke I. R. Infrared evidence for an oxo-bridged (Fe-O-Fe) haemin dimer. Nature. 1969 Aug 30;223(5209):960–961. doi: 10.1038/223960a0. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Lantzke I. R. Solution structures of ferrihaem in some dipolar aprotic solvents and their binary aqueous mixtures. Biochem J. 1969 Nov;115(2):279–285. doi: 10.1042/bj1150279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleran E., Carra P. O. Non-enzymic nature of the pyridine haemochrome-cleaving activity of mammalian tissue extracts ('haem alpha-methenyl oxygenase'). Biochem J. 1970 Oct;119(5):905–911. doi: 10.1042/bj1190905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. H., Nicholson D. C., Tipton G. Degradation of haem compounds to bile pigments. Nat New Biol. 1972 Sep 6;239(88):5–8. doi: 10.1038/newbio239005a0. [DOI] [PubMed] [Google Scholar]
- Jones P., Prudhoe K., Robson T. Oxidation of deuteroferrihaem by hydrogen peroxide. Biochem J. 1973 Oct;135(2):361–365. doi: 10.1042/bj1350361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P., Robson T., Brown S. B. The catalase activity of ferrihaems. Biochem J. 1973 Oct;135(2):353–359. doi: 10.1042/bj1350353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENCH J. E. Bile pigment formation in vitro from haematin and haem derivatives. Biochem J. 1954 Apr;56(4):669–677. doi: 10.1042/bj0560669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe G. H. The degradation of haem by mammals and its excretion as conjugated bilirubin. Essays Biochem. 1972;8:107–148. [PubMed] [Google Scholar]
- Tenhunen R., Marver H., Pimstone N. R., Trager W. F., Cooper D. Y., Schmid R. Enzymatic degradation of heme. Oxygenative cleavage requiring cytochrome P-450. Biochemistry. 1972 Apr 25;11(9):1716–1720. doi: 10.1021/bi00759a029. [DOI] [PubMed] [Google Scholar]


