Abstract
In a hyperconnected world, framing and managing biological invasions poses complex and contentious challenges, affecting socioeconomic and environmental sectors. This complexity distinguishes the field and fuels polarized debates. In the present article, we synthesize four contentious issues in invasion science that are rarely addressed together: vocabulary usage, the potential benefits of nonnative species, perceptions shifting because of global change, and rewilding practices and biological invasions. Researchers have predominantly focused on single issues; few have addressed multiple components of the debate within or across disciplinary boundaries. Ignoring the interconnected nature of these issues risks overlooking crucial cross-links. We advocate for interdisciplinary approaches that better integrate social and natural sciences. Although they are challenging, interdisciplinary collaborations offer hope to overcome polarization issues in invasion science. These may bridge disagreements, facilitate knowledge exchange, and reshape invasion science narratives. Finally, we present a contemporary agenda to advance future research, management, and constructive dialogue.
Keywords: conservation biology, human–wildlife interactions, invasive species, invasion science, natural resource management
Human-aided introductions of species to geographical regions outside their native range is one of the most distinguishable features of the Anthropocene era (Ricciardi 2007), with no evidence of reaching saturation over time (Mormul et al. 2022). Although only a subset of nonnative species subsequently become established and invasive (Blackburn et al. 2011, Roy et al. 2023, Soto et al. 2024), biological invasions can have important environmental (Pyšek et al. 2020), socioeconomic (Bacher et al. 2018), and human health effects (Zhang et al. 2022). Moreover, the replacement of native species by largely generalist nonnative biota is reshaping patterns of global biodiversity via the process of biotic homogenization (Olden et al. 2018).
The study of biological invasions is generally referred to under the terms invasion biology and invasion ecology (Elton 1958), which emphasize the natural sciences; however, invasive species are inherently embedded in complex socioecological systems (e.g., Bacher et al. 2023), leading to the more recent use of invasion science. This all-encompassing term aims to reflect the “full spectrum of fields of enquiry that address issues pertaining to alien species and biological invasions” (Richardson 2011, p. 415). Biological invasions should therefore be considered a pervasive global phenomenon that may act on a broad range of intersectional themes, requiring cross-fertilization of techniques and expertise to better address the challenges posed.
Invasion science is a fertile ground to integrate a range of scientific approaches, including citizen, social, and environmental science research, and all of these approaches may stimulate considerable debate. Despite the broad academic interest, the topic remains inadequately acknowledged by the public, not fully accepted by some decision-makers, and a matter of ongoing polarization among stakeholders (e.g., Richardson and Ricciardi 2013, Courchamp et al. 2017, Haubrock et al. 2024a). For instance, major debates revolve around values and perceptions such as whether biological invasions could be considered contemporary features of the globalized era, the consequences of valuing the contributions of nonnative species and whether the terminology within the field is adequately defined and applied (e.g., Young and Larson 2011, Shackleton et al. 2022, Pelicice et al. 2023). These differences in perspectives are propelled by cultural and demographic values influencing perception (Shackleton et al. 2019). Deficits between public perceptions and national policy definitions cause difficulties in developing mutually accepted conservation goals (van Eeden et al. 2020), whereas media pressure and political debates may have greater capacity to drive policy changes regarding nonnative species than scientific evidence alone (Gozlan et al. 2013).
The novel change and biogeographical implications associated with the Anthropocene (e.g., commercial globalization, new species introduction pathways and human–wildlife interactions) will continually exacerbate conflicting opinions and will contribute to fluctuations in debates surrounding the fundamental concepts and definitions that underpin the field. Anticipating and better understanding these challenges is an urgent issue for both research and legislation to mitigate future disconnections and conflicts across the field of invasion science.
Although there has been considerable progress in synthesizing knowledge and theoretical or technological advancements in invasion science (Drake et al. 1989, Richardson 2011, Fricke and Olden 2023, Stevenson et al. 2023), research has predominantly been focused on individual aspects, and few studies have addressed multiple components of the debate within or across disciplinary boundaries. It is timely to frame priority research areas in the field that draw on past experiences to identify pathways forward and an integrative framework to address the multifaceted interrelationships among nature conservation, invasion science, and society. In the present article, we specifically review and synthesize four topics insufficiently or historically addressed on their own in the literature as examples of contentious and emerging issues across the breadth of invasion science: controversial and contentious vocabulary, potential positive effects of nonnative species, new conservation or policy frameworks prompted by global environmental change, and rewilding practices and their associations with biological invasions. The issues critically reviewed in the present article, along with the insights they provide, should be integrated in the contemporary interdisciplinary agenda on invasion science to help advance the field. This should foster productive dialogue among stakeholders, facilitate the wide dissemination of knowledge, and highlight new perspectives in the field to those who will increasingly have to engage with invasion science.
Challenges and polarized issues
Language in invasion science as a pending task for multiple sectors
Linguistics is a central issue for the effective communication and management of nonnative and invasive species. Considerable effort has been given to standardizing glossaries and definitions across invasion science and policies (e.g., Blackburn et al. 2011, Essl et al. 2018, Soto et al. 2024), but debates and specific terminology are still naturally evolving alongside the vocabulary (e.g., Lemoine and Svenning 2022 or the new challenges prompted by global change; see below). In the present article, we focus on the challenges and implications posed by the use of value-laden language, which appears almost exclusively dichotomous in the field (for further debate, see the next subsection below).
Value-laden vocabulary is widespread in society, media, and science; therefore, it is not solely an issue associated with biological invasions (Kueffer and Larson 2014). Invasion science research tends to employ a higher frequency of militaristic language per article (e.g., enemy, combat, attack) than does research on other topics (see Janovsky and Larson 2019). Nevertheless, this may inadvertently lead to misrepresentation of the original intention in some instances (e.g., a charge of xenophobia; Simberloff et al. 2013), given that invasion science lacks an easy or a single iconic challenge on which to focus, unlike many other fields (Courchamp et al. 2017). Metaphors are widely used in research (e.g., Kueffer and Larson 2014), and military terms appear to draw short-term public attention to invasive species. For example, in 2019 the Environmental Audit Committee of the UK Parliament called for a citizen army of 1.3 million people to tackle biosecurity risks from invasive alien species (BBC 2019). The nationalization of nature can be traced in the nineteenth century, when civil law terminology was applied to the natural world (see Antonsich 2021 for a wider historical perspective) relying on a nation's boundaries to classify species. However, nationalistic and militaristic idiolects applied to nature have the potential to be misconstrued, contributing to social misunderstanding and driving counterproductive conflict in conservation activities (Larson 2005, Young and Larson 2011).
Because invasive nonnative species are more likely to be implicated in recent species extinctions than are native species (Blackburn et al. 2019), it is crucial to acknowledge the relevance of coevolutionary history and biogeographical origins, including the role of prey naïveté (the failure of prey to recognize novel predators as threats; e.g., Pauchard et al. 2018, Anton et al. 2020). Most invasive species (often abbreviated to INNS, for invasive nonnative species) checklists are based on administrative boundaries or at the country level that fund them and that have legislative jurisdiction regarding management (Early et al. 2016, Essl et al. 2018). These typically aim to preserve the natural heritage of the country and reflect the national jurisdiction on border control and investments. However, they can also unintentionally confer a national status to species, which may complicate international definitions (Essl et al. 2018) or bypass instances in which a species is invasive and native to different regions of the same country (Nelufule et al. 2022). Shackleton and colleagues (2022) highlighted that the majority of scientists and practitioners find the use of country borders to define a species to be problematic, and in this context, EU Regulation 1143/2014 specifically aimed to extend the management of INNS beyond national borders. Nonetheless, transcendence of national boundaries in these respects can be attained; for example, the European LIFE INVASQUA project produced collaborative species lists at the biogeographic scale of the entire Iberian Peninsula (Spain and Portugal) while avoiding loaded vocabulary (Oficialdegui et al. 2023).
In a review, Khan (2021) stressed how the use of noninclusive terms in life sciences literature is ongoing. A common example is the historical use of dichotomous black and white lists to define groups of regulated (i.e., those that require control or policy implementation) and approved species, respectively. Although these terms are largely avoided now, translations between languages may result in further variations of perceptions. Lepczyk (2022) went one step further by suggesting that words such as alien take on political and cultural connotations and recommended simplifying the terminology to simply nonnative or invasive, depending on the context (as we have done in the present article except where the original authors used alternative terms). In this context, Soto and colleagues (2024) suggested allochthonous (contra autochthonous) as a possible alternative that is not (yet) politically charged and used in multiple scientific fields. Ovid and Phaka (2022) further highlighted the racialization of vocabulary relating to some invasive species (e.g., Xenopus laevis, a frog originating in Sub-Saharan Africa and introduced in regions of the Americas, Europe, and Asia), as well as changes in attitudes associated with their economic value, recommending that the significant role society plays in species becoming invasive should be emphasized.
In addition to the technical linguistics of invasion science, a cultural linguistic barrier can appear where an invasive species may not only be phylogenetically new but culturally and socially new. An interesting and recent example is Cherax quadricarinatus (redclaw crayfish, native to Australia), which has been intentionally introduced for aquaculture purposes to southern Africa (Madzivanzira et al. 2020). There are no native crayfish species on the African continent, and landlocked countries such as Zimbabwe and Zambia have no functional crustacean equivalents. In the absence of an equivalent name, communities in the Barotse floodplain refer to the redclaw crayfish as Chinese nkala—a direct reference to the Chinese road workers building the Mongu–Kalabo floodplain road who introduced the species, and nkala, meaning “crab.” In these instances, there are no words in the recipient country's language to easily name or identify the new nonnative species, leading to marked differences in values and perceptions ascribed to them. It is likely that similar examples will be increasingly common in the future and that this scenario will ultimately promote discrepancies (and further challenges) in those species’ monitoring, regulation, and societal value.
Responsible communication among scientists (e.g., within peer-reviewed literature) and across wider society sectors (e.g., dissemination and policy) also poses a significant challenge, and the choice of language has the potential to influence public perceptions regarding invasive species (Golebie et al. 2022). Indeed, vocabulary is an evolving issue that encompasses multiple debates in both the field and society (see figure 1). The contemporary and global nature of biological invasions would benefit from the use of a contemporary and unbiased language, avoiding sensational, value-laden or military terminology beyond the Anglophone-speaking arena. To avoid popular names strictly referring to a single nation (such as New Zealand mud snail or Chinese mitten crab) may also help in reducing misunderstanding when disseminating outputs in popular science. Similarly, although the scientific community is not charging the terms with any moral significance, more inclusive terminology to describe nonnative species using terms such as priority list; attention, concern, or watch list; and observation list should be considered while ensuring links to historic data and established knowledge are maintained (e.g., Bayón and Vilà 2019, Oficialdegui et al. 2023, Kumschick et al. 2024). This would also help in avoiding criticism at a later point and would allow policymakers to easily integrate information from the past.
Figure 1.
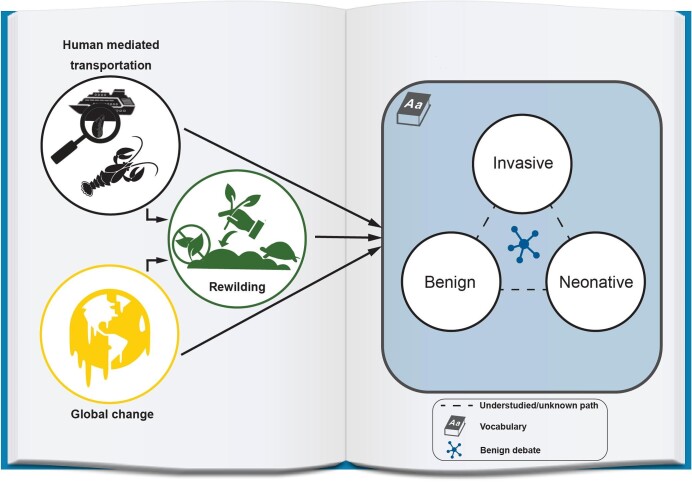
Schematic flow diagram indicating the process of biological invasions: from pathways and processes of introductions to debates, labels, and vocabulary. Two potential introduction pathways are highlighted: human-mediated transportation (nonnative species) and colonization due to global change (e.g., adaptive spread, in yellow). The example of rewilding (in green) is shown to illustrate how this practice may contribute to the arrival of species via either of the two processes. Once the species arrives in a new region (the blue square on the right), it may result in differential effects in the recipient ecosystem and may be labeled with different vocabulary options (summarized as benign sensu Vimercati et al. 2022, invasive sensu Roy et al. 2023 and neonative sensu Essl et al. 2019). The dashed lines highlight understudied pathways and potential challenges in terminology and ecosystem implications that require further research. Species labeled as benign (but this can apply for all three terms) should be associated with further information available to stakeholders, because they may also present unknown, heterogenous, or unintended consequences (benign species debate in the blue and the dashed lines). The entire diagram is placed on a book to denote that all these issues and processes are firmly situated within the backdrop of vocabulary, which remains an ongoing issue. Terms can vary over time and context, whereas connector lines do not necessarily reflect linear time.
Benign nonnative species debate: Oxymoron versus possibility.
As Goodenough (2010) argued, the narrative “native good, nonnative bad” does not fully recognize the complexity of invasion science and the environmental gradients experienced in the field. In many instances, only a small fraction of nonnative species may reach an invasive behavior or status, and the magnitude and direction of their implications can be highly context dependent (e.g., resident community composition and biotic interactions; Catford et al. 2022). Interestingly, Blackburn and colleagues (2011) and Soto and colleagues (2024) considered that ecological impacts were beyond the scope of the definition of invasive species (they can occur at any of the stages during the process of invasion), but they are considered in Roy and colleagues’ (2023) recent glossary.
Given the dominance of invasive nonnative species at some locations, to the extent that eradication may no longer be feasible (at least in the short term), it has been postulated that novel communities should be accepted and that research efforts should be focused on better understanding their ecoevolutionary implications and roles (e.g., Thomas 2013). This view may be supported by a range of factors, from limited financial resources for research and management to conflicting stakeholder interests in species eradication or the lack of realistic short-term options to prevent the current rate of introductions worldwide. For example, Davis and colleagues (2011) suggested, in what is still considered a contentious proposition over 10 years later, that conservationists should focus much more on the functions of species and much less on where they originated from. More recently, Bolpagni (2021) hypothesized a near future where invasive plants play dominant roles in driving freshwater ecosystems functioning within a biohistorical horizon termed the Exocene.
Rapid change and increased inequality and disparity in human well-being may increase the likelihood that nonnative species will be viewed as benign (e.g., with some beneficial outcomes) over time. Despite uncertainty surrounding the effects of many nonnative species (e.g., unpredicted or not immediately positive or negative), some authors anticipated that some will be regarded as benign over time (e.g., serving as functional substitutes for extinct taxa, food or providing desirable economic services; Schlaepfer et al. 2011, Sax et al. 2022). For instance, the importance of nonnative birds in Hawaii has been highlighted by Foster and Robinson (2007) for the dispersal of native plant seeds, especially given that almost all native seed dispersers are locally extinct. Similarly, marine nonnative polychaetes (Marenzelleria spp.) can enhance phosphorus retention in sediments, therefore reducing eutrophication and hypoxia (e.g., northern Baltic Sea: Norkko et al. 2012). Even species with recognizable negative implications within the ecosystem, such as invasive crayfish, have been identified as a food source for some native faunal groups (e.g., Eurasian otter Lutra lutra in South Europe; Dettori et al. 2021). The latter highlights that single positive and isolated effects (e.g., on a single species or over a short period of time) should not be enough to define a species as benign because a single nonnative species can have multiple and simultaneous interactions at different levels of ecological complexity (Simberloff et al. 2013, Saunders et al. 2016). Moreover, implications, values, and labels may change over time, and this dynamism may also drive shifts in environmental baselines (Soga and Gaston 2018). Shifts in social acceptance may also affect environmental policies and baseline definitions, which may subsequently promote the conservation of highly appreciated nonnative species, especially if the species is introduced before living memory and before a subsequent nonnative species threatens it (e.g., Clavero 2014; see also box 1).
Box 1. Multifaceted challenges associated with nonnative species: Nile tilapia.
Individual of O. niloticus (credits: Denis Tweddle)
Nile tilapia (Oreochromis niloticus) has a complex history in southern Africa where it was introduced during the 1980s by donor-funded Western organizations to address food security on the basis of its fast growth and stress tolerance (Zengeya et al. 2015, Moyo and Rapatsa 2021). Subsequently, the establishment of aquaculture based on O. niloticus has expanded—for example, in Zambia and Zimbabwe—driven by its domestication traits with high feed-conversion efficiency, and high-quality animal protein (Zengeya et al. 2015, Basiita et al. 2022). However, the persistent limited market profitability in domestic aquaculture, driven by imports from Asian markets (Moyo and Rapatsa 2021), has been accompanied by negative ecological impacts from O. niloticus’s spread. These include competition and hybridization with native congeners, leading to food web disruption, a decline in native species populations, and the loss of genetic integrity (Zengeya et al. 2015, Jere et al. 2021).
Local dambo ecosystems (i.e., complex shallow wetlands) and other water bodies may become susceptible to invasion by cultured fish species via escape during harvest and rainy season flooding. Moreover, Chakandinakira and colleagues (2023) revealed a novel interaction in Lake Kariba (Zambezi Basin) where the rapidly spreading invasive Cherax quadricarinatus may damage O. niloticus, causing further cascading implications on local fisheries.
Contrasting policies between countries regarding O. niloticus management causes a problematic dynamic in southern Africa (e.g., because of many catchments being shared between countries) but also across other areas of the Global South. For example, in South Africa the current legislation recognizes ecological damage and prohibits O. niloticus aquaculture unless the species is already present in the catchment (e.g., Moyo and Rapatsa 2021). However, in Brazil, legislation (e.g., 79/2016, on fish cage culture in hydroelectric reservoirs) has proposed the naturalization of nonnative tilapias regardless of their ecological impacts (e.g., Padial et al. 2017). This sets a shortsighted dangerous precedence on the basis of prioritization of economies rather than other ecosystem values and potential implications. Therefore, the social narrative and perceptions associated with nonnative aquaculture species (e.g., O. niloticus as benign) should be considered alongside the potential implications for wider nature conservation, social interests, and local economies.
The classical invasional meltdown hypothesis postulates that the presence of nonnative species might facilitate the invasion of another or others (see Braga et al. 2018). However, it is also likely that multiple co-occurrent nonnative species in a region will interact (e.g., Fricke and Svenning 2020, Guareschi et al. 2021), leading to scenarios where, in highly invaded sites, one species could potentially mitigate the negative effects of another. This will be possible, for example, by relieving the predatory pressure on a native prey by becoming the new preferred prey in a system or by consuming previously introduced invasive taxa (e.g., Liu et al. 2018, Céspedes et al. 2024). Similar situations (with turnover and accumulation) are likely to become more frequent in the future, representing a research priority in invasion science to advance understanding of the field and to better inform conservation decisions.
Nevertheless, so far, the benignity of a nonnative species primarily reflects an anthropocentric and utilitarian perspective. Sax and colleagues (2022) stressed how beneficial outcomes of some nonnative species may be common and important for human well-being (e.g., relational, instrumental, and intrinsic values). However, different stakeholders may have contrasting perspectives (i.e., environmental, economic, social, ethic, or aesthetic), different assessment systems, and different priorities, making it difficult to achieve feasible trade-offs (Woodford et al. 2016, Oficialdegui et al. 2020, Novoa et al. 2024). These perspectives are value laden and may not align with a simplistic for or against narrative. These wicked problems (e.g., Woodford et al. 2016) should be carefully acknowledged as soon as possible and dialogue initiated to prevent conflicts among stakeholders. Stresses relating to climate forced changes in species distribution, food and water security mean that conflicting agendas could occur more frequently in the future. Therefore, anticipating these and engaging multiple parties increases the possibility of reaching mutually suitable outcomes over time.
Perceived values vary depending on geographic context. For example, some African and Indian stakeholders or communities are more likely to acknowledge the benefits of some invasive species alongside negative aspects (Shackleton et al. 2019, Singh et al. 2022; but see also Bacher et al. 2023). This could be, at least partially, attributed to the initial purposes of introduction (e.g., food security or economic bolstering in the Global South compared with pet or aquarium pathways of introduction in many locations in the Global North; Turbelin et al. 2017). In contexts where a dependence on nonnative species has been fostered through other systemic inequities, individuals often prioritize personal well-being (i.e., physiological needs sensu Maslow's hierarchy; Maslow 1943) over environmental causes. Anticipating the consequences of eradication programs (and species escape) on food resources, especially in emerging economies and impoverished nations, is therefore crucial. In this context, lessons from other fields of conservation biology, such as coexistence with large mammals through equity and long-term planning payments (Hamm et al. 2023), may provide valuable insights.
From an economic perspective (not necessarily concordant with nature conservation priorities), the most common way to assign a monetary value to a species and, as a result, to reach a potential benign status is to have a specific market for it (e.g., harvesting for food, exotic pet trade, fishing competitions, or domestic gardening or horticulture trade). However, creating markets for INNS may drive a socioecological trap where individuals and industries may try to recreate the market in new previously noninvaded regions with unpredictable consequences (box 1; Nuñez et al. 2012). In southern Spain, although the crayfish aquaculture industry extracts around 150 million red swamp crayfish (Procambarus clarkii) annually for international food markets, the population has shown no evidence of declining (Oficialdegui et al. 2020). As a result, the economic market did not solve nor properly address the environmental issues. The commodification of invasive species can also bring further socioecological cascade dilemmas, such as those reported for the Indo-Pacific lionfish (Pterois spp.) fishery in Mexico where an overfishing campaign led to the collapse of the lionfish fishery but also to social problems relating to sustainability and fishery industry disenchantment (Quintana et al. 2023). The need to apply values to abstract services or effects (e.g., those of pristine ecosystems or native species, human well-being and species interactions) requires the application of nonmarket valuation approaches, which have recently been outlined specifically for invasive species management (Hanley and Roberts 2019).
Despite the high and rising economic costs of biological invasions worldwide (Diagne et al. 2021), economic interests may also act as strong deterrents to the implementation of mitigation or eradication policies (Lambertucci and Speziale 2011). For example, the eastern Australian Eucalyptus globulus, widely cultivated on the Iberian Peninsula for paper pulp production, has been a matter of wide bureaucratic litigation (Cidrás and González-Hidalgo 2022). Despite the species being recognized as nonnative and causing many detrimental ecological effects, it is currently not recorded in the national Catalogue of Invasive Species (e.g., Spain: RD 630/2013). Similarly, the policy listing of brown and rainbow trout (Salmo trutta, Oncorhynchus mykiss) as invasive in South Africa remains unresolved because of stakeholder conflicts (South et al. 2022).
Evaluating and quantifying INNS impacts or risks in a standard way has greatly improved (e.g., Vimercati et al. 2022), although it remains challenging in some cases. For example, aquatic invaders are less visible and more likely to go undetected and, as a result, are not regularly assessed (Moorhouse and Macdonald 2015), thereby representing a critical emerging issue in the field. Factors such as crypticity (i.e., species complexes comprising both native and nonnative taxa), in addition to endangered species that also have nonnative populations, further complicates assessments and management (see numerous examples in Marchetti and Engstrom 2016, Jarić et al. 2019). In extreme instances if a nonnative species hybridizes with a native species, the hybrid could be considered endemic or new, and therein a further challenge in terms of conservation management may arise (e.g., Senecio eboracensis in the United Kingdom; Lowe and Abbott 2015). Assessments at the species level are further confounded by the fact that the invasion stages of a specific species should reflect individual populations rather than being generalized across all regions. As was argued by Colautti and MacIsaac (2004), the field of biological invasions will benefit from a greater focus at the population level, rather than the species level, which has not been sufficiently explored (see new evidence from Haubrock et al. 2024b). A contemporary perspective of sleeper populations embodies this call (Spear et al. 2021). These are characterized by initially small population that may become invasive (e.g., a sudden shift from low to high abundance causing impact generation) after an environmental trigger facilitates rapid proliferation.
In the current globalized context, it seems reasonable and unavoidable to simultaneously investigate both the benign and detrimental implications of nonnative taxa on biodiversity or on anthropogenic societal well-being to complement our understanding and better guide management (e.g., Goodenough 2010, Vimercati et al. 2022). However, at the same time, emphasizing the positive contributions of INNS to society and nature may misguide conservation actions (e.g., Pelicice et al. 2023). The examples discussed in the present article underscore the significance of assessing the implications and impacts of nonnative taxa across both the species and population level. Benign implications should clearly be contextualized within the terms of the areas or regions considered, the target audience, and the overall framework—clarifying who benefits benignly and under which circumstances. This should be considered because incidental benefits may also come at a price and may affect communities and regions differently over time (Carneiro et al. 2024). Overall, this information may help guide the development of triage systems with prioritization levels customized to specific contexts (e.g., Downey et al. 2010).
Global change: Expanding species, new interactions, and additional challenges
The outcomes and implications of anthropogenically driven climate change may directly influence biological invasions through the creation of previously inaccessible trading routes, by creating favorable conditions for generalist invasive species (e.g., extreme events increasing stress on resident taxa), and by modifying trophic networks or species life cycles (e.g., populational outbreaks; e.g., Hulme 2017, van Wilgen et al. 2022). For these reasons, the management of invasive species would benefit from the incorporation of climate change scenarios as a prerequisite to facilitate better decision-making processes (Robinson et al. 2020, Bradley et al. 2022). Likewise, climate change adaptation planning should consider the likelihood that current nonnative species may become of increasing concern in the future, given that the geographical ranges of some species will increase or change and that stressed habitats may become more vulnerable to the spread of tolerant species (e.g., Gallardo et al. 2017, Bradley et al. 2024). This seems supported by Gu and colleagues (2023), who recently revealed how nonnative animals appear less sensitive than native taxa to extreme weather conditions globally. Biological communities may suffer the combined effects of invasive species and climate change and, as a result, native or endangered species may find themselves in a precarious position between multiple interacting stressors (Lopez et al. 2022). Cornerstone conservation tools, including protected areas, may not be able to effectively address these co-occurring, highly dynamic, pressures (e.g., Cerasoli et al. 2019).
Polar and alpine areas are particularly sensitive to a warmer climate, which may weaken their climatic barriers and facilitate the spread of species into new environments that were previously unsuitable (e.g., Antarctic, Duffy et al. 2017; Arctic, Alsos et al. 2015). For instance, the combined threat of climate change and the range expansion of a competitor species had a negative effect on the arctic fox (Vulpes lagopus) as the red fox (Vulpes vulpes) expanded its niche into new, warming areas of the arctic. This range shift competitively excluded the arctic fox (Angerbjörn et al. 2013), prompting the need for active management of red fox populations. In a different environmental context, similar faunal shifts have been reported for some dragonflies (Insecta: Odonata), such as the afrotropical orange-winged dropwing, Trithemis kirbyi, originally recorded in arid areas of Africa and southern Asia but increasingly recorded within European Mediterranean countries (Herrera-Grao et al. 2012) because of recent warmer and drier summers. Reflecting these changes, an international survey encompassing 18 countries showed that the majority of experts working on freshwater ecosystems reported that native species, which had expanded their range as a result of climate change, were not considered alien or nonnative (Boon et al. 2020) in line with the EU Regulation 1143/2014 on the prevention and management of the introduction and spread of invasive alien species (article 2, chapter 1).
In a hyperconnected world, historic biogeographical barriers have become more permeable, in some instances without physical anthropic intervention but through the creation of new habitats and environmental conditions favorable for colonization by both native and nonnative species. This has further polarized new terminology. Tentative terms used for these species in the literature include tracking species, range expanders, and range shifters, which reflects their new or increased distribution because of favorable environmental conditions emerging (see also Parmesan 2006). This range shift will result in new interactions in the new recipient ecosystem or area but likely in a more gradual way than directly human-mediated introduced species (Essl et al. 2019). In this instance, species will also expand their range with many of their coevolved natural enemies, making invasive behavior less likely. In some instances, these range shifts may be crucial to species persistence. Strict range shifters can be difficult to define as invasive, as was highlighted in recent public surveys and scientific bibliographies (e.g., Cranston et al. 2022). Essl and colleagues (2019) concluded that these species will become a common feature and issue for biodiversity conservation in the Anthropocene and used the term neonative to describe them. This interesting neologism was rapidly criticized by Wilson (2020) because of the potential confusion it adds to the debate. Urban (2020) supported the development of a framework centered on climate-tracking species with these also being labeled as refugees of climate change; although this term may still be considered controversial. The concept of climatic travelers may also share similarities with the range expansions of many species following the Pleistocene glaciations from southern refugia toward the north (Hewitt 2000). Nevertheless, a key difference is the rapid increase in the rate of dispersal (i.e., speed, distance, and time) coupled with the anthropogenic origin that underlies climate change (IPCC 2022). It cannot be ignored that neonative or climate-tracking species can also, in some context-specific conditions, spread widely with invasive behaviors; however, this remains an ongoing matter of research (as is indicated by the dashed line in figure 1). Special attention should be given to species with broader implications in their historical native range (e.g., predators and ecosystem engineering species; Wallingford et al. 2020), as well as to those known for their implications in other geographic areas. In addition, ecosystems, regions, and contexts that exhibit heightened susceptibility to the accumulation of nonnative taxa require particular attention. Long-term monitoring of range-expanding species, including temporal patterns and the distribution of natural competitors and predators, is essential to avoid overlooking their trajectories of change in biogeographical research. It also ensures that the ecological consequences of their spread can be comprehensively studied and anticipated for the effective implementation of environmental policies.
Marine systems offer a particularly controversial example where terms such as neonative are inapplicable, because the range expansion does not directly respond to new climatic availability alone. For example, so-called Lessepsian species (named after Ferdinand de Lesseps, responsible for construction of the Suez Canal) migrate from the Red Sea into the Eastern Mediterranean through the Suez Canal, which provides a direct link between two previously unconnected biogeographic regions (Por 1978, Azzurro et al. 2016). Increasing temperatures in the Mediterranean Sea have facilitated the rising rates of establishment of primarily tropical species moving unaided through anthropogenic infrastructure. A similar phenomenon occurs in inland water bodies where interbasin water transfers provide an infrastructure-based pathway of introduction, with impacts measured predominantly in the recipient location (Gallardo and Aldridge 2018).
Post-Fordist global economies, where processes of production are organized at the global scale, are associated with increased international connectivity and anthropogenic mobility (Nyström et al. 2019). As much as globalization has eroded political borders, so too has climate change through the expansion, contraction, or modification of suitable habitats for numerous species. Ongoing global environmental change (e.g., new environmental conditions, altered rainfall patterns) has already highlighted climate‐change winners and losers related to species distributions (e.g., headwater communities, Hossack et al. 2023; alpine plants, Geppert et al. 2023) as well as implications in pathogen vectors (e.g., mosquito Aedes albopictus; Brugueras et al. 2020). However, nonnative species do not always benefit from climate change. For example, the distributions of some invasive ants, European plant species, and trout species in Africa are expected to potentially decline in some future climate change scenarios (e.g., Peterson et al. 2008, Bertelsmeier et al. 2015, van Wilgen et al. 2022). The examples discussed in the present article, spanning different environments, illustrate the difficulty of simple generalizations and therefore the importance of adjusting the lens of invasion science when focusing on the implications of climate-driven range shifts.
Novel restoration approaches and rewilding: Potential Trojan horses for biological invasions?
To effectively address the escalating biodiversity crisis and meet the post-2020 biodiversity goals within the United Nations–designated decade of ecosystem restoration (2021–2030), bold and innovative approaches to conservation and restoration are imperative. This aligns with the Kunming–Montreal Global Biodiversity Framework (CBD/COP/15), with goal A calling for maintaining, enhancing, or restoring the integrity, connectivity, and resilience of all ecosystems, which also includes targeted objectives for addressing invasive species (no. 6). In this context, the concept of rewilding as a restoration approach has received considerable attention and today it exemplifies an emerging polarizing issue gaining traction in science and policy, including (and probably peaking) in the field of biological invasions (e.g., Guerisoli et al. 2023). It has, for instance, been referred to as Pandora's box (Nogués-Bravo et al. 2016) or as a wolf in sheep's clothing (Rubenstein and Rubenstein 2016). These criticisms have specifically highlighted the potential for rewilding to facilitate the introduction of nonnative species or even to support the presence and functions that invasive nonnative species may bring to rewilded ecosystems. The polarization surrounding the issue, including biological invasions, could be largely mitigated by embracing a broader understanding of rewilding's definition, approaches, and principles.
There has been an explosion of definitions and interpretations of what rewilding encompasses, in both the scientific and practitioners’ literatures, and this itself may have caused confusion and misunderstanding (Nogues-Bravo et al. 2016). As a relatively recent approach, the definition and implementation of rewilding have evolved and have been adapted over the past two decades from an initial emphasis on protecting large, connected areas for carnivore conservation to adopting dynamic, process-oriented approaches (Jørgensen 2015, Svenning et al. 2016, Perino et al. 2019). There is now a growing consensus to define rewilding as the restoration of self-sustaining and complex ecosystems and the ecological processes within them while minimizing and ultimately phasing out anthropogenic interventions (Perino et al. 2019, Carver et al. 2021). However, in practice, much of the debate and discussions around rewilding have largely been focused on species introductions and reintroductions, which can drive the perception—and sometimes misconceptions—of what rewilding is. Specifically, rewilding may involve the arrival of new species within an ecosystem (figure 1), via passive colonization or recolonization or via human-mediated introductions or reintroductions (e.g., to restore trophic complexity and disturbance regimes). Considerable public and scientific attention is often focused on proposals for the introduction of large or charismatic species, as was seen in Pleistocene rewilding (Donlan et al. 2006, Rubenstein and Rubenstein 2016), and on the natural return of species absent from specific regions for centuries, as has been experienced with some mammals in Europe (Navarro and Pereira 2012, Passoni et al. 2023).
When population reinforcement, reintroductions, or even introductions are part of the rewilding management plan, these come with inherent ecological and social uncertainty, including, ultimately, potential invasive behavior (Nogués-Bravo et al. 2016, Fernández et al. 2017; see also figure 1 and the dashed lines that can link different labels). The ecological uncertainties are strongly related to the ecological baseline that is selected for the implementation of rewilding and whether this baseline is temporal (i.e., historical) or functional (Fernández et al. 2017). For introductions or even reintroductions, the selected temporal baseline for rewilding will influence how the natural conditions of an ecosystem (its composition, structure, and functioning) may have changed over time (Genovesi and Simberloff 2020). In some cases, the species that are naturally recolonizing or that are being reintroduced may interact with species, communities, and land-use practices that are completely different from those present before local extinction, which has the potential for completely new conflicts with stakeholders within and outside of the area (e.g., Guerisoli et al. 2023). If the baseline considered for rewilding is strictly functional, it has been postulated that a nonnative species could be considered as an ecological equivalent or taxon substitute. This has been the case with the introductions of nonnative tortoises on islands (e.g., Aldabrachelys gigantea in Mauritius and Centrochelys sulcate in Hawaii) to restore seed dispersal and disturbance regimes (Svenning et al. 2016, Falcón and Hansen 2018; more examples in Guerisoli et al. 2023). Similarly, Gordon and colleagues (2021) have also debated the role that livestock play in maintaining disturbances in the absence of native herbivores.
Additional criticisms and dilemmas may also emerge when considering that intentionally introduced species should be disease or parasite free (i.e., after a period of quarantine for plants) and, therefore, may lack naturally coevolved competitors that have previously controlled population levels. This is unlikely to represent a problem when native species are used (particularly when precautions regarding unintentional hitchhiker species are taken) but could be an additional issue of concern if a functional baseline is applied and if nonnative species are considered.
Nevertheless, restoring a lost function or historical community should not be considered at all costs, and the potential negative effects of new species should be carefully assessed as part of a stepwise transparent and adaptative process to avoid or mitigate unexpected outcomes and ecosystem degradation (van Meerbeek et al. 2019, Carver et al. 2021). Similarly, the use of nonnative species in restoration strategies just to meet sustainability goals (e.g., via preferences for fast-growing nonnative plants to quickly gain carbon credits) should be discouraged.
Overall, introductions or reintroductions are just one of several approaches in the rewilding toolbox. Rewilding, by definition, emphasizes ecosystem-wide restoration approaches, focusing on ecological processes including dispersal, connectivity, stochasticity, disturbance regimes, and trophic interactions, rather than individual species and habitats (Perino et al. 2019). Successful rewilding should enhance ecosystem resistance and resilience against future invasions and other stressors (e.g., Derham et al. 2018) and rewilding management activities may include early removal of invasive species (Ripple et al. 2022).
When it comes to implementation, neither the underlying motivation nor the mechanisms should ignore the potential impact of biological invasions and should make no exception in adhering to internationally endorsed principles and guidelines to mitigate unexpected negative effects, including those linked to introductions and biological invasions (IUCN SSC 2013, Aslan et al. 2014, Guyton et al. 2020, Roy et al. 2023).
Restoration of connectivity may also come with some additional ecological uncertainty, and Häkkinen and colleagues (2023) recently highlighted how the landscape connectivity of suitable environments could favor the spread of terrestrial invasive species. In this regard, the planning and implementation of blue-green infrastructure (i.e., interconnected network of natural and seminatural areas, including water bodies, to support ecosystem services) should explicitly address this challenge to prevent and mitigate unintended introductions of species (e.g., Essl et al. 2019). This seems particularly important for aquatic and freshwater ecosystems (e.g., inland water networks) on which less emphasis has been placed when it comes to rewilding practices and theories (figure 2; Dobel et al. 2020) and where heterogenous interests may lead to unexpected pathways for dispersal at the basin level (e.g., controversial live release of aquatic species; Everard et al. 2019, South et al. 2022).
Figure 2.
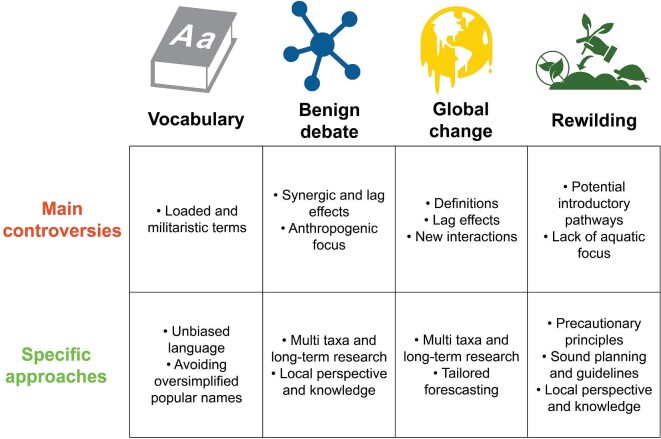
Polarized issues in invasion science discussed in this article with a summary of some of their main controversies and suggestions for possible approaches to obtain a more interdisciplinary perspective to overcome them.
Ultimately, by recognizing that it is precisely the restoration of multiple interacting ecosystem processes that should be considered to limit risks associated with rewilding practices, an interdisciplinary focus may help in bridging gaps between different fields of research and management (within the natural and social sciences and beyond), to ensure society is aware of the potential wider implications. Overall, the implementation of rewilding practices needs to be based on sound planning, clearly explained intentions and definitions (e.g., short and long-term goals), accessible and unbiased vocabulary, incorporated existing local knowledge, and the recognized relevance of interdisciplinarity in addressing the ongoing challenges within the Anthropocene (figure 2).
Lessons from the past, contemporary agenda, and future perspectives
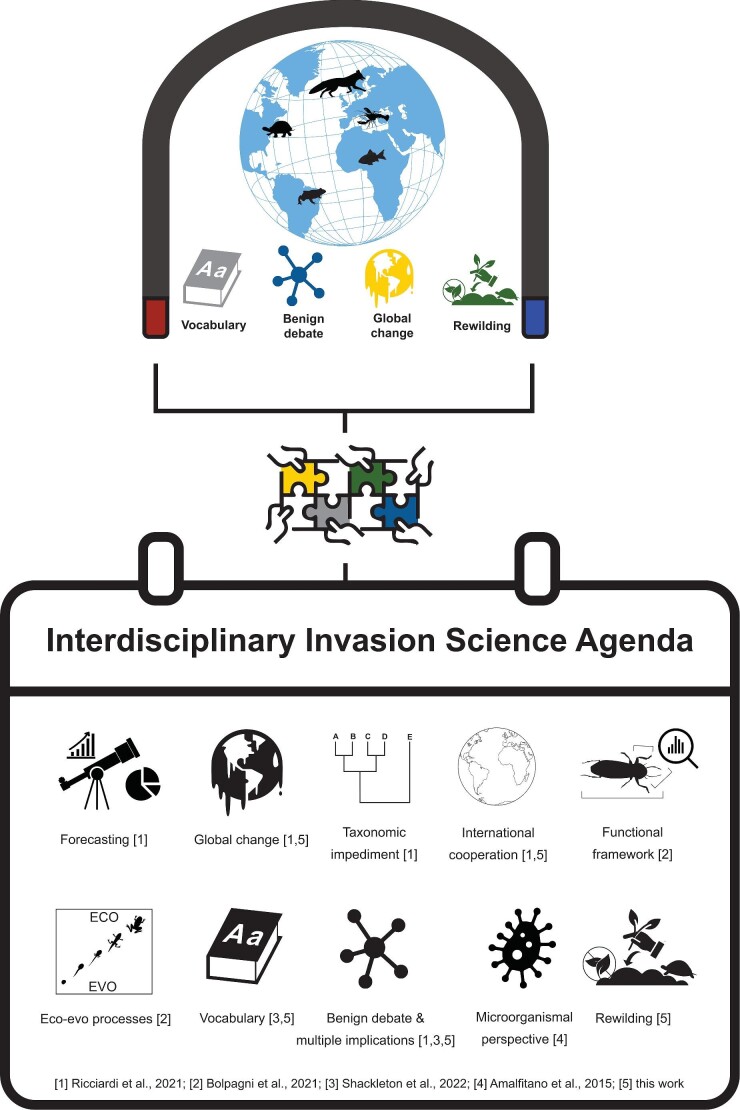
The polarized issues discussed in this article (summarized in figure 2) are interconnected, spanning the field of invasion science (figure 1). They complement the priority areas identified by Ricciardi and colleagues (2021): prediction capacities; multiple, co-occurring stressors; taxonomic impediment; and multistakeholder biosecurity cooperation. Integrating the recent reviews, questionnaires, and topics discussed in the present article will help guide future research and establish the foundation for a contemporary agenda in invasion science in a globalized world (e.g., Bolpagni 2021, Shackleton et al. 2022). A dynamic list of at least 10 core issues, presented in figure 3, can be clearly identified. It should be regularly updated to reflect technological and scientific advancements and would benefit from interdisciplinary approaches to address them.
Figure 3.
Polarized topics discussed in the main text and listed in figure 2 are integrated in the present figure as part of a dynamic and synthesized proposal of 10 issues for a contemporary agenda of invasion science in a hyperconnected world. The numbers represent some examples (not exhaustive) of relevant literature that were focused on the specific issues that are discussed in the main text.
Calls for greater interdisciplinarity when investigating biological invasions and their implications are mounting (figure 3; e.g., Hulme 2022). Such interdisciplinarity, as was advocated for in other global challenges (e.g., water crisis, Martin-Ortega 2023; extreme event research, Aoki et al. 2022), would help obtain new perspectives and improvements in the way society and academia perceive and engage with invasion science. Better integration between the social and life sciences may facilitate this progress (Vaz et al. 2017, Bortolus and Schwindt 2022), but it may also be challenging. For example, the first steps toward interdisciplinarity would benefit from actions within the individual disciplines of social and natural sciences themselves. On some occasions, a single invasive species may result in unexpected consequences that span multiple ecosystems, taxa, and processes (e.g., multiple interactions, Graham et al. 2018). Nevertheless, some experts in the macro field of natural science may not find it straightforward to collaborate, and in fact, true cross-fertilization between highly specialized fields is still rarely implemented. Similar difficulties may exist among experts in the field of social science (e.g., historians, human geographers, economists). These initial barriers still need to be first recognized and overcome before integration between the life and social sciences can be fully achieved (e.g., Moon and Blackman 2014) without losing highly specialized expertise.
In the field of natural science, for example, interdisciplinarity will facilitate multiple-taxa research and cross-ecosystem studies, enabling the recognition of new species interactions, novel human–wildlife connections, and providing guidance for environmental managers. Microbiological, physiological, behavioral, and functional aspects of biological invasions remain poorly investigated (e.g., Amalfitano et al. 2015, Nuñez et al. 2015, Bolpagni 2021), and more space in the agenda of the field should be found (figure 3). This would provide greater understanding of the invasion mechanisms involved in different contexts, further supporting both biological conservation and rewilding management.
Interdisciplinarity should, however, not be limited to linking different fields of knowledge but should also encompass the integration of complementary perceptions, tools, methods, and the people that use and apply them. Advances in bioinformatics and eScience infrastructures (including Bayesian approaches, neural networking, and artificial intelligence-based methods; e.g., Fricke and Olden 2023) may provide a suitable framework for integrating heterogeneous data sets (e.g., metadata across multiple languages) into informative models for forecasting and preventing or mitigating invasion events.
Varying proficiencies in a shared common working language among researchers (e.g., English; Bortolus 2012) may further impede cooperation among researchers from different geographic regions. As a result, the importance of multilingual decision-support tools is emerging as a possible solution to inform conservation managers (e.g., Copp et al. 2021, Amano et al. 2023). Moreover, consideration of multilingualism potentially provides benefits for nonspecialist stakeholders and end users in countries where English is rarely used outside of academia and where literacy rates may be low.
A dogmatic approach is generally not recommended in conservation biology (Martínez-Abraín and Oro 2013), and this also appears true of the field of invasion science, because it is characterized by gradients more than dichotomies, and therefore, decision-making processes should be better tailored to specific taxonomic and geographical levels (e.g., species or populations and regions or locations). Greater awareness and knowledge within specific emerging economy countries (where INNS are likely to increase with globalization; Early et al. 2016), through the more effective use and integration of indigenous and local knowledge or perspectives (Caceres-Escobar et al. 2019) and abstaining from parachute science (Asase et al. 2022) are recommended to effectively tackle contemporary challenges and prevent biological invasions outrunning the implementation of environmental policies.
Finally, it has been recognized that SARS-CoV-2, the virus responsible for the COVID-19 disease, displayed characteristics that mirror those of a successful invasive species, including rapid adaptation and large-scale dispersion via human transportation networks (Nuñez et al. 2020). A conscientious society should learn from such a global phenomenon. In this context, the forecasting and management tools used to address epidemics could be applied to biological invasions and vice versa (Vilà et al. 2021) in a cross-disciplinary approach (e.g., collaborations between biomedical researchers and ecologists). Curiously, at the beginning of the book The Ecology of Invasions by Animals and Plants, Charles S. Elton (1958, p. 1) defined ecological explosions using a highly infectious virus as the main example, saying that they “differ from some of the rest by not making such a loud noise and in taking longer to happen.”
Decades of debates in invasion science have provided useful lessons and theories that have sought to increase our general and scientific understanding. Issues such as those associated with new vocabulary, benign species, global climate change, and rewilding practices should be incorporated and comprehensively addressed in the contemporary agenda of biological invasion science (figure 3). These interconnected emerging topics have been shown to be part of historical and ongoing polarized debates and would benefit from bridging gaps between disciplines, which are inclusive rather than exclusive. Drawing more on integrative thinking paradigms and research approaches that incorporate social and natural elements would be highly beneficial to promote interdisciplinarity through new collaborations and favor conceptual advancements in the field. Indeed, there are still large knowledge gaps and questions that need to be investigated and appropriately disseminated in the biological invasion arena, and, as commonly happens in numerous fields, the best answers we collectively implement will almost certainly trigger even further new and stimulating scientific questions.
Acknowledgments
This article represents the output of meetings and discussion from the project “Biological invasions in a changing world: Reconceptualizing frameworks for a multidisciplinary audience” funded by the Royal Society. Thanks to Anna-Maria Sourelli, Judy England, Alex Laini, and Marco Bartoli for comments on an early version of the manuscript. SG was supported by a Newton International alumni fellowship from the Royal Society (code no. AL\221015). AN was supported by the Czech Science Foundation (project no. 23–07278S), the Czech Academy of Sciences (project no. RVO 67985939), the MCIN/AEI/10.13039/501100011033, and the FSE+ (grant no. RYC2022-037905-I). The views expressed within this article are those of the authors and not necessarily of their organizations. We thank the constructive and positive comments by three anonymous reviewers that improved the clarity of the discussion points within.
Author Biography
S. Guareschi (s.guareschi@lboro.ac.uk, simone.guareschi@unito.it) is affiliated with the Department of Life Sciences and Systems Biology at the University of Turin, in Turin, Italy; with the Department of Geography and Environment at Loughborough University, in Loughborough, England, in the United Kingdom; and with the Estación Biológica de Doñana, in Sevilla, Spain. K. L. Mathers, M. Antonsich, and P. J. Wood are affiliated with the Department of Geography and Environment at Loughborough University, in Loughborough, England, in the United Kingdom. J. South is affiliated with the Faculty of Biological Sciences at the University of Leeds, in Leeds, England, in the United Kingdom, and with the South African Institute for Aquatic Biodiversity, in Makhanda, South Africa. L. M. Navarro is affiliated with the Estación Biológica de Doñana, in Sevilla, Spain. T. Renals and A. Hiley are affiliated with the Environment Agency of Bristol, England, in the United Kingdom. R. Bolpagni is affiliated with the Department of Chemistry, Life Sciences, and Environmental Sustainability at the University of Parma, in Parma, Italy. A. Bortolus is affiliated with the Instituto Patagonico para el Estudio de los Ecosistemas Continentales, in Chubut, Argentina. P. Genovesi is affiliated with the Institute for Environmental Protection and Research, in Roma, Italy. A. Jere is affiliated with the School of Applied Science and Open Learning at Kapasa Makasa University, in Chinsali, Zambia. T. C. Madzivanzira is affiliated with the Department of Ichthyology and Fisheries Science at Rhodes University and with the South African Institute for Aquatic Biodiversity, in Makhanda, South Africa. F. M. Phaka is affiliated with the South African Institute for Aquatic Biodiversity, in Makhanda, South Africa; with the African Amphibian Conservation Research Group, Unit for Environmental Sciences and Management, of North-West University, in Potchefstroom, South Africa; and with the Research Group on Zoology: Biodiversity and Toxicology at the Centre for Environmental Sciences at Hasselt University, in Diepenbeek, Belgium. A. Novoa is affiliated with the Institute of Botany of the Czech Academy of Sciences, in Prague, in the Czech Republic, and with the Estación Experimental de Zonas Áridas, in Almería, Spain. J. D. Olden is affiliated with the School of Aquatic and Fishery Sciences at the University of Washington, in Seattle, Washington, in the United States, and with the Department of Wildlife, Fish, and Environmental Studies at the Swedish University of Agricultural Sciences, in Uppsala, Sweden. M. Saccó is affiliated with the Department of Chemistry, Life Sciences, and Environmental Sustainability at the University of Parma, in Parma, Italy, and with the School of Molecular and Life Science at Curtin University, in Perth, Western Australia, in Australia. R. T. Shackleton is affiliated with the Swiss Federal Institute for Forest, Snow, and Landscape Research, in Birmensdorf, Switzerland, and with the Centre for Invasion Biology, in the Department of Botany and Zoology at Stellenbosch University, in Stellenbosch, South Africa. M. Vilà is affiliated with the Estación Biológica de Doñana and with the Department of Plant Biology and Ecology at the University of Sevilla, in Sevilla, Spain.
Contributor Information
Simone Guareschi, Department of Life Sciences and Systems Biology at the University of Turin, Turin, Italy; Department of Geography and Environment at Loughborough University, Loughborough, England, United Kingdom; Estación Biológica de Doñana, Sevilla, Spain.
Kate L Mathers, Department of Geography and Environment at Loughborough University, Loughborough, England, United Kingdom.
Josie South, Faculty of Biological Sciences at the University of Leeds, Leeds, England, United Kingdom; South African Institute for Aquatic Biodiversity, Makhanda, South Africa.
Laetitia M Navarro, Estación Biológica de Doñana, Sevilla, Spain.
Trevor Renals, Environment Agency of Bristol, England, United Kingdom.
Alice Hiley, Environment Agency of Bristol, England, United Kingdom.
Marco Antonsich, Department of Geography and Environment at Loughborough University, Loughborough, England, United Kingdom.
Rossano Bolpagni, Department of Chemistry, Life Sciences, Environmental Sustainability at the University of Parma, Parma, Italy.
Alejandro Bortolus, Instituto Patagonico para el Estudio de los Ecosistemas Continentales, Chubut, Argentina.
Piero Genovesi, Institute for Environmental Protection and Research, Roma, Italy.
Arthertone Jere, School of Applied Science and Open Learning at Kapasa Makasa University, Chinsali, Zambia.
Takudzwa C Madzivanzira, Department of Ichthyology and Fisheries Science at Rhodes University; South African Institute for Aquatic Biodiversity, Makhanda, South Africa.
Fortunate M Phaka, South African Institute for Aquatic Biodiversity, Makhanda, South Africa; African Amphibian Conservation Research Group, Unit for Environmental Sciences and Management of North-West University, Potchefstroom, South Africa; Research Group on Zoology: Biodiversity and Toxicology at the Centre for Environmental Sciences at Hasselt University, Diepenbeek, Belgium.
Ana Novoa, Institute of Botany of the Czech Academy of Sciences, Prague the Czech Republic; Estación Experimental de Zonas Áridas, Almería, Spain.
Julian D Olden, School of Aquatic and Fishery Sciences at the University of Washington, Seattle, Washington, United States; Department of Wildlife, Fish, Environmental Studies at the Swedish University of Agricultural Sciences, Uppsala.
Mattia Saccó, Department of Chemistry, Life Sciences, Environmental Sustainability at the University of Parma, Parma, Italy; School of Molecular and Life Science at Curtin University, Perth, Western Australia, Australia.
Ross T Shackleton, Swiss Federal Institute for Forest, Snow, and Landscape Research, Birmensdorf, Switzerland; Centre for Invasion Biology, Department of Botany and Zoology at Stellenbosch University, Stellenbosch, South Africa.
Montserrat Vilà, Estación Biológica de Doñana; Department of Plant Biology and Ecology at the University of Sevilla, Sevilla, Spain.
Paul J Wood, Department of Geography and Environment at Loughborough University, Loughborough, England, United Kingdom.
Author contributions
S. Guareschi (Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing), K.L. Mathers (Conceptualization, Investigation, Writing – original draft, Writing – review & editing), J. South (Conceptualization, Investigation, Writing – original draft, Writing – review & editing), L.M. Navarro (Conceptualization, Investigation, Writing – original draft, Writing – review & editing), T. Renals (Investigation, Writing – review & editing), A.Hiley (Investigation, Writing – review & editing), M. Antonsich (Investigation, Writing – review & editing), R. Bolpagni (Investigation, Writing – review & editing), A. Bortolus (Investigation, Writing – original draft, Writing – review & editing), P. Genovesi (Investigation, Writing – review & editing), A. Jere (Investigation, Writing – review & editing), T.C. Madzivanzira (Investigation, Writing – review & editing), F.M. Phaka (Investigation, Writing – review & editing), A. Novoa (Investigation, Writing – original draft, Writing – review & editing), J.D. Olden (Investigation, Writing – original draft, Writing – review & editing), M. Saccó (Investigation, Visualization, Writing – review & editing), R.T. Shackleton (Investigation, Writing – review & editing), M. Vilà (Investigation, Writing – original draft, Writing – review & editing), and P.J. Wood (Conceptualization, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing).
Data availability
No new data were generated or analyzed in support of this research.
References cited
- Alsos IG, Ware C, Elven R. 2015. Past Arctic aliens have passed away, current ones may stay. Biological Invasions 17: 3113–3123. [Google Scholar]
- Amalfitano S, Coci M, Corno G, Luna GM. 2015. A microbial perspective on biological invasions in aquatic ecosystems. Hydrobiologia 746: 13–22. [Google Scholar]
- Amano T et al. 2023. The role of non-English-language science in informing national biodiversity assessments. Nature Sustainability 6: 845–854. [Google Scholar]
- Angerbjörn A et al. 2013. Carnivore conservation in practice: Replicated management actions on a large spatial scale. Journal of Applied Ecology 50: 59–67. [Google Scholar]
- Anton A, Geraldi NR, Ricciardi A, Dick JT. 2020. Global determinants of prey naïveté to exotic predators. Proceedings of the Royal Society B 287: 20192978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsich M. 2021. Natives and aliens: Who and what belongs in nature and in the nation? Area 53: 303–310. [Google Scholar]
- Aoki LR et al. 2022. Preparing aquatic research for an extreme future: Call for improved definitions and responsive, multidisciplinary approaches. BioScience 72: 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asase A, Mzumara-Gawa TI, Owino JO, Peterson AT, Saupe E. 2022. Replacing “parachute science” with “global science” in ecology and conservation biology. Conservation Science and Practice 4: e517. [Google Scholar]
- Aslan CE, Aslan A, Croll D, Tershy B, Zavaleta E. 2014. Building taxon substitution guidelines on a biological control foundation. Restoration Ecology 22: 437–441. [Google Scholar]
- Azzurro E, Maynou F, Belmaker J, Golani D, Crooks JA. 2016. Lag times in Lessepsian fish invasion. Biological Invasions 18: 2761–2772. [Google Scholar]
- Bacher S et al. 2018. Socio-economic impact classification of alien taxa (SEICAT). Methods in Ecology and Evolution 9: 159–168. [Google Scholar]
- Bacher S et al. 2023. Impacts of invasive alien species on nature, nature's contributions to people, and good quality of life. Pages 397–559 in Roy HE, Pauchard A, Stoett P, Renard Truong T eds. Thematic Assessment Report on Invasive Alien Species and Their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. 10.5281/zenodo.7430731. [DOI] [Google Scholar]
- Basiita RK et al. 2022. Performance of Oreochromis niloticus and Oreochromis andersonii in controlled laboratory conditions in Zambia. Aquaculture Reports 27: 101338. [Google Scholar]
- Bayón Á, Vilà M. 2019. Horizon scanning to identify invasion risk of ornamental plants marketed in Spain. NeoBiota 52: 47–86. [Google Scholar]
- BBC . 2019. Invasive species: MPs call for a million people's help. BBC (24 October 2019). www.bbc.co.uk/news/uk-politics-50173577.
- Bertelsmeier C, Luque GM, Hoffmann BD, Courchamp F. 2015. Worldwide ant invasions under climate change. Biodiversity and Conservation 24: 117–128. [Google Scholar]
- Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, Wilson JR, Richardson DM. 2011. A proposed unified framework for biological invasions. Trends in Ecology and Evolution 26: 333–339. [DOI] [PubMed] [Google Scholar]
- Blackburn TM, Bellard C, Ricciardi A. 2019. Alien versus native species as drivers of recent extinctions. Frontiers in Ecology and the Environment 17: 203–207. [Google Scholar]
- Bolpagni R. 2021. Towards global dominance of invasive alien plants in freshwater ecosystems: The dawn of the Exocene? Hydrobiologia 848: 2259–2279. [Google Scholar]
- Boon PJ, Clarke SA, Copp GH. 2020. Alien species and the EU Water Framework Directive: A comparative assessment of European approaches. Biological Invasions 22: 1497–1512. [Google Scholar]
- Bortolus A. 2012. Running like Alice and losing good ideas: On the quasi-compulsive use of English by non-native English-speaking scientists. Ambio 41: 769–772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bortolus A, Schwindt E. 2022. Biological invasions and human dimensions: We still need to work hard on our social perspectives. Ecología Austral 32: 767–783. [Google Scholar]
- Bradley BA, Beaury EM, Fusco EJ, Lopez BE. 2022. Invasive species policy must embrace a changing climate. BioScience 73: 124–133. [Google Scholar]
- Bradley BA et al. 2024. Observed and potential range shifts of native and nonnative species with climate change. Annual Review of Ecology, Evolution, and Systematics 55: 013135. 10.1146/annurev-ecolsys-102722-013135. [DOI] [Google Scholar]
- Braga RR, Gómez Aparicio L, Heger T, Vitule JRS, Jeschke JM. 2018. Invasional meltdown hypothesis. Pages 79–91 in Jeschke JM, Heger T, eds. Invasion Biology: Hypotheses and Evidence. CABI. [Google Scholar]
- Brugueras S et al. 2020. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in southern Europe: A systematic review. Environmental Research 191: 110038. [DOI] [PubMed] [Google Scholar]
- Caceres-Escobar H, Kark S, Atkinson SC, Possingham HP, Davis KJ. 2019. Integrating local knowledge to prioritise invasive species management. People and Nature 1: 220–233. [Google Scholar]
- Carneiro L et al. 2024. Benefits do not balance costs of biological invasions. BioScience 74: 340–344. 10.1093/biosci/biae010. [DOI] [Google Scholar]
- Carver S et al. 2021. Guiding principles for rewilding. Conservation Biology 35: 1882–1893. [DOI] [PubMed] [Google Scholar]
- Catford JA, Wilson JR, Pyšek P, Hulme PE, Duncan RP. 2022. Addressing context dependence in ecology. Trends in Ecology and Evolution 37: 158–170 [DOI] [PubMed] [Google Scholar]
- Cerasoli F, Iannella M, Biondi M. 2019. Between the hammer and the anvil: How the combined effect of global warming and the non-native common slider could threaten the European pond turtle. Management of Biological Invasions 10: 428. [Google Scholar]
- Céspedes V, Bernardo-Madrid R, Picazo F, Vilà M, Rubio C, García M, Sanz I, Gallardo B. 2024. Massive decline of invasive apple snail populations after blue crab invasion in the Ebro River, Spain. Biological Invasions 2024: 03334. [Google Scholar]
- Chakandinakira AT, Madzivanzira TC, Mashonga S, Muzvondiwa JV, Ndlovu N, South J. 2023. Socioeconomic impacts of Australian redclaw crayfish Cherax quadricarinatus in Lake Kariba. Biological Invasions 25: 2801–2812. [Google Scholar]
- Cidrás D, González-Hidalgo M. 2022. Defining invasive alien species from the roots up: Lessons from the “de-eucalyptising brigades” in Galicia, Spain. Political Geography 99: 102746. [Google Scholar]
- Clavero M. 2014. Shifting baselines and the conservation of non-native species. Conservation Biology 28: 1434–1436. [Google Scholar]
- Colautti RI, MacIsaac HJ. 2004. A neutral terminology to define “invasive” species. Diversity and Distributions 10: 135–141. [Google Scholar]
- Copp GH et al. 2021. Speaking their language–development of a multilingual decision-support tool for communicating invasive species risks to decision makers and stakeholders. Environmental Modelling and Software 135: 104900. [Google Scholar]
- Courchamp F, Fournier A, Bellard C, Bertelsmeier C, Bonnaud E, Jeschke JM, Russell JC. 2017. Invasion biology: Specific problems and possible solutions. Trends in Ecology and Evolution 32: 13–22. [DOI] [PubMed] [Google Scholar]
- Cranston J, Crowley SL, Early R. 2022. UK wildlife recorders cautiously welcome range-shifting species but incline against intervention to promote or control their establishment. People and Nature 4: 879–892. [Google Scholar]
- Davis MA et al. 2011. Don't judge species on their origins. Nature 474: 153–154. [DOI] [PubMed] [Google Scholar]
- Derham TT, Duncan RP, Johnson CN, Jones ME. 2018. Hope and caution: Rewilding to mitigate the impacts of biological invasions. Philosophical Transactions of the Royal Society B 373: 20180127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettori EE, Balestrieri A, Zapata-Perez VM, Bruno D, Rubio-Saura N, Robledano-Aymerich F. 2021. Distribution and diet of recovering Eurasian otter (Lutra lutra) along the natural-to-urban habitat gradient (river Segura, SE Spain). Urban Ecosystems 24: 1221–1230. [Google Scholar]
- Diagne C, Leroy B, Vaissière AC, Gozlan RE, Roiz D, Jarić I, Salles JM, Bradshaw CJ, Courchamp F. 2021. High and rising economic costs of biological invasions worldwide. Nature 592: 571–576. [DOI] [PubMed] [Google Scholar]
- Dobel AJ, Willby N, Ives SC, May L, Carvalho L, Olszewska J, Pickard A, Spears BM. 2020. Rewilding revisited: Don't forget about freshwater ecology. Science 364: 5570. [Google Scholar]
- Donlan CJ et al. 2006. Pleistocene rewilding: An optimistic agenda for twenty-first century conservation. American Naturalist 168: 660–681. [DOI] [PubMed] [Google Scholar]
- Downey PO, Williams MC, Whiffen LK, Auld BA, Hamilton MA, Burley AL, Turner PJ. 2010. Managing alien plants for biodiversity outcomes: The need for triage. Invasive Plant Science and Management 3: 1–11. [Google Scholar]
- Drake JA, Mooney HA, diCastri F, Groves RH, Kruger FJ, Rejmánek M, Williamson M. 1989. Biological Invasions: A Global Perspective. Wiley. [Google Scholar]
- Duffy GA, Coetzee BW, Latombe G, Akerman AH, McGeoch MA, Chown SL. 2017. Barriers to globally invasive species are weakening across the Antarctic. Diversity and Distributions 23: 982–996. [Google Scholar]
- Early R et al. 2016. Global threats from invasive alien species in the twenty-first century and national response capacities. Nature Communications 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton CS. 1958. The Ecology of Invasions by Animals and Plants. Methuen. [Google Scholar]
- Essl F et al. 2018. Which taxa are alien? Criteria, applications, and uncertainties. BioScience 68: 496–509. [Google Scholar]
- Essl F et al. 2019. A conceptual framework for range-expanding species that track human-induced environmental change. BioScience 69: 908–919. [Google Scholar]
- Everard M, Pinder AC, Raghavan R, Kataria G. 2019. Are well-intended Buddhist practices an under-appreciated threat to global aquatic biodiversity? Aquatic Conservation: Marine and Freshwater Ecosystems 29: 136–141. [Google Scholar]
- Falcón W, Hansen DM. 2018. Island rewilding with giant tortoises in an era of climate change. Philosophical Transactions of the Royal Society B 373: 20170442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández N, Navarro LM, Pereira HM. 2017. Rewilding: A call for boosting ecological complexity in conservation. Conservation Letters 10: 276–278. [Google Scholar]
- Foster JT, Robinson SK. 2007. Introduced birds and the fate of Hawaiian rainforests. Conservation Biology 21: 1248–1257. [DOI] [PubMed] [Google Scholar]
- Fricke RM, Olden JD. 2023. Technological innovations enhance invasive species management in the Anthropocene. BioScience 73: 261–279. [Google Scholar]
- Fricke EC, Svenning JC. 2020. Accelerating homogenization of the global plant–frugivore meta-network. Nature 585: 74–78. [DOI] [PubMed] [Google Scholar]
- Gallardo B, Aldridge DC. 2018. Inter-basin water transfers and the expansion of aquatic invasive species. Water Research 143: 282–291. [DOI] [PubMed] [Google Scholar]
- Gallardo B, Aldridge DA, González-Moreno P, Pergl J, Pizarro M, Pyšek P, Thuiller W, Yesson C, Vilà M. 2017. Protected areas offer refuge from invasive species spreading under climate change. Global Change Biology 23: 5331–5343. [DOI] [PubMed] [Google Scholar]
- Genovesi P, Simberloff D. 2020. “De-extinction” in conservation: Assessing risks of releasing “resurrected” species. Journal for Nature Conservation 56: 125838. [Google Scholar]
- Geppert C, Bertolli A, Prosser F, Marini L. 2023. Red-listed plants are contracting their elevational range faster than common plants in the European Alps. Proceedings of the National Academy of Sciences 120: e2211531120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebie EJ, van Riper CJ, Arlinghaus R, Gaddy M, Jang S, Kochalski S, Lu Y, Olden JD, Stedman R, Suski C. 2022. Words matter: A systematic review of communication in non-native aquatic species literature. NeoBiota 74: 1–28. [Google Scholar]
- Goodenough A. 2010. Are the ecological impacts of alien species misrepresented? A review of the “native good, alien bad” philosophy. Community Ecology 11: 13–21. [Google Scholar]
- Gordon IJ, Pérez-Barbería FJ, Manning AD. 2021. Rewilding lite: Using traditional domestic livestock to achieve rewilding outcomes. Sustainability 13: 3347. [Google Scholar]
- Gozlan RE, Burnard D, Andreou D, Britton JR. 2013. Understanding the threats posed by non-native species: Public vs. conservation managers. PLOS ONE 8: e53200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NAJ et al. 2018. Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 559: 250–253. [DOI] [PubMed] [Google Scholar]
- Gu S, Qi T, Rohr JR, Liu X. 2023. Meta-analysis reveals less sensitivity of non-native animals than natives to extreme weather worldwide. Nature Ecology and Evolution 6: 1–24. [DOI] [PubMed] [Google Scholar]
- Guareschi S, Laini A, England J, Barrett J, Wood PJ. 2021. Multiple co-occurrent alien invaders constrain aquatic biodiversity in rivers. Ecological Applications 31: e02385. [DOI] [PubMed] [Google Scholar]
- Guerisoli MM et al. 2023. Reflexiones acerca del «reasilvestramiento» en la Argentina. Mastozoología Neotropical 30: e0946. [Google Scholar]
- Guyton JA et al. 2020. Trophic rewilding revives biotic resistance to shrub invasion. Nature Ecology and Evolution 4: 712–724. [DOI] [PubMed] [Google Scholar]
- Häkkinen H, Hodgson D, Early R. 2023. Global terrestrial invasions: Where naturalised birds, mammals, and plants might spread next and what affects this process. PLOS Biology 21: e3002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J, Holmes G, Martin-Ortega J. 2023. The importance of equity in payments to encourage coexistence with large mammals. Conservation Biology 38: e14207. 10.1111/cobi.14207. [DOI] [PubMed] [Google Scholar]
- Hanley N, Roberts M. 2019. The economic benefits of invasive species management. People and Nature 1: 124–137. [Google Scholar]
- Haubrock PJ et al. 2024a. Discrepancies between non-native and invasive species classifications. Biological Invasions 26: 371–384. [Google Scholar]
- Haubrock PJ et al. 2024b. Biological invasions are a population-level rather than a species-level phenomenon. Global Change Biology 30: e17312. [DOI] [PubMed] [Google Scholar]
- Herrera-Grao T, Bonada N, Gavira O, Blanco Garrido F. 2012. First record of Trithemis kirbyi Sélys, 1891 in Catalonia (Odonata, Libellulidae. Boletín de la Asociación Española de Entomología 36: 457–459. [Google Scholar]
- Hewitt G. 2000. The genetic legacy of the quaternary ice ages. Nature 405: 907–912. [DOI] [PubMed] [Google Scholar]
- Hossack BR, LeMoine MT, Oja EB, Eby LA. 2023. Cryptic declines of small, cold-water specialists highlight potential vulnerabilities of headwater streams as climate refugia. Biological Conservation 277: 109868. [Google Scholar]
- Hulme PE. 2017. Climate change and biological invasions: Evidence, expectations, and response options. Biological Reviews 92: 1297–1313. [DOI] [PubMed] [Google Scholar]
- Hulme PE. 2022. Importance of greater interdisciplinarity and geographic scope when tackling the driving forces behind biological invasions. Conservation Biology 36: e13817. [DOI] [PubMed] [Google Scholar]
- [IPCC] Intergovernmental Panel on Climate Change . 2022. Climate Change 2022: Mitigation of Climate Change. Cambridge University Press. doi: 10.1017/9781009157926 [DOI] [Google Scholar]
- [IUCN SSC] International Union for Conservation of Nature Species Survival Commission . 2013. Guidelines for Reintroductions and Other Conservation Translocations, vers. 1.0. IUCN SSC. https://portals.iucn.org/library/efiles/documents/2013-009.pdf. [Google Scholar]
- Janovsky RM, Larson ER. 2019. Does invasive species research use more militaristic language than other ecology and conservation biology literature? NeoBiota 44: 27–38. [Google Scholar]
- Jarić I, Heger T, Monzon FC, Jeschke JM, Kowarik I, McConkey KR, Pyšek P, Sagouis A, Essl F. 2019. Crypticity in biological invasions. Trends in Ecology and Evolution 34: 291–302. [DOI] [PubMed] [Google Scholar]
- Jere A, Jere WW, Mtethiwa A, Kassam D. 2021. Breeding pattern of Oreochromis niloticus (Linnaeus, 1758) versus native congeneric species, Oreochromis macrochir (Boulinger, 1912), in the upper Kabompo River, northwest of Zambia. Ecology and Evolution 11: 17447–17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen D. 2015. Rethinking rewilding. Geoforum 65: 482–488. [Google Scholar]
- Khan A. 2021. Equity, Diversity and Inclusion: A call to eradicate non-inclusive terms from the life sciences. Elife 10: e65604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueffer C, Larson BM. 2014. Responsible use of language in scientific writing and science communication. BioScience 64: 719–724. [Google Scholar]
- Kumschick S et al. 2024. Considerations for developing and implementing a safe list for alien taxa. BioScience 74: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertucci SA, Speziale KL. 2011. Protecting invaders for profit. Science 332: 35–35. [DOI] [PubMed] [Google Scholar]
- Larson BM. 2005. The war of the roses: Demilitarizing invasion biology. Frontiers in Ecology and the Environment 3: 495–500. [Google Scholar]
- Lemoine RT, Svenning JC. 2022. Nativeness is not binary: A graduated terminology for native and non-native species in the Anthropocene. Restoration Ecology 30: e13636 [Google Scholar]
- Lepczyk CA. 2022. Time to retire “alien” from the invasion ecology lexicon. Frontiers in Ecology and the Environment 20: 447–447. [Google Scholar]
- Liu X et al. 2018. More invaders do not result in heavier impacts: The effects of non-native bullfrogs on native anurans are mitigated by high densities of non-native crayfish. Journal of Animal Ecology 87: 850–862. [DOI] [PubMed] [Google Scholar]
- López BE et al. 2022. Global environmental changes more frequently offset than intensify detrimental effects of biological invasions. Proceedings of the National Academy of Sciences 119: e2117389119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AJ, Abbott RJ. 2015. Hybrid swarms: Catalysts for multiple evolutionary events in Senecio in the British Isles. Plant Ecology and Diversity 8: 449–463. [Google Scholar]
- Madzivanzira TC, South J, Wood LE, Nunes AL, Weyl OL. 2020. A review of freshwater crayfish introductions in Africa. Reviews in Fisheries Science and Aquaculture 29: 218–241. [Google Scholar]
- Marchetti MP, Engstrom T 2016. The conservation paradox of endangered and invasive species. Conservation Biology 30, 434–437. [DOI] [PubMed] [Google Scholar]
- Martínez-Abraín A, Oro D. 2013. Preventing the development of dogmatic approaches in conservation biology: A review. Biological Conservation 159: 539–547. [Google Scholar]
- Martin-Ortega J. 2023. We cannot address global water challenges without social sciences. Nature Water 1: 2–3. [Google Scholar]
- Maslow AH. 1943. A theory of human motivation. Psychological Review 50: 370–396. [Google Scholar]
- Moon K, Blackman D. 2014. A guide to understanding social science research for natural scientists. Conservation Biology 28: 1167–1177. [DOI] [PubMed] [Google Scholar]
- Moorhouse TP, Macdonald DW. 2015. Are invasives worse in freshwater than terrestrial ecosystems?. Wiley Interdisciplinary Reviews: Water 2: 1–8. [Google Scholar]
- Mormul RP et al. 2022. Invasive alien species records are exponentially rising across the Earth. Biological Invasions 24: 3249–3261. [Google Scholar]
- Moyo NA, Rapatsa MM. 2021. A review of the factors affecting tilapia aquaculture production in Southern Africa. Aquaculture 535: 736386. [Google Scholar]
- Navarro LM, Pereira HM. 2012. Rewilding abandoned landscapes in Europe. Ecosystems 15: 900–912. 10.1007/s10021-012-9558-7. [DOI] [Google Scholar]
- Nelufule T, Robertson MP, Wilson JRU, Faulkner KT. 2022. Native-alien populations: An apparent oxymoron that requires specific conservation attention. NeoBiota 74: 57–74. [Google Scholar]
- Nogués-Bravo D, Simberloff D, Rahbek C, Sanders NJ. 2016. Rewilding is the new Pandora's box in conservation. Current Biology 26: R87–R91. [DOI] [PubMed] [Google Scholar]
- Norkko J, Reed DC, Timmermann K, Norkko A, Gustafsson BG, Bonsdorff E, Slomp CP, Carstensen J, Conley DJ. 2012. A welcome can of worms? Hypoxia mitigation by an invasive species. Global Change Biology 18: 422–434. [Google Scholar]
- Novoa A et al. 2024. Stakeholders’ views on the global guidelines for the sustainable use of non-native trees. People and Nature 6: 10670. 10.1002/pan3.10670. [DOI] [Google Scholar]
- Nuñez MA, Kuebbing S, Dimarco RD, Simberloff D. 2012. Invasive species: To eat or not to eat, that is the question. Conservation Letters 5: 334–341. [Google Scholar]
- Nuñez MA, Dimarco RD, Dickie IA, Pauchard A. 2015. What can possibly go wrong? The risks of introducing soil microorganisms from Antarctica into South America. Bosque 36: 343–346. [Google Scholar]
- Nuñez MA, Pauchard A, Ricciardi A. 2020. Invasion science and the global spread of SARS-CoV-2. Trends in Ecology and Evolution 35: 642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström M, Jouffray JB, Norström AV, Crona B, Søgaard Jørgensen P, Carpenter SR, Bodin Ö, Galaz V, Folke C. 2019. Anatomy and resilience of the global production ecosystem. Nature 575: 98–108. [DOI] [PubMed] [Google Scholar]
- Oficialdegui FJ, Delibes-Mateos M, Green AJ, Sánchez MI, Boyero L, Clavero M. 2020.. Rigid laws and invasive species management. Conservation Biology 34: 1047–1050. [DOI] [PubMed] [Google Scholar]
- Oficialdegui FJ et al. 2023. A horizon scan exercise for aquatic invasive alien species in Iberian inland waters. Science of the Total Environment 869: 161798. [DOI] [PubMed] [Google Scholar]
- Olden JD, Comte L, Giam X. 2018. The Homogocene: A research prospectus for the study of biotic homogenisation. NeoBiota 37: 23–36. [Google Scholar]
- Ovid D, Phaka FM. 2022. Idwi, Xenopus laevis, and African clawed frog: Teaching counternarratives of invasive species in postcolonial ecology. Journal of Environmental Education 53: 69–86. [Google Scholar]
- Padial AA et al. 2017. The “tilapia law” encouraging non-native fish threatens Amazonian River basins. Biodiversity and Conservation 26: 243–246. [Google Scholar]
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 37: 637–669. [Google Scholar]
- Passoni G, Coulson T, Cagnacci F. 2023. Celebrating wildlife population recovery through education. Trends in Ecology and Evolution 39: 101–105. 10.1016/j.tree.2023.10.004 [DOI] [PubMed] [Google Scholar]
- Pauchard A et al. 2018. Biodiversity assessments: Origin matters. PLOS Biology 16: e2006686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicice FM et al. 2023. Unintended consequences of valuing the contributions of non-native species: Misguided conservation initiatives in a megadiverse region. Biodiversity and Conservation 32: 3915–3938. [Google Scholar]
- Perino A et al. 2019. Rewilding complex ecosystems. Science 364: eaav5570. [DOI] [PubMed] [Google Scholar]
- Peterson AT, Stewart A, Mohamed KI, Araújo MB. 2008. Shifting global invasive potential of European plants with climate change. PLOS ONE 3: e2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Por FD. 1978. Lessepsian Migration: the Influx of Red Sea Biota into the Mediterranean by Way of the Suez Canal. Ecological Studies, vol. 23. Springer. [Google Scholar]
- Pyšek P et al. 2020. Scientists’ warning on invasive alien species. Biological Reviews 95: 1511–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Marcos S, Malpica-Cruz L, Tamayo L, Canto Noh JÁ, Fernández-Rivera Melo F, Fulton S. 2023. Socioeconomic dilemmas of commercial markets for invasive species: Lessons from lionfish in Mexico. ICES Journal of Marine Science 80: 31–39. [Google Scholar]
- Ricciardi A. 2007. Are modern biological invasions an unprecedented form of global change? Conservation Biology 21: 329–336. [DOI] [PubMed] [Google Scholar]
- Ricciardi A et al. 2021. Four priority areas to advance invasion science in the face of rapid environmental change. Environmental Reviews 29: 119–141. [Google Scholar]
- Richardson DM, ed. 2011. Fifty Years of Invasion Ecology: The Legacy of Charles Elton. Blackwell. [Google Scholar]
- Richardson DM, Ricciardi A. 2013. Misleading criticisms of invasion science: A field guide. Diversity and Distributions 19: 1461–1467. [Google Scholar]
- Ripple WJ et al. 2022. Rewilding the American West. BioScience 72: 931–935. [Google Scholar]
- Robinson TB, Martin N, Loureiro TG, Matikinca P, Robertson MP. 2020. Double trouble: The implications of climate change for biological invasions. NeoBiota 62: 463–487. [Google Scholar]
- Roy HE et al., eds. 2023. IPBES Invasive Alien Species Assessment: Summary for Policymakers. Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. 10.5281/zenodo.7430692. [DOI] [Google Scholar]
- Rubenstein DR, Rubenstein DI. 2016. From Pleistocene to trophic rewilding: A wolf in sheep's clothing. Proceedings of the National Academy of Sciences 113: E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders ME, Peisley RK, Rader R, Luck GW. 2016. Pollinators, pests, and predators: Recognizing ecological trade-offs in agroecosystems. Ambio 45: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax DF, Schlaepfer MA, Olden JD. 2022. Valuing the contributions of non-native species to people and nature. Trends in Ecology and Evolution 37: 1058–1066. [DOI] [PubMed] [Google Scholar]
- Schlaepfer MA, Sax DF, Olden JD. 2011. The potential conservation value of non-native species. Conservation Biology 25: 428–437. [DOI] [PubMed] [Google Scholar]
- Shackleton RT, Shackleton CM, Kull CA. 2019. The role of invasive alien species in shaping local livelihoods and human well-being: A review. Journal of Environmental Management 229: 145–157. [DOI] [PubMed] [Google Scholar]
- Shackleton RT, Vimercati G, Probert AF, Bacher S, Kull CA, Novoa A. 2022. Consensus and controversy in the discipline of invasion science. Conservation Biology 36: e13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberloff D et al. 2013. Impacts of biological invasions: What's what and the way forward. Trends in Ecology and Evolution 28: 58–66. [DOI] [PubMed] [Google Scholar]
- Singh R, Tiwari AK, Singh GS. 2022. Perceptions of the impacts of invasive alien plants in the riparian zone of the Ganga River: Insights from Varanasi, India. River Research and Applications 38: 1495–1509. [Google Scholar]
- Soga M, Gaston KJ. 2018. Shifting baseline syndrome: Causes, consequences, and implications. Frontiers in Ecology and the Environment 16: 222–230. [Google Scholar]
- Soto I et al. 2024. Taming the terminological tempest in invasion science. Biological Reviews 99: 1357–1390. [DOI] [PubMed] [Google Scholar]
- South J, Charvet P, Khosa D, Smith ERC, Woodford DJ. 2022. Recreational fishing as a major pathway for the introduction of invasive species. Pages 49–58 in Barros A, Shackleton R, Rew L, Pizarro C, Pauchard A, eds. Tourism, Recreation, and Biological Invasions. Wallingford, CABI. [Google Scholar]
- Spear MJ, Walsh JR, Ricciardi A, Zanden MJV. 2021. The invasion ecology of sleeper populations: Prevalence, persistence, and abrupt shifts. BioScience 71: 357–369. [Google Scholar]
- Stevenson EA et al. 2023. Synthesising 35 years of invasive non-native species research. Biological Invasions 25: 2423–2438. [Google Scholar]
- Svenning JC et al. 2016. Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proceedings of the National Academy of Sciences 113: 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. 2013. The Anthropocene could raise biological diversity. Nature 502: 7. [DOI] [PubMed] [Google Scholar]
- Turbelin AJ, Malamud BD, Francis RA. 2017. Mapping the global state of invasive alien species: Patterns of invasion and policy responses. Global Ecology and Biogeography 26: 78–92. [Google Scholar]
- Urban MC. 2020. Climate-tracking species are not invasive. Nature Climate Change 10: 382–384. [Google Scholar]
- van Eeden LM, Newsome TM, Crowther MS, Dickman CR, Bruskotter J. 2020. Diverse public perceptions of species’ status and management align with conflicting conservation frameworks. Biological Conservation 242: 108416. [Google Scholar]
- Van Meerbeek K, Muys B, Schowanek SD, Svenning JC. 2019. Reconciling conflicting paradigms of biodiversity conservation: Human intervention and rewilding. BioScience 69: 997–1007. [Google Scholar]
- van Wilgen NJ, Faulkner KT, Robinson TB, South J, Beckett H, Janion-Scheepers C, Measey J, Midgley GF, Richardson DM. 2022. Climate change and biological invasions in South Africa. Pages 158–187 in Ziska L, ed. Invasive Species and Global Climate Change. CABI. [Google Scholar]
- Vaz AS et al. 2017. The progress of interdisciplinarity in invasion science. Ambio 46: 428–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilà M et al. 2021. Viewing emerging human infectious epidemics through the lens of invasion biology. BioScience 71: 722–740. [Google Scholar]
- Vimercati G et al. 2022. The EICAT+ framework enables classification of positive impacts of alien taxa on native biodiversity. PLOS Biology 20: e3001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford PD et al. 2020. Adjusting the lens of invasion biology to focus on the impacts of climate-driven range shifts. Nature Climate Change 10: 398–405. [Google Scholar]
- Wilson JR. 2020. Definitions can confuse: Why the “neonative” neologism is bad for conservation. BioScience 70: 110–111. [Google Scholar]
- Woodford DJ, Richardson DM, MacIsaac HJ, Mandrak NE, van Wilgen BW, Wilson JRU, Weyl OLF. 2016. Confronting the wicked problem of managing biological invasions. NeoBiota 31: 63–86. [Google Scholar]
- Young AM, Larson BM. 2011. Clarifying debates in invasion biology: A survey of invasion biologists. Environmental Research 111: 893–898. [DOI] [PubMed] [Google Scholar]
- Zengeya TA, Booth AJ, Chimimba CT. 2015. Broad niche overlap between invasive Nile tilapia Oreochromis niloticus and indigenous congenerics in Southern Africa: Should we be concerned? Entropy 17: 4959–4973. [Google Scholar]
- Zhang L et al. 2022. Biological invasions facilitate zoonotic disease emergences. Nature Communications 13: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.