Abstract
Background
The relationship between serum uric acid level (SUA) and periodontal diseases (PD) is still controversial, and few studies have been carried out in population with no element of metabolic syndrome especially in sub-Saharan Africa. The aim of this study was to assess the relationship between PD and SUA in Cameroonian adults not suffering from metabolic syndrome.
Methods
We carried out a cross-sectional study including Cameroonians aged over 18 years recruited in the general population and free of metabolic syndrome elements. They were assessed for frequency of consumption of purine-rich foods, periodontal indices (plaque index, gingival index, calculus index, pocket depth and clinical loss of attachment), and SUA. The diagnosis of PD was based on the American Academy of Periodontology criteria revised in 2015, and hyperuricemia was defined for values exceeding 70 and 60mg/L in men and women respectively. Comparison of SUA means was performed with the ANOVA test. Association between hyperuricemia and PD were evaluated using Fischer's exact test. The threshold of significance was 0.05.
Results
One hundred and seventy-four participants were included (57.5% women, mean age 29 (10.39) years). The frequencies of PD were 75.9%, gingivitis (59.1%) and periodontitis (40.1%). Hyperuricemia was found in 20.45% of people with PD, with no difference in frequency comparing with those without PD. The frequency of consumption of purine-rich foods was similar in individuals with and without PD. Serum uric acid levels were not different in individuals with and without PD, and differed among periodontal indices only for calculus index, where SUA were higher in participants with the highest score (p=0.026). We found no association between hyperuricemia and PD.
Conclusion
In individuals without elements of metabolic syndrome, hyperuricemia affects one in five people with PD. There appears to be no link between SUA and overall periodontal status in this population. Further studies are needed to better understand the salivary interaction between uric acid and periodontium in our population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-05352-w.
Keywords: Periodontal disease, Serum uric acid levels, Cameroonian
Clinical relevance
Scientific rationale for study
The relationship between periodontal disease and hyperuricemia remains poorly studied in Sub-Saharan Africa and Cameroon, especially among individuals without metabolic syndrome. A better understanding of this relationship will improve knowledge and strategies for the prevention and management of these affections.
Principal findings
We found that periodontal disease affects three out of four adults not suffering from metabolic syndrome, and hyperuricemia affects one-fifth. There appears to be no link between serum uric acid levels and periodontal disease in this group.
Practical implications
The relationship between serum uric acid levels and periodontal disease may be dependent on elements of the metabolic syndrome. In the absence of these elements, it may not be necessary to assess for hyperuricemia. Further studies are needed to better understand the salivary interaction between uric acid and periodontium in our population.
Background
Periodontal diseases (PD) are disorders affecting the supporting tissues of the teeth. They are caused by excessive plaque formation, mainly due to infection, leading to inflammation and progressive destruction of the periodontium [1]. Gingivitis and periodontitis are the main types of PD [2]. PD is a worldwide oral health problem, affecting over 1.5 billion people [3, 4]. In Africa, with limited access to dental care, these conditions pose an additional public health challenge [5]. In Cameroon, around 62.2% of the population suffers from gingivitis and 15% from periodontitis [6]. They represent the main cause of tooth loss, which can compromise mastication, aesthetics, self-confidence and quality of life [2]. Looking beyond the mouth, PD has been associated with a number of risk factors and conditions, of which cardiovascular risk factors such as hypertension, diabetes and dyslipidemia are particularly prominent, making it a significant and often overlooked contributor to morbidity [6–8]. Some of the biomarkers involved in the spectrum of cardiovascular disease such as uric acid, have been proposed as factors associated with PD, [9].
Uric acid derived from the catabolism of endogenous but mainly exogenous purines from the diet [10]. Hyperuricemia, which refers to elevated serum uric acid levels (SUA), is the main metabolic abnormality associated with uric acid, and is a risk factor for gout and cardiovascular disease [11]. The relationship between SUA and PD is still a matter of controversy. Evidence from fundamental studies suggests that uric acid, and particularly hyperuricemia, plays a role in the pathogenesis of PD. The imbalance of the oral microbiome during periodontal disease is thought to be responsible for chronic low-grade systemic inflammation, which has been associated with the development of metabolic syndrome and hyperuricemia [9]. On the other hand, hyperuricemia may disrupt salivary and oral balance, leading to onset and progression of PD [12]. However, in the light of epidemiological data, there is a real contradiction, with some studies suggesting that hypouricemia may have a harmful effect on the periodontal tissue. Thus, Tsai et al. showed that higher serum uric acid levels were associated with a greater risk of periodontitis [13]; Sato et al. corroborated this by showing that hyperuricemia could be a cause of alveolar bone destruction in obesity-related periodontitis [14]. Nevertheles, some authors, such as Sreeram et al., Brotto et al. and Narenda et al., have found no relationship between uricemia and PD [15–17]. Moreover, PD and hyperuricemia share a number of common risk factors, particularly the elements of the metabolic syndrome [18, 19]. It would therefore be crucial to evaluate the relationship between PD and SUA taking into consideration the role of metabolic syndrome. To the best of our knowledge, few studies have been carried out in the adult population with no element of metabolic syndrome.
In Sub-Saharan Africa in general, and in Cameroon more specifically, the epidemiological importance of PD is certain, but little work is available on its relationship with uricemia. The aim of the present study was to assess the relationship between PD and SUA in Cameroonian adults with no evidence of metabolic syndrome, in order to enhance the state of knowledge on this subject.
Methods
Study design and setting
This was a cross-sectional study conducted from December 2023 to May 2024 at the Implantology and Periodontology Laboratory of the Faculty of Medicine and Biomedical Sciences, University of Yaoundé I (Cameroon). Biological assays were performed at the Biochemistry Laboratory of the University Hospital Centre of Yaoundé (Cameroon).
Participants
We included Cameroonians aged 18 and above residing in the city of Yaoundé, Cameroon. They were invited to participate by announcements in the general population in public places. We excluded any participant with at least one element of the metabolic syndrome, namely: abdominal obesity, overweight or general obesity, hypertension, diabetes, HDL or LDL dyslipidemia. People with gout, chronic kidney disease (with glomerular filtration rate below 60 ml/min/1.73m2), pregnant women, HIV infection, and participants receiving hypo- or hyperuricemic medication were also excluded.
Sample size estimation
The minimum sample size was estimated at 167, using the size calculation formula with respect to our study type contained in the manual by Whitley and Ball [20]. We considered the prevalence of hyperuricemia in people with periodontal disease in the study by Joo et al. (30.6%), with a power of 95% and an error rate of 7% [21].
Clinical data collection
After obtaining administrative authorizations from the various study sites, and ethical clearance, we invited each potential study participant, who was informed through an information notice available in official languages (English and French). All eligible participants completed an informed consent form prior to inclusion. Data were collected using a data collection sheet. These included
Sociodemographic data: age, sex;
Oral hygiene habits: daily frequency of tooth brushing, brushing period, type of toothbrush, brushing technique, type of toothpaste and frequency of oral hygiene visits;
Lifestyle informations: alcohol consumption, tobacco consumption, frequency of consumption of purine-rich foods. To assess the consumption of purine-rich foods, in the absence of validated tools in the Cameroonian population, we carried out a semi-quantitative assessment based on frequency of consumption, using the recommendations of Cade et al. [22]. Purine-rich foods were selected on the basis of data from Central and West African populations with the highest purine content [23]. A nutritionist was consulted at this stage. Consumption frequencies were assessed on a daily, weekly and monthly basis, and then weighted from 0 to 8 for a total score of 64. This made it possible to compare participants' consumption frequencies. The questionnaire is presented in Supplementary Table 1. The frequency of purine-rich food consumption was stratified into low (score below the 25th quartile), moderate (score between the 25th and 75th quartiles), and high (score above the 75th quartile).
Periodontal examination: the methodology was described in one of our previous publications [7]. The oral examination was complete and performed on each sextant (17–14, 13–23, 24–27, 37–34, 33–43, 44–47) using mirrors, tweezers and Williams periodontal probe graduated from 1 to 15 mm. The periodontal indices estimated were: the Silness and Loe plaque index, the Green and Vermillon calculus index (direct assessment of oral hygiene), and the Loe and Silness gingival index [24–26]. Each index was stratified (scores 0, 1, 2, and 3) as presented in Periodontal examination: the methodology was described in one of our previous publications [7]. The oral examination was complete and performed on each sextant (17–14, 13–23, 24–27, 37–34, 33–43, 44–47) using mirrors, tweezers and Williams periodontal probe graduated from 1 to 15 mm. The periodontal indices estimated were: the Silness and Loe plaque index, the Green and Vermillon calculus index (direct assessment of oral hygiene), and the Loe and Silness gingival index [24–26]. Each index was stratified (scores 0, 1, 2, and 3) as presented in Supplementary Table 2. For each participant, we considered the highest score for each index. Periodontal pocket depth was assessed from the distance between the bottom of the periodontal pocket and the gingival margin. This was measured at six sites on each tooth: mesiobuccal, distobuccal, mesio-lingual or mesio-palatal, disto-lingual or disto-palatal and lingual or palatal. Pocket depth and clinical attachment loss were stratified from 0 to 3, as shown in Supplementary Table 3. Based on the criteria of the American Academy of Periodontology 1999, revised in 2015, we retained the diagnosis of gingivitis for a gingival index score of at least 1 present on at least two non-adjacent teeth [27]. The diagnosis of periodontitis was based on evidence of loss of attachment and a pocket depth of at least 3 mm on two or more non-contiguous teeth. A patient with gingivitis and periodontitis was classified with the most severe condition, periodontitis.
Biological data
Uricemia was determined using the uricase method, using a reagent supplied by Biolabo®. It was performed on a venous blood sample taken after an 8-h fast. SUA was expressed in mg/L and hyperuricemia was defined for a value in men ≥ 70 mg/L, and in women ≥ 60 mg/L [11].
Statistical analysis
Data were analyzed using SPSS software version 23.0. It was also used to design the graphs. Continuous quantitative variables were presented with mean and standard deviation, while those not following the normal distribution were presented with median and interquartile range [quartile 25; quartile 75]. Categorical variables are presented with their counts and percentages. Means were compared using the one-factor ANOVA test. The association between periodontal disease and hyperuricemia was investigated by comparing frequencies using Fischer's exact test, and measuring the odds ratio along with its 95% confidence interval (OR [95%CI]). For all tests used, the significance threshold was 0.05.
Results
Characteristics of the sample
We received 217 participants during the study period, of whom 174 were eligible for the study and were finally included. The 43 participants who were not included had at least one component of metabolic syndrome. The average age of the participants was 29 (10.39) years, ranging from 18 to 65 years. The sample included 100 (57.5%) women. Regarding their oral hygiene habits, the majority used medium-bristle toothbrushes (52.9%), brushed once a day (42.5%) or twice a day (53.4%), mostly used fluoride toothpaste (83.9%), and went more than a year without visiting the dentist (53.5%). Alcohol consumption was reported by 81 (46.1%) participants. The frequency of consumption of purine-rich foods was moderate and high in 92 (52.9%) and 47 (27%) participants respectively. These data are presented in Table 1.
Table 1.
Information on participants' oral hygiene habits and lifestyles
| Variables | Effectives (%) |
|---|---|
| Toothbrush type, n (%) | |
| Hard | 4 (2.3) |
| Medium | 92 (52.9) |
| Soft | 73 (42) |
| Traditional | 5 (2.9) |
| Brushing technique, n (%) | |
| Horizontal | 26 (14.9) |
| Vertical | 33 (19) |
| Mixed | 115 (66.1) |
| Brushing period, n (%) | |
| Before meal | 78 (44.8) |
| After meal | 96 (55.2) |
| Toothpaste type, n (%) | |
| Fluoride | 146 (83.9) |
| Non-fluoride | 28 (16.1) |
| Daily brushing frequency, n (%) | |
| 0 | 2 (1.1) |
| 1 | 74 (42.1) |
| 2 | 93 (53.4) |
| 3 | 5 (2.9) |
| Annual frequency of visits to the dentist, n (%) | |
| 0 | 93 (53.4) |
| 1 | 52 (29.9) |
| 2 | 23 (13.2) |
| 3 | 2 (1.1) |
| 4 | 4 (2.3) |
| Score for frequency of consumption of purine-rich foods | |
| Median [IQR] | 14 [10; 21] |
| Low frequency (< Q25), n (%) | 35 (20.1) |
| Moderate frequency ([Q25; Q75[, n (%) | 92 (52.9) |
| High frequency (≥ Q75), n (%) | 47 (27) |
| Alcohol consumption, n (%) | 81 (46.1) |
| Tobacco consumption, n (%) | 5 (2.9) |
IQR interquartile range [Q25; Q75], Q25 25th quartile, Q75 75th quartile
Prevalence of periodontal diseases
Table 2 shows the periodontal index scores. Most participants had a plaque and gingival index score of 1 or 2, while most participants had a loss of attachment and pocket depth index score of 1. As for the calculus index, most participants had a score of 0 or 1. Periodontal disease was found in 132 (75.9%) participants, of whom 78 (59.1%) had gingivitis, 54 (40.1%) had periodontitis.
Table 2.
Distribution of the population according to periodontal index scores
| Score | Plaque index (Silness and Loe) n (%) |
Calculus index (Green and Vermilion) n (%) |
Gingival index (Loe and Silness) n (%) |
Pocket depth n (%) |
Clinical attachment loss n (%) |
|---|---|---|---|---|---|
| 0 | 7 (4) | 52 (29.9) | 47 (27) | 119 (64.8) | 106 (60.9) |
| 1 | 91 (52.3) | 82 (47.1) | 85 (48.9) | 53 (30.5) | 26 (14.9) |
| 2 | 75 (43.1) | 29 (16.7) | 41 (23.6) | 2 (1.1) | 32 (18.4) |
| 3 | 1 (6) | 11 (6.3) | 1 (0.6) | 0 (0) | 10 (5.7) |
Prevalence of hyperuricemia
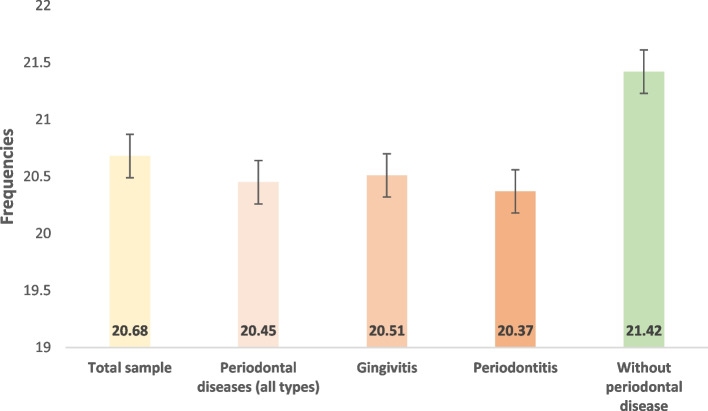
Participants' mean SUA was 54.98 (16.86) mg/L. Values ranged from 24 to 98 mg/L. The frequency of hyperuricemia was 20.7% in the total sample. It was significantly higher in men compared with women (62.89 (14.7) vs. 46.63 (13.2) mg/L, p < 0.001), and in individuals with a purine-rich food frequency score ≥ 18 compared with the < 18 score group (56.9 (15.9) vs. 51.73 (15.86) mg/L, p = 0.043). The frequencies of hyperuricemia in participants with periodontal disease, gingivitis and periodontitis were respectively 20.45%, 20.51% and 20.37%. The frequency of hyperuricemia was 21.42% in individuals without periodontal disease (Fig. 1).
Fig. 1.
Frequency of hyperuricemia in different sample groups
Association between serum uric acid levels and periodontal diseases
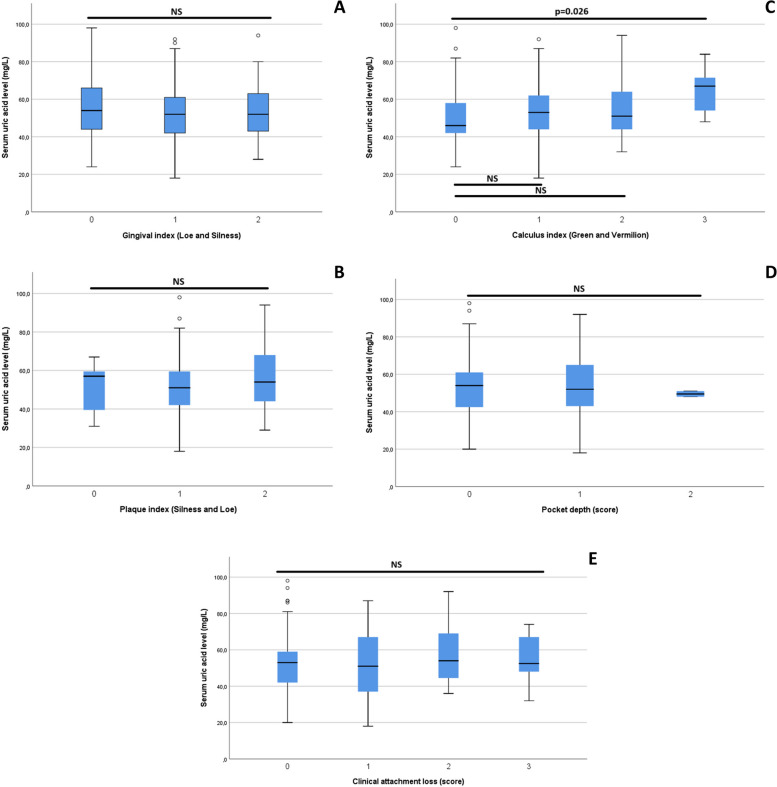
We first compared the mean serum uric acid levels in the different periodontal index score groups (Fig. 2 A-E), and found only that the uricemia of participants with calculus index score 3 was significantly higher compared to those with score 0 (Fig. 2-C; p = 0.026). Subsequently, we compared the mean serum uric acid levels between participants with and without periodontal disease, as well as those of the different periodontal disease groups, and found no significant difference. We also compared the mean scores for the frequency of purine-rich food consumption in these different groups, taking the group with no periodontal disease as a reference, without finding any significant difference. These results are presented in Table 3. We also assessed the association between hyperuricemia and periodontal disease, taken together and separately, without finding any significant association (Table 4).
Fig. 2.
A-E Comparison of serum uric acid levels in different periodontal index score groups. A: comparison of serum uric acid levels in the gingival index score groups (0, 1, and 2); B: comparison of serum uric acid levels in the plaque index score groups (0, 1 and 2); C: comparison of serum uric acid levels in the calculus index score groups (0, 1, 2 and 3); D: comparison of serum uric acid levels in pocket depth index score groups (0, 1 and 2); E: comparison of serum uric acid levels in the clinical attachment loss index score groups (0, 1, 2 and 3)
Table 3.
Comparison of means of purine-rich food frequency score and serum uric acid levels within periodontal disease groups
| Groups | Purine rich diet frequency score, mean (SD) | Serum uric acid level, mean (SD), mg/L |
|---|---|---|
| Without periodontal disease | 14.9 (5.7) | 54.4 (16.9) |
| Periodontal disease | 16.7 (8.5) | 53.3 (15.8) |
| Gingivitis | 17.5 (8.4) | 52.9 (16) |
| Periodontitis | 15.5 (8.5) | 53.9 (15.6) |
| p-value | 0.16 | 0.87 |
Table 4.
Evaluation of the association between hyperuricemia and periodontal disease
| Variables | Hyperuricemia | OR [95%CI] | p value | |
|---|---|---|---|---|
| Yes (n = 36) | No (n = 138) | |||
| Periodontal diseases (all types) | ||||
| Yes | 27 (20.5) | 105 (79.5) | 0.943 [0.403; 2.205] | 1 |
| No | 9 (21.4) | 33 (78.6) | 1 | |
| Gingivitis | ||||
| Yes | 16 (20.5) | 62 (79.5) | 0.98 [0.469; 2.051] | 1 |
| No | 20 (20.8) | 76 (79.2) | 1 | |
| Periodontitis | ||||
| Yes | 11 (20.4) | 43 (79.6) | 0.972 [0.439; 2.153] | 1 |
| No | 25 (20.8) | 95 (79.2) | 1 | |
OR [95%CI]: odds ratio and its 95% confidence interval
Discussion
The aim of the present study was to investigate the relationship between periodontal disease and serum uric acid levels in a group of Cameroonian adults with no evidence of metabolic syndrome. We found that, in this group, the frequency of hyperuricemia did not appear to differ between people with periodontal disease and those without. To the best of our knowledge, this is the first study of its kind in sub-Saharan Africa.
Like PD, serum uric acid levels are also linked to metabolic syndrome. Several data in the literature point to the shared risk factors that largely explain the association between these two entities. Hypertension, diabetes, dyslipidemia and obesity are all important metabolic factors that share with hyperuricemia and PD a contingent of genetic, epigenetic and environmental risk factors, notably the oral and intestinal microbiome, and lifestyle attitudes such as alcohol consumption, smoking and a sedentary lifestyle [28–32]. These pathways converge towards dysbiosis, chronic low-grade inflammation at tissue and vascular level [9]. In order to better characterize the link between SUA and PD, it is important to assess this relationship while taking cardiometabolic risk factors into account. However, there are currently few data on this subject in the literature, particularly in populations free of metabolic syndrome. We therefore considered it appropriate to carry out this study in a population with no evidence of metabolic syndrome, residing in sub-Saharan Africa, also given the scarcity of data regarding this association in this region.
We found that hyperuricemia affected 20.7% of participants with periodontal disease, with no difference between types of PD, and no difference from individuals without PD. What's more, no association was found between hyperuricemia and PD in general, or with PD types separately. These results corroborate those of Brotto et al. who found no association between uricemia and periodontal disease in the general population [16]. However, these findings differ from those of Tsai et al. who found that higher serum uric acid levels were associated with a greater risk of periodontitis [13]. The association between SUA and PD remains controversial. Uppin et al. in a systematic review of 6 studies in 2023 found that SUA were significantly altered in individuals with PD compared with those without. However, they reported that, with current evidence, it remains difficult to conclude whether they are significantly higher or lower in PD [33]. While Byun et al. in a Korean study between 2004 and 2016 found that hyperuricemia would be a protective factor in periodontal disease [19]. Results also corroborated by Xu et al. in the National Health and Nutrition Examination Survey (NHANES) of data collected between 2011 and 2014 [34]. Joo et al. also found in a Korean study between 2016 and 2018 that hypouricemia increased the risk of periodontal disease by (OR = 1.62; 95% CI [1.13; 2.23]), while hyperuricemia did not [21]. However, given our sample size, we were unable to perform the analysis for hypouricemia.
We found that the highest calculus index had significantly higher serum uric acid levels (Fig. 2-C), but no difference in other periodontal indices. The literature reports more severe periodontal damage in individuals with hyperuricemia [12]. In the absence of the metabolic syndrome, the hypothesis of gingival damage in relation to elevated salivary uric acid levels seems more plausible, as demonstrated in studies in rats [12]. In humans, a meta-analysis of 14 studies by Uppin et al. in 2022 found that individuals with PD had lower crevicular and salivary uric acid levels, in contrast to blood levels, a situation that remains unexplained to this day [35]. These findings concur with those of Ye et al. in a more recent meta-analysis in 2023 [36]. In addition, elevated salivary uric acid levels would presumably be an anti-inflammatory marker after treatment of PD [37]. It would therefore be important to be able to jointly assess serum and salivary levels in a larger sample within our population in order to improve the state of knowledge on this subject.
Certain limitations must be borne in mind when interpreting the data from our study. The lack of evaluation of alveolar bone loss by radiography, which is an important element in defining the stage of periodontitis. Another limitation is the small sample size, which was highly selective in order to limit metabolic bias. Surely, a longitudinal study would provide a better answer to the question, with clustering according to the elements of the metabolic syndrome, in order to assess their individual influences, and the effect of treatments. Finally, it would be useful to consider the degree of inflammation, which can be assessed using indices such as the “Periodontal inflamed surface area (PISA)”, in order to get a more precise idea [38].
Conclusion
In this sample of adults without metabolic syndrome components, periodontal disease accounted for 75%. The prevalence of hyperuricemia among patients with periodontal disease was 20%. No association was found between serum uric acid levels and periodontal disease. The relationship described between uricemia and periodontal disease seems to be more dependent on metabolic syndrome. However, further studies are needed with a longitudinal design and measurement of salivary uric acid levels in this population.
Supplementary Information
Additional file 1. Supplementary Table 1: food frequency questionnaire for purine rich diet. Supplementary Table 2: Stratification of plaque index (Silness and Loe), calculus index (Green and Vermilion), and gingival index (Loe and Silness). Supplementary Table 3: Stratification of pocket depth and clinical attachment loss.
Acknowledgements
The authors would like to thank the staff of the Biochemistry Laboratory of the Yaoundé University Hospital Centre, and the Implantology and Periodontology Laboratory of the Faculty of Medicine and Biomedical Sciences for their participation in data collection for the study.
Abbreviations
- SUA
Serum uric acid level
- PD
Periodontal diseases
Authors’ contributions
JRN, LATK and VJAM conceived the study. JRN, JJN, GFMM, MEO, JXEN and OT collected data. JRN, GFMM, DTN, and CBYM analyzed data. JRN and GFMM drafted the manuscript. JXEN and MIC edited the manuscript. VJAM supervized the work. All authors reviewed the final manuscript.
Funding
This research did not receive funds from any organization.
Data availability
All the dataset generated from this study are available from the corresponding author on request.
Declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the Faculty of Medicine and Biomedical Sciences, University of Yaoundé I (N°92/UY1/FMSB/VDRC/DAASR/CSD/2024). All the participants read and signed a written informed consent before their inclusion in the study. This study was conducted in compliance with the principles and standards of the Declaration of Helsinki, revised in October 2013.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guthmiller JM, Novak KF. Periodontal diseases. Polymicrobial Diseases. ASM Press; 2002. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2496/. Cited 2024 Jun 15.
- 2.Gasner NS, Schure RS. Periodontal Disease. StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/books/NBK554590/. Cited 2024 Jun 15.
- 3.Sanz M. European workshop in periodontal health and cardiovascular disease. Eur Heart J Suppl. 2010;12(suppl_B):B2. [Google Scholar]
- 4.Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Lond Engl. 2017;390(10100):1211–59. [DOI] [PMC free article] [PubMed]
- 5.Kamagate A, Coulibaly NT, Kone D, Brou E, Bakayoko LR. Prévalence des parodontites. Les parodontites en Afrique noire. Influences des facteurs socio-économiques et habitudes culturelles. Odonto-Stomatol Trop Trop Dent J Dakar : Secretariat Of Dental Health In Africa. 2001;24(94):37–41. [PubMed] [Google Scholar]
- 6.Belinga LEE, Ngan WB, Lemougoum D, Nlo’o ASPE, Bongue B, Ngono A, et al. Association between periodontal diseases and cardiovascular diseases in Cameroon. J Public Health Afr. 2018;9(1):761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngoude JXE, Moor VJA, Nadia-Flore TT, Agoons BB, Marcelle GGC, MacBrain EE, et al. Relationship between periodontal diseases and newly-diagnosed metabolic syndrome components in a sub-Saharan population: a cross sectional study. BMC Oral Health. 2021;29(21):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bissong M, Azodo CC, Agbor MA, Nkuo-Akenji T, Fon PN. Oral health status of diabetes mellitus patients in Southwest Cameroon. Odonto-Stomatol Trop Trop Dent J. 2015;38(150):49–57. [PubMed] [Google Scholar]
- 9.Hou W, Xia X, Li Y, Lv H, Liu J, Li X. Recent progress and perspectives on the relationship between hyperuricemia and periodontitis. Front Immunol. 2022;16(13):995582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Ridi R, Tallima H. Physiological functions and pathogenic potential of uric acid: A review. J Adv Res. 2017;8(5):487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michael A, Becker MJ. Hyperuricemia and associated diseases. Rheum Dis Clin North Am. 2006;32(2):275–93. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Ye L, Zhao L, Liang Z, Yu T, Gao J. Hyperuricemia as a potential plausible risk factor for periodontitis. Med Hypotheses. 2020;1(137):109591. [DOI] [PubMed] [Google Scholar]
- 13.Tsai K-Z, Su F-Y, Cheng W-C, Huang R-Y, Lin Y-P, Lin G-M. Associations between metabolic biomarkers and localized stage II/III periodontitis in young adults: The CHIEF Oral Health study. J Clin Periodontol. 2021;48(12):1549–58. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Yamazaki K, Kato T, Nakanishi Y, Tsuzuno T, Yokoji-Takeuchi M, et al. Obesity-related gut microbiota aggravates alveolar bone destruction in experimental periodontitis through elevation of uric acid. mBio. 2021;12(3):e0077121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sreeram M, Suryakar AN, Dani NH. Is gamma-glutamyl transpeptidase a biomarker for oxidative stress in periodontitis? J Indian Soc Periodontol. 2015;19(2):150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brotto RS, Vendramini RC, Brunetti IL, Marcantonio RAC, Ramos APP, Pepato MT. Lack of correlation between periodontitis and renal dysfunction in systemically healthy patients. Eur J Dent. 2011;5(1):8–18. [PMC free article] [PubMed] [Google Scholar]
- 17.Narendra S, Das UK, Tripathy SK, Sahani NC. Superoxide dismutase, uric acid, total antioxidant status, and lipid peroxidation assay in chronic and aggressive periodontitis patients. J Contemp Dent Pract. 2018;19(7):874–80. [PubMed] [Google Scholar]
- 18.Ding C, Du F, Li L, Chen Y. Synergistic effect of blood lipids and uric acid on periodontitis in patients with type 2 diabetes. Am J Transl Res. 2023;15(2):1430–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Byun S-H, Yoo D-M, Lee J-W, Choi H-G. Analyzing the association between hyperuricemia and periodontitis: a cross-sectional study using KoGES HEXA Data. Int J Environ Res Public Health. 2020;17(13):4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitley E, Ball J. Statistics review 4: sample size calculations. Crit Care Lond Engl. 2002;6(4):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joo J-Y, Park HR, Cho Y, Noh Y, Lee CH, Lee S-G. Increased prevalence of periodontitis with hypouricemic status: findings from the Korean National Health and Nutrition Examination Survey, 2016–2018. J Periodontal Implant Sci. 2023;53(4):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food-frequency questionnaires - a review. Public Health Nutr. 2002;5(4):567–87. [DOI] [PubMed] [Google Scholar]
- 23.Vincent A, Grande F, Compaoré E. FAO/INFOODS food composition table for western Africa (2019). User Guide Condens Food Compos Table Rome Food Agric Organ U N. 2020;
- 24.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;1939(68):7–13. [DOI] [PubMed] [Google Scholar]
- 25.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. [DOI] [PubMed] [Google Scholar]
- 26.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Periodontology Task Force Report on the Update to the 1999 Classification of Periodontal Diseases and Conditions. J Periodontol. 2015;86(7):835–8. [DOI] [PubMed]
- 28.Aizenbud I, Wilensky A, Almoznino G. Periodontal disease and its association with metabolic syndrome—a comprehensive review. Int J Mol Sci. Multidisciplinary Digital Publishing Institute. 2023;24(16):13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava MC, Srivastava R, Verma PK, Gautam A. Metabolic syndrome and periodontal disease: an overview for physicians. J Fam Med Prim Care. 2019;8(11):3492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamster IB, Pagan M. Periodontal disease and the metabolic syndrome. Int Dent J. 2020;67(2):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raya-Cano E, Molina-Luque R, Vaquero-Abellán M, Molina-Recio G, Jiménez-Mérida R, Romero-Saldaña M. Metabolic syndrome and transaminases: systematic review and meta-analysis. Diabetol Metab Syndr. 2023;30(15):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billiet L, Doaty S, Katz JD, Velasquez MT. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol. 2014;16(2014):852954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uppin RB, Varghese SS. Association of uric acid in oral health, periodontal disease, and systemic disorders: a systematic review. J Datta Meghe Inst Med Sci Univ. 2023;18(3):524. [Google Scholar]
- 34.Xu J, Jia Y, Mao Z, Wei X, Qiu T, Hu M. Association between serum uric acid, hyperuricemia and periodontitis: a cross-sectional study using NHANES data. BMC Oral Health. 2023;30(23):610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uppin RB, Varghese SS. Estimation of serum, salivary, and gingival crevicular uric acid of individuals with and without periodontal disease: a systematic review and meta-analysis. J Int Soc Prev Community Dent. 2022;12(4):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye L, Zhao L, Mei Z, Zhou Y, Yu T. Association between periodontitis and uric acid levels in blood and oral fluids: a systematic review and meta-analysis. BMC Oral Health. 2023;28(23):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Priya KL, Mahendra J, Mahendra L, Kanakamedala A, Alsharif KF, Mugri MH, et al. Salivary biomarkers in periodontitis post scaling and root planing. J Clin Med. 2022;11(23):7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nesse W, Abbas F, Van Der Ploeg I, Spijkervet FKL, Dijkstra PU, Vissink A. Periodontal inflamed surface area: quantifying inflammatory burden. J Clin Periodontol. 2008;35(8):668–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Table 1: food frequency questionnaire for purine rich diet. Supplementary Table 2: Stratification of plaque index (Silness and Loe), calculus index (Green and Vermilion), and gingival index (Loe and Silness). Supplementary Table 3: Stratification of pocket depth and clinical attachment loss.
Data Availability Statement
All the dataset generated from this study are available from the corresponding author on request.