Abstract
Objective
This study aims to establish a new prognostic index using machine learning models to predict the clinical outcomes of triple-negative breast cancer (TNBC) patients receiving neoadjuvant therapy.
Methods
In this study, we collected data from the electronic medical records system of Harbin Medical University Cancer Hospital to establish a training set of 501 breast cancer patients who received neoadjuvant therapy from January 2017 to December 2021. Additionally, we collected data from Harbin Medical University Affiliated Cancer Hospital, Harbin Medical University Affiliated Second Hospital, and Harbin Medical University Affiliated Sixth Hospital to establish a validation set of 1533 patients during the same period. All patients underwent blood tests, and the following inflammatory and immune indices were calculated for each patient: neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), systemic immune-inflammatory index (SII), systemic inflammatory response index (SIRI), and advanced lung cancer inflammation index (ALI). The observed outcomes included Disease-free survival (DFS) and overall survival (OS). Survival analysis was performed using Kaplan‒Meier survival curves, Cox survival analysis, propensity score matching analysis (PSM), and a nomogram to comprehensively investigate the impact of inflammatory status on patient survival.
Results
The training set comprised 501 patients with a mean age of 48.63 (9.41) years, while the validation set comprised 1533 patients with a mean age of 49.01 (9.51) years. The formula for ANLR established through Lasso regression analysis on the training set is: ANLR index = NLR − 0.04 × ALB (g/L). In both the training and validation sets, ANLR was significantly associated with patient DFS and OS (all P < 0.05). Additionally, ANLR was found to be an independent prognostic factor in this study. PSM analysis further confirmed its significant correlation with patient DFS and OS (76 cases vs. 76 cases, χ2 = 2.179, P = 0.001 and χ2 = 2.063, P = 0.002). The nomogram containing ANLR also demonstrated high prognostic value. The C-index for the nomogram in the training set was 0.742 (0.619–0.886) for DFS and 0.758 (0.607–0.821) for OS, while in the validation set, the C-index was 0.733 (0.655–0.791) for DFS and 0.714 (0.634-0.800) for OS.
Conclusion
ANLR was associated with the prognosis of TNBC patients receiving neoadjuvant therapy and could identify high-risk postoperative patients.
Keywords: Triple-negative breast cancer, Neoadjuvant therapy, Inflammation status, Machine learning, Prognosis
Introduction
In recent decades, significant progress has been made in the research and treatment of breast cancer, particularly for hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-positive (HER2+) breast cancer [1]. With the widespread application of targeted therapies, hormonal treatments, and personalized medicine, patient survival rates have markedly improved, and breast cancer is increasingly viewed as a chronic disease that can be managed long-term [2]. However, triple-negative breast cancer (TNBC) remains a highly aggressive subtype, lacking the expression of estrogen receptors (ER), progesterone receptors (PR), and HER2, rendering traditional endocrine therapies and HER2-targeted treatments ineffective [3]. Even with effective treatment, TNBC patients face a high risk of recurrence and distant metastasis, with prognoses significantly poorer than those of other breast cancer subtypes [4]. A further challenge is that TNBC patients frequently present with early occult metastases—meaning that at the time of diagnosis, tumor cells have already spread to other sites but have not yet manifested clinical symptoms or been detected by conventional imaging techniques [5]. This hidden metastasis complicates treatment and can lead to poor prognoses due to recurrence or metastasis after treatment completion [6]. TNBC accounts for approximately 15–20% of all breast cancers, making it a critical area in need of breakthroughs in treatment [7]. Therefore, exploring novel therapeutic strategies is of significant clinical and public health importance for improving patient outcomes and quality of life.
Neoadjuvant therapy has emerged as a crucial approach in managing early-stage TNBC, aiming to reduce tumor size and enhance surgical outcomes [8]. Despite advancements in treatment modalities, TNBC remains a formidable subtype with limited options, underscoring the urgent need for robust prognostic markers that can guide clinical decision-making and tailor therapy [9]. Recent studies have elucidated the significant role of inflammation in cancer progression, with various inflammatory markers showing associations with clinical outcomes in breast cancer [10]. Indices such as the neutrophil-to-lymphocyte ratio (NLR) and the systemic immune-inflammatory index (SII) have shown promise in predicting prognosis [11–13]. However, these markers are based on prior research and may not fully align with the rapid advancements in treatment approaches. Thus, establishing new inflammatory markers specifically for TNBC patients undergoing neoadjuvant therapy is of great significance.
In the context of big data and advanced analytics, machine learning presents an innovative opportunity to analyze complex datasets and develop predictive models. This study aims to establish a novel prognostic index that incorporates inflammatory status using machine learning techniques. By leveraging electronic medical records and inflammatory indices, this research seeks to identify high-risk patients among those undergoing neoadjuvant therapy, ultimately contributing to more personalized treatment approaches and improved patient outcomes.
Patients and materials
Patients
In this study, we collected data from the electronic medical records system of Harbin Medical University Cancer Hospital to establish a training set of 501 breast cancer patients who received neoadjuvant therapy from January 2017 to December 2021. Additionally, we collected data from Harbin Medical University Affiliated Cancer Hospital, Harbin Medical University Affiliated Second Hospital, and Harbin Medical University Affiliated Sixth Hospital to establish a validation set of 1533 patients during the same period. All patients were confirmed by pathology to be negative for ER, PR, and HER2, and underwent comprehensive surgical resection. The exclusion criteria for this study included incomplete clinical data, concurrent other tumors, the presence of acute or chronic inflammatory diseases, and loss to follow-up. This study was approved by the Ethics Committee of Sixth Affiliated Hospital of Harbin Medical University (LC2024-052).
Data collection and follow-up
On the day before treatment, blood samples were collected from patients and relevant blood and biochemical parameters were tested. Regular telephone follow-ups were conducted to determine disease-free survival (DFS) and overall survival (OS) over a follow-up period of 60 months. DFS was defined as the time from treatment initiation or diagnosis to disease progression, while OS was defined as the time from treatment initiation or diagnosis to patient death. Evidence of disease progression was determined through rigorous imaging and pathological examinations.
Inflammatory status
To more accurately assess patients’ inflammatory status, we calculated the NLR, platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), SII and systemic inflammation response index (SIRI) based on blood parameters. The calculation formulas are provided in Supplementary Table 1.
Statistical analysis
Statistical analyses were performed using R (version 4.3.1) and SPSS (version 25). Baseline characteristics were summarized as means ± standard deviations (SDs) for continuous variables and frequencies with percentages for categorical variables. Independent sample t-tests and Chi-square tests were used for correlation analyses. Kaplan–Meier survival curves and log-rank tests were applied to compare survival probabilities across ANLR groups, providing visual and statistical assessments of its association with survival outcomes. Cox proportional hazards regression identified independent prognostic factors and estimated hazard ratios (HRs). LASSO regression was used to construct a refined ANLR-based prognostic indicator by selecting the most predictive inflammatory indices while addressing potential multicollinearity. LASSO was chosen for its efficiency in variable selection and its ability to develop concise, interpretable models suitable for clinical application. A prognostic model and nomograms were then developed to further quantify and visualize the prognostic significance of the new ANLR indicator. Decision curve analysis (DCA) evaluated the clinical utility of the model. To minimize confounding factors, propensity score matching (PSM) was performed, and survival analyses were repeated in the matched cohort to validate the reliability and robustness of the new ANLR indicator as a prognostic marker.
Results
Patient characteristics
The training set comprised 501 patients with an average age of 48.63 (9.41) years, while the validation set consisted of 1,533 patients with an average age of 49.01 (9.51) years. Among them, 1,097 patients (53.9%) were menopause, and 179 patients (8.8%) had a family history. A total of 1,063 patients (52.3%) were classified as TNM stage II, and 998 patients (49.1%) were classified as TNM stage III. There were no significant differences in all pathological parameters between the two groups of patients (all P > 0.05), as shown in Table 1.
Table 1.
Patients’ characteristics
| Training set | Validation set | P | |
|---|---|---|---|
| Items | n = 501 | n = 1533 | |
| Age (years), mean (SD) | 48.63(9.41) | 49.01(9.51) | 0.439 |
| BMI (Kg/m2), mean (SD) | 25.23(4.87) | 24.05(5.06) | 0.534 |
| Menopause, n (%) | 0.550 | ||
| Yes | 276(55.1) | 821(53.6) | |
| No | 225(44.9) | 712(46.4) | |
| Family history, n (%) | 0.737 | ||
| Yes | 37(7.4) | 142(9.3) | |
| No | 464(92.6) | 1391(90.7) | |
| Blood type, n (%) | 0.981 | ||
| A | 117(23.4) | 361(23.5) | |
| B | 164(32.7) | 495(32.3) | |
| AB | 63(12.6) | 185(12.1) | |
| O | 157(31.3) | 492(32.1) | |
| Tumor site, n (%) | 0.387 | ||
| Right | 233(46.5) | 679(44.3) | |
| Left | 268(53.5) | 854(55.7) | |
| LNP, n (%) | 0.800 | ||
| Positive | 326(65.1) | 1007(65.7) | |
| Negative | 175(34.9) | 526(34.3) | |
| Tumor size, n (%) | 0.093 | ||
| < 30 mm | 181(36.1) | 614(40.1) | |
| 30–50 mm | 219(43.7) | 637(41.6) | |
| > 50 mm | 101(20.2) | 282(18.4) | |
| Histological Grading, n (%) | 0.614 | ||
| Grade I | 110(22.0) | 296(19.3) | |
| Grade II | 333(66.5) | 1080(70.5) | |
| Grade III | 58(11.5) | 157(10.2) | |
| TNM stage, n (%) | 0.614 | ||
| II | 265(52.9) | 771(50.3) | |
| III | 236(47.1) | 762(49.7) |
SD: standard deviation; BMI: body mass index; LNP: lymph node positivity
Construction of risk index
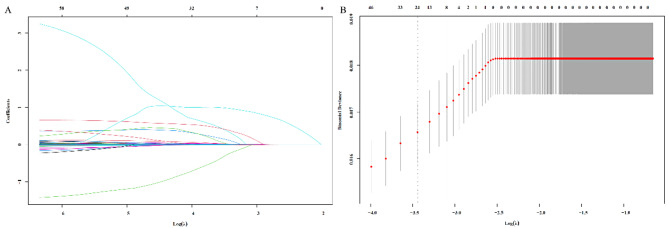
All blood parameters were included in the Lasso regression model for analysis, with the regularization strength λ systematically adjusted to optimize variable selection (Fig. 1A, B). As λ increased, less significant parameters were excluded, leaving only the most predictive factors. When λ = 0.001, only ALB and NLR remained significant, indicating their strong contribution to the model. Based on the Lasso regression coefficients, the ANLR index was constructed as follows: ANLR index = NLR − 0.04 × ALB (g/L).
Fig. 1.
Establishment of the Lasso model (A) and calculation of the optimal λ value (B)
The prognostic value of ANLR index
The ANLR index achieved a maximum Youden index of approximately 0.247 when calculated using an ROC curve focused on mortality, with an optimal cutoff value of 1.05 (Fig. 2).
Fig. 2.
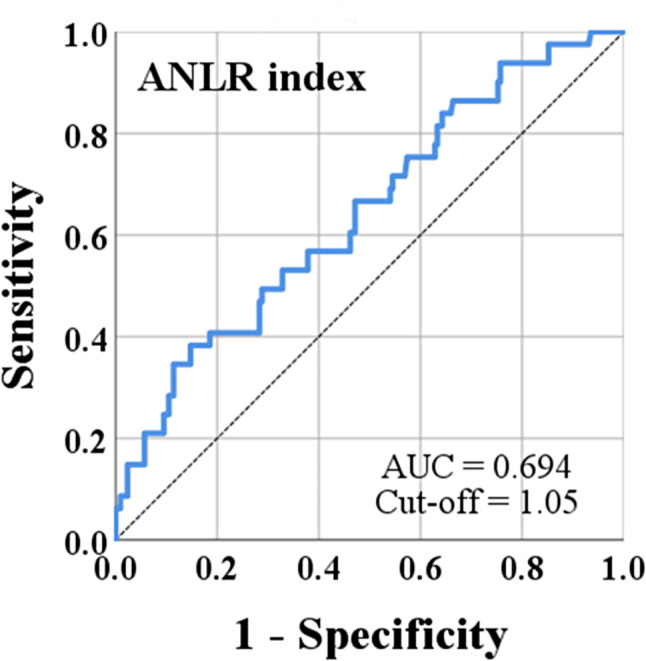
The ROC curve of ANLR
Furthermore, compared with other classical inflammatory markers, ANLR exhibited the highest AUC of 0.694 (Table 2).
Table 2.
The AUC of ANLR and classical inflammatory markers
| Items | AUC | 95%CI |
|---|---|---|
| ANLR | 0.694 | 0.607–0.772 |
| NLR | 0.657 | 0.534–0.691 |
| PLR | 0.639 | 0.555–0.723 |
| LMR | 0.648 | 0,549-0.716 |
| SII | 0.601 | 0.533–0.744 |
| SIRI | 0.635 | 0.528–0.692 |
AUC: Area under the curve; CI: Confidence interval; ANLR: Alb and Neutrophil-Lymphocyte Ratio; NLR: Neutrophil-Lymphocyte Ratio; PLR: Platelet-Lymphocyte Ratio; LMR: Lymphocyte-Monocyte Ratio; SII: Systemic Immune-Inflammation Index; SIRI: Systemic Inflammation Response Index
Survival analysis of ANLR index
Survival analysis in training set
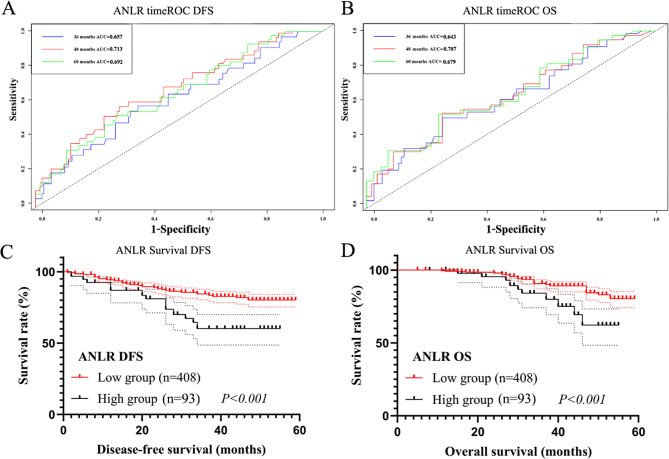
The timeROC analysis for ANLR showed that the 3-, 4-, and 5-year AUCs for DFS were 0.657, 0.713, and 0.692, and for OS were 0.643, 0.707, and 0.679, respectively (Fig. 3A, B). Patients with higher ANLR had significantly shorter DFS (408 cases vs. 93 cases, χ2 = 3.735, P < 0.001) and OS (408 cases vs. 93 cases, χ2 = 4.117, P < 0.001, Fig. 3C, D).
Fig. 3.
Survival analysis in Training set. (A) The timeROC of ANLR in DFS; (B) The timeROC of ANLR in OS; (C) The survival curve of ANLR in DFS; (D) The survival curve of ANLR in OS
Survival analysis in test set
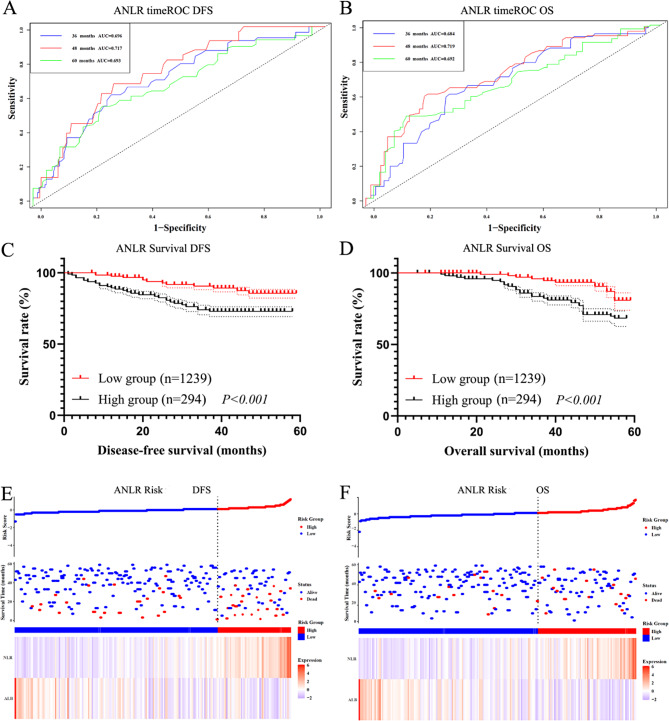
In the validation set, timeROC analysis for ANLR revealed 3-, 4-, and 5-year AUCs of 0.696, 0.717, and 0.693 for DFS, and 0.684, 0.719, and 0.692 for OS (Fig. 4A, B). Patients with higher ANLR values exhibited significantly shorter DFS and OS (1239 cases vs. 294 cases, χ2 = 3.889, P < 0.001 and χ2 = 4.746, P < 0.001, Fig. 4C, D). The risk analysis of ANLR index also revealed significant differences between high-risk and low-risk groups (Fig. 4E, F).
Fig. 4.
Survival analysis in Test set. (A) The timeROC of ANLR in DFS; (B) The timeROC of ANLR in OS; (C) The survival curve of ANLR in DFS; (D) The survival curve of ANLR in OS; (E) The risk analysis of ANLR in DFS; (F) The risk analysis of ANLR in OS
Cox survival analysis
In the validation set, univariate analysis revealed that tumor size, lymph node positivity (LNP), histological grading, TNM stage, and ANLR index were all associated with DFS and OS (all P < 0.05). Further multivariate analysis identified LNP (HR = 1.544, P < 0.001 and HR = 1.995, P < 0.001), TNM stage (HR = 2.582, P < 0.001 and HR = 2.796, P < 0.001) and ANLR (HR = 3.551, P < 0.001 and HR = 3.798, P < 0.001) as independent prognostic factors for both DFS and OS (Tables 3 and 4).
Table 3.
Univariate Cox analysis of DFS and OS
| DFS | OS | |||
|---|---|---|---|---|
| Items | HR (95%CI) | P | HR (95%CI) | P |
| Age | 1.019(1.008–1.030) | < 0.001 | 1.016(1.004–1.028) | 0.010 |
| BMI | 1.047(1.034–1.059) | < 0.001 | 1.037(1.021–1.053) | < 0.001 |
| ANLR | ||||
| < 1.05 | Ref | Ref | ||
| ≥ 1.05 | 4.134(3.713–4.658) | < 0.001 | 4.326(3.597–5.016) | < 0.001 |
| Menopause | ||||
| Yes | Ref | Ref | ||
| No | 0.946(0.821–1.091) | 0.449 | 0.913(0.792–1.052) | 0.438 |
| Family history | ||||
| Yes | Ref | Ref | ||
| No | 1.060(0.752–1.495) | 0.739 | 1.079(0.946–1.114) | 0.534 |
| Primary tumor site | ||||
| Right | Ref | Ref | ||
| Left | 1.012(0.874–1.266) | 0.705 | 1.070(0.915–1.348) | 0.653 |
| Tumor size | ||||
| < 30 mm | Ref | Ref | ||
| 30–50 mm | 1.086(0.876–1.077) | 0.282 | 1.008(0.771–1.070) | 0.250 |
| > 50 mm | 1.681(1.400-2.018) | < 0.001 | 1.756(14.62–2.110) | < 0.001 |
| LNP | ||||
| Negative | Ref | Ref | ||
| Positive | 2.160(1.703–2.740) | < 0.001 | 3.168(2.357–4.260) | < 0.001 |
| Histological Grading | ||||
| Grade I | Ref | Ref | ||
| Grade II | 1.390(1.017-1.900) | 0.039 | 1.469(1.075–2.008) | 0.016 |
| Grade III | 1.580(1.198–2.085) | 0.001 | 1.545(1.171–2.038) | 0.002 |
| TNM stage | ||||
| II | Ref | Ref | ||
| III | 3.501(2.008–5.114) | < 0.001 | 4.595(3.383–5.839) | < 0.001 |
DFS: Disease-Free Survival; OS: Overall survival; HR: Hazard ratio; BMI: Body mass index; ANLR: Alb and Neutrophil-Lymphocyte Ratio; LNP: lymph node positivity
Table 4.
Multivariate Cox analysis of DFS and OS
| DFS | OS | |||
|---|---|---|---|---|
| Items | HR (95%CI) | P | HR (95%CI) | P |
| Age | 1.002(0.994–1.010) | 0.563 | 1.004(0.996–1.012) | 0.338 |
| BMI | 1.005(0.975–1.014) | 0.589 | 1.006(0.974–1.014) | 0.554 |
| ANLR | ||||
| < 1.05 | Ref | Ref | ||
| ≥ 1.05 | 3.551(2.547–4.982) | < 0.001 | 3.798(2.987–4.679) | < 0.001 |
| Tumor size | ||||
| < 30 mm | Ref | Ref | ||
| 30–50 mm | 1.057(0.898–1.255) | 0.523 | 1.185(0.787–1.332) | 0.222 |
| > 50 mm | 1.139(0.850–1.525) | 0.383 | 1.360(0.997–1.959) | 0.063 |
| LNP | ||||
| Negative | Ref | Ref | ||
| Positive | 1.544(1.238–2.421) | < 0.001 | 1.995(1.595–2.496) | < 0.001 |
| Histological Grading | ||||
| Grade I | Ref | Ref | ||
| Grade II | 1.389(0.926–2.083) | 0.147 | 1.120(0.835–1.501) | 0.450 |
| Grade III | 1.164(0.783–1.852) | 0.253 | 1.055(0.878–1.517) | 0.737 |
| TNM stage | ||||
| II | Ref | Ref | ||
| III | 2.582(1.771–4.674) | < 0.001 | 2.796(1.555–4.021) | < 0.001 |
DFS: Disease-Free Survival; OS: Overall survival; HR: Hazard ratio; BMI: Body mass index; ANLR: Alb and Neutrophil-Lymphocyte Ratio; LNP: lymph node positivity
Propensity score matching analysis for ANLR
In the validation set, correlation analysis showed that the ANLR index was significantly associated with age, BMI, family history, PLN, tumor size, histological grading, and TNM stage (all P < 0.05). Based on these correlation analysis results, we selected variables with significant statistical associations for PSM. A 1:1 nearest-neighbor matching method without replacement was applied to ensure that patients with similar prognostic characteristics were paired. Through this matching process, a total of 152 patients were included, with 76 in the high ANLR group and 76 in the low ANLR group. After matching, there were no significant differences in baseline clinical characteristics between the two ANLR groups (all P > 0.05, Table 5), confirming the balance and comparability of the matched cohorts. This rigorous matching process ensures that the observed prognostic differences are primarily attributable to the ANLR index.
Table 5.
Propensity score matching analysis
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Low ANLR | High ANLR | Low ANLR | High ANLR | |||
| Items | n = 1239 | n = 294 | P | n = 76 | n = 76 | P |
| Age (years), mean (SD) | 50.64(9.19) | 49.18(9.14) | 0.016 | 49.37(9.08) | 49.45(9.24) | 0.717 |
| BMI (Kg/m2), mean (SD) | 25.27(5.32) | 24.15(3.69) | 0.001 | 24.44(4.26) | 24.28(3.72) | 0.608 |
| Menopause, n (%) | 0.864 | 0.138 | ||||
| Yes | 114(9.2) | 28(9.5) | 7(9.2) | 9(11.8) | ||
| No | 1125(90.8) | 266(90.5) | 69(90.8) | 67(88.2) | ||
| Family history, n (%) | < 0.001 | 0.351 | ||||
| Yes | 565(45.6) | 184(62.6) | 37(48.7) | 36(47.4) | ||
| No | 674(54.4) | 110(37.4) | 39(51.3) | 40(52.6) | ||
| Blood type, n (%) | 0.136 | 0.762 | ||||
| A | 483(39.0) | 101(34.4) | 28(36.8) | 29(38.2) | ||
| B | 319(25.7) | 68(23.1) | 18(23.7) | 17(22.4) | ||
| AB | 115(9.3) | 35(11.9) | 7(9.2) | 8(10.5) | ||
| O | 322(26.0) | 90(30.6) | 23(30.3) | 22(28.9) | ||
| Tumor site, n (%) | 0.063 | 0.209 | ||||
| Right | 574(46.3) | 105(35.7) | 34(44.7) | 32(42.1) | ||
| Left | 665(53.7) | 189(64.3) | 42(55.3) | 44(57.9) | ||
| PLN, n (%) | 0.002 | 0.091 | ||||
| Positive | 748(60.4) | 206(70.1) | 51(67.1) | 49(64.3) | ||
| Negative | 491(39.6) | 88(29.9) | 25(32.9) | 27(35.7) | ||
| Tumor size, n (%) | < 0.001 | 0.155 | ||||
| < 30 mm | 531(42.9) | 83(28.2) | 24(31.6) | 22(28.9) | ||
| 30–50 mm | 526(42.5) | 111(37.8) | 31(40.8) | 31(40.8) | ||
| > 50 mm | 182(14.6) | 100(34.0) | 21(27.6) | 23(30.3) | ||
| Histological Grading, n (%) | < 0.001 | 0.537 | ||||
| Grade I | 263(21.2) | 33(11.2) | 12(15.8) | 11(14.5) | ||
| Grade II | 864(69.7) | 216(73.5) | 57(75.0) | 57(75.0) | ||
| Grade III | 112(9.1) | 45(15.3) | 7(9.2) | 8(10.5) | ||
| TNM stage, n (%) | < 0.001 | 0.766 | ||||
| II | 668(53.9) | 103(35.0) | 40(52.6) | 39(51.3) | ||
| III | 571(46.1) | 191(65.0) | 36(47.4) | 37(48.7) | ||
PSM: Propensity score matching; ANLR: Alb and Neutrophil-Lymphocyte Ratio; BMI: Body mass index; LNP: lymph node positivity
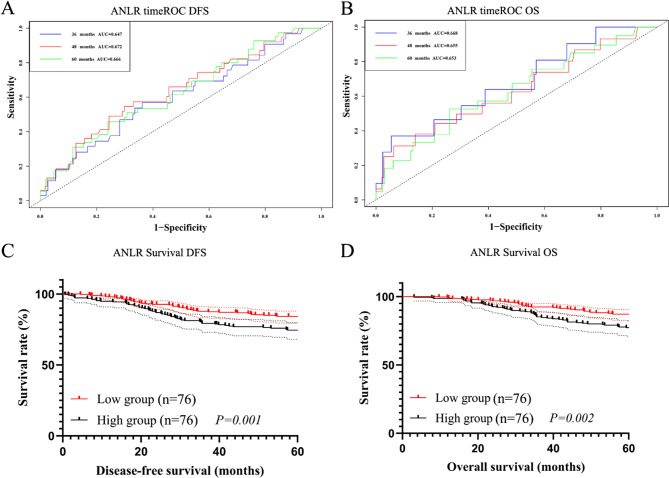
After PSM, the 3-, 4-, and 5-year AUCs for DFS of ANLR were 0.647, 0.672, and 0.666, respectively, and for OS were 0.668, 0.655, and 0.653, respectively (Fig. 5A, B). Higher ANLR remained associated with shorter DFS and OS (76 cases vs. 76 cases, χ2 = 2.179, P = 0.001 and χ2 = 2.063, P = 0.002, Fig. 5C, D).
Fig. 5.
Survival analysis after PSM. (A) The timeROC of ANLR in DFS; (B) The timeROC of ANLR in OS; (C) The survival curve of ANLR in DFS; (D) The survival curve of ANLR in OS
Comparison of prognostic value of ANLR in different periods
By collecting the levels of ALB, NEU, and LYM the day before surgery, the preoperative ANLR index (pANLR) was calculated. The timeROC curve analysis showed that the AUC of ANLR for both DFS and OS was higher than that of pANLR at all stages (Fig. 6).
Fig. 6.
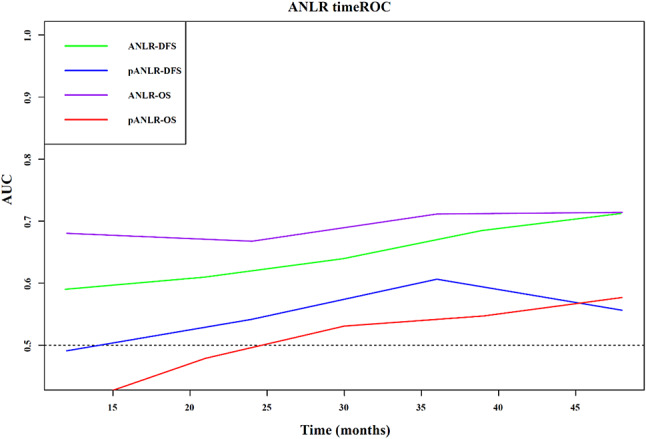
The timeROC curve of ANLR and pANLR
Construction of nomograms
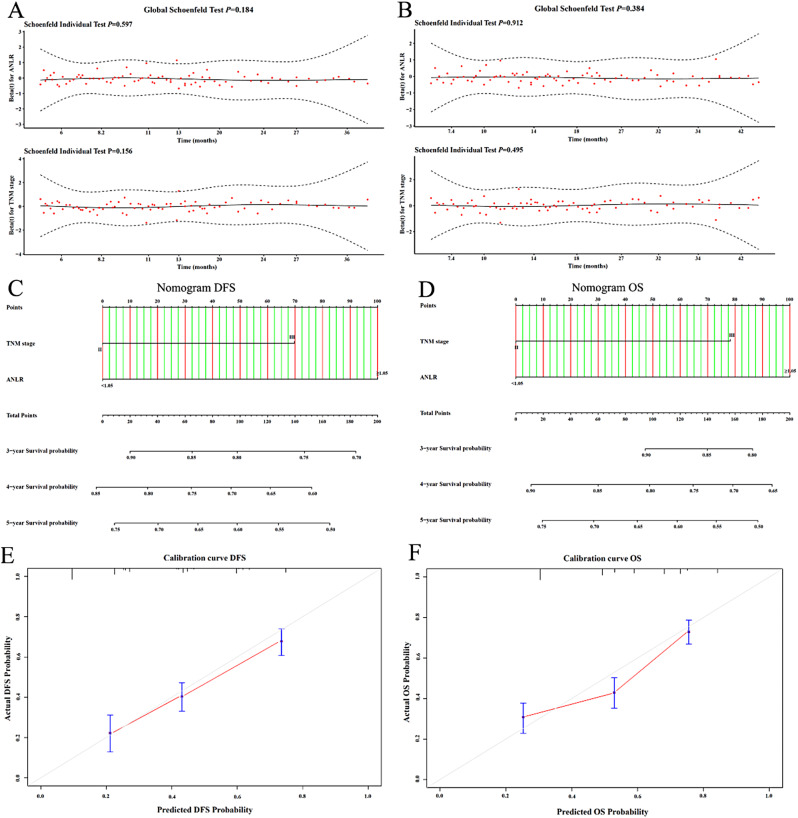
The Schoenfeld residual plots indicated that both TNM stage and the ANLR index met the proportional hazards assumption (all P > 0.05, Fig. 7A, B). The nomograms incorporating TNM stage and the ANLR index were developed in the training set (Fig. 7C, D). The C-index for the nomogram in the training set was 0.742 (0.619–0.886) for DFS and 0.758 (0.607–0.821) for OS, while in the validation set, the C-index was 0.733 (0.655–0.791) for DFS and 0.714 (0.634-0.800) for OS. Calibration curves based on the validation set demonstrated good agreement of the nomogram (Fig. 7E, F).
Fig. 7.
Construction of nomograms. (A) Schoenfeld residual plots of DFS; (B) Schoenfeld residual plots of OS; (C) Nomogram of DFS; (D) Nomogram of OS; (E Calibration curve of DFS; (F) Calibration curve of OS
Discussion
The association between inflammation and cancer progression is well-documented, with various inflammatory markers reflecting the host’s immune response to tumorigenesis [14, 15]. In TNBC, where traditional hormone-targeted therapies are ineffective, understanding the role of inflammation becomes particularly crucial [16]. TNBC is characterized by its aggressive behavior and poor prognosis, making it imperative to identify reliable prognostic indicators that can guide treatment decisions [17].
Research on the impact of inflammatory status on cancer patients has become quite mature. Classic inflammatory markers such as NLR, PLR, LMR, SII, SIRI, and ALI have been found to be associated with the prognosis of various cancers. Melanoma is one of the earliest solid tumors to utilize immune checkpoint inhibitors. Capone and colleagues analyzed the relationship between NLR and its derived marker dNLR with the prognosis of advanced melanoma patients treated with nivolumab. They collected and analyzed data from 97 patients and found that when baseline levels were below a critical threshold, both NLR and dNLR were associated with improved survival rates [18]. Additionally, Mandaliya and his colleagues analyzed the prognostic predictive capabilities of NLR, PLR, LMR, and ALI in advanced non-small cell lung cancer. They collected data from 279 advanced patients over a five-year period for analysis. The results indicated that high baseline NLR, high PLR, low LMR, and low ALI were significantly associated with poor OS. Similar findings were observed across various subgroups, including age [19]. In another study, Guo and colleagues investigated the predictive value of preoperative inflammatory markers for early recurrence after surgery in hepatitis B virus-related hepatocellular carcinoma (HCC). They conducted a retrospective analysis of 162 patients who underwent HCC resection, determining the optimal cutoff values for the NLR, PLR, SIRI, and SII. The results indicated that tumor diameter, tumor differentiation, vascular invasion, and elevated inflammatory markers were associated with an increased risk of early recurrence. The combined index developed in the study demonstrated strong predictive capability (AUC of 0.804), outperforming individual markers [20]. Inflammatory status is also widely used in breast cancer, especially TNBC. In a retrospective study, Nakamoto and colleagues explored the link between systemic immune markers and outcomes of atezolizumab treatment in advanced TNBC patients. Analyzing 36 patients across eight Japanese institutions, they found that low baseline NLR and a decrease in NLR at the second treatment cycle were associated with longer OS, while high baseline absolute lymphocyte count (ALC) and reduced NLR predicted longer time to treatment failure (TTF). The study supports the efficacy and safety of atezolizumab and underscores the predictive value of immune markers in advanced TNBC [21]. Another study conducted by Wang Ping and colleagues in 2019 explored the relationship between the SII and the Breast Imaging Reporting and Data System (BI-RADS) classification with the prognosis of 215 patients with TNBC. The results showed that low SII was associated with better survival rates: the median OS for patients with low SII was 60.9 months, while for those with high SII it was 40.3 months (HR = 3.78, P < 0.001); the median DFS was 22.4 months and 14.4 months, respectively (HR = 3.16, P < 0.001). Patients with BI-RADS 5 classification also had shorter survival times. Multivariable analysis indicated that high SII is an independent predictor of poor prognosis (OS: HR = 2.96, P < 0.001; DFS: HR = 2.85, P = 0.005). The study concluded that pre-treatment SII and BI-RADS 5 are important prognostic indicators for TNBC patients, suggesting the need for further research on the potential role of SII in clinical decision-making [22]. These studies highlight the significant potential of inflammatory status in TNBC.
In this study, we established a novel inflammatory marker, ANLR, composed of ALB and the NLR, through a grouped analysis of 2,034 TNBC patients receiving neoadjuvant therapy. ANLR was not only significantly associated with patient prognosis but also demonstrated a higher prognostic value compared to other classical inflammatory markers such as SII and PLR. Furthermore, ANLR served as an independent prognostic factor for both DFS and OS, showing predictive power comparable to TNM staging and outperforming other inflammatory markers in multivariable analysis. Notably, ANLR has also shown greater prognostic value in studies focusing on TNBC compared to other inflammatory markers reported in previous research [23–25]. Subsequent PSM analysis further validated ANLR’s substantial prognostic value by minimizing potential confounding factors, highlighting its significant advantage and clinical relevance in this patient cohort. These findings underscore the potential of ANLR as a reliable and practical tool for personalized prognostic assessment in TNBC.
ANLR is a novel inflammatory biomarker whose mechanism for predicting prognosis can be analyzed through the specific roles and interactions of its components. Firstly, albumin plays a crucial physiological role in plasma, including maintaining oncotic pressure and transporting nutrients and medications [26–28]. A decrease in albumin levels is often associated with chronic inflammation, malnutrition, and tumor-related cachexia [29]. Particularly in cancer patients, low albumin levels indicate systemic inflammation and a reduced ability to combat tumors [30]. Research has demonstrated that low albumin levels correlate with poor prognosis in cancer patients, highlighting a weakened anti-tumor response [31, 32]. Thus, albumin not only reflects a patient’s nutritional status but is also closely linked to overall health and quality of life [33]. Secondly, the NLR serves as an indicator of the body’s inflammatory state and provides insights into the tumor microenvironment [34, 35]. An increase in neutrophils generally reflects the body’s response to tumor cells, while lymphocytes are key components of the immune response [36, 37]. Elevated NLR signifies an enhanced inflammatory response, which is often associated with increased risks of tumor progression, metastasis, and recurrence [38]. In cancer patients, a high NLR is frequently linked to poorer survival rates and prognosis, indicating a state of immune suppression within the tumor microenvironment [39, 40]. By combining albumin with NLR, ANLR enables a comprehensive assessment of both inflammatory and nutritional statuses. This holistic approach allows ANLR to reflect not only the body’s inflammatory response but also overall health and anti-tumor capacity. Consequently, ANLR serves as a more effective prognostic indicator for TNBC patients.
Compared to traditional inflammatory biomarkers, ANLR presents several clear advantages. Traditional single inflammatory markers can be influenced by various factors, including infections, comorbid conditions, and physiological states [41, 42]. In contrast, ANLR combines two distinct biological markers, providing a more stable and comprehensive evaluation that mitigates the impact of these external factors. Additionally, the calculation of ANLR is relatively straightforward and easy to implement in clinical practice. Clinicians can derive the necessary data from routine blood tests to quickly compute ANLR and adjust treatment strategies as needed. This ease of use enhances the potential for ANLR to be integrated into standard clinical protocols, offering a valuable tool for predicting outcomes in TNBC patients.
Despite the promising results, our study is not without limitations. The retrospective nature of the analysis may introduce biases, and the generalizability of our findings should be validated in larger, prospective cohorts. Additionally, while ANLR demonstrated strong prognostic value, further studies are needed to explore its biological mechanisms, particularly how ANLR interacts with tumor microenvironments and immune responses. Furthermore, the study’s reliance on a single-center dataset may limit its applicability to broader populations, and potential confounding factors, despite efforts to control them through propensity score matching, cannot be entirely ruled out. Future research should also investigate the integration of ANLR with other novel inflammatory or immune markers to enhance prognostic accuracy.
Conclusion
In conclusion, our study demonstrates that the ANLR is a valuable prognostic marker for TNBC patients undergoing neoadjuvant therapy. The ANLR shows strong predictive ability for patient outcomes and can effectively identify high-risk patients postoperatively.
Author contributions
Writing-original draft and Writing-review & editing: H.S. and J.L.; Data curation and Investigation: S.X., X.Z., Mingqiang Ding and J.Z.; Methodology and Supervision: A.N., T.L. and G.L., Y.G. and Y.L.; Resources, Funding acquisition, and Project administration: L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China (52272273). Scientific research project of China Medical Education Association (2022KTM025). Graduate Practice Innovation Project of Harbin Medical University (YJSCX2024-111HYD).
Data availability
The data supporting this study can be provided by the corresponding author upon reasonable request.
Declarations
Ethical approval
This study was conducted in accordance with the Helsinki Declaration and received approval from the ethics committee of Sixth Affiliated Hospital of Harbin Medical University (LC2024-052). All patients signed an “Informed Consent Form for the Secondary Use of Medical History Data/Biological Specimens”. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Sun and Jian Liang contributed equally to this work.
References
- 1.Barzaman K, Karami J, Zarei Z, et al. Breast cancer: Biology, biomarkers, and treatments. INT IMMUNOPHARMACOL. 2020;84:106535. 10.1016/j.intimp.2020.106535. [DOI] [PubMed] [Google Scholar]
- 2.Kawiak A. Molecular Research and treatment of breast Cancer. Int J Mol Sci. 2022;23(17). 10.3390/ijms23179617. [DOI] [PMC free article] [PubMed]
- 3.Obidiro O, Battogtokh G, Akala EO. Triple negative breast Cancer Treatment options and limitations: Future Outlook. Pharmaceutics. 2023;15(7). 10.3390/pharmaceutics15071796. [DOI] [PMC free article] [PubMed]
- 4.Zagami P, Carey LA. Triple negative breast cancer: pitfalls and progress. NPJ Breast Cancer. 2022;8(1):95. 10.1038/s41523-022-00468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houvenaeghel G, Sabatier R, Reyal F, et al. Axillary lymph node micrometastases decrease triple-negative early breast cancer survival. BRIT J CANCER. 2016;115(9):1024–31. 10.1038/bjc.2016.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. BREAST CANCER RES TR. 2016;161(2):279–87. 10.1007/s10549-016-4059-6. [DOI] [PubMed] [Google Scholar]
- 7.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J CLIN ONCOL. 2008;26(8):1275–81. 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 8.Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast Cancer: ASCO Guideline. J CLIN ONCOL. 2021;39(13):1485–505. 10.1200/JCO.20.03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. ANN ONCOL. 2018;29(7):1497–508. 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 10.van den Ende NS, Nguyen AH, Jager A, et al. Triple-negative breast Cancer and predictive markers of response to Neoadjuvant Chemotherapy: a systematic review. Int J Mol Sci. 2023;24(3). 10.3390/ijms24032969. [DOI] [PMC free article] [PubMed]
- 11.Kusama H, Kittaka N, Soma A, et al. Predictive factors for response to neoadjuvant chemotherapy: inflammatory and immune markers in triple-negative breast cancer. BREAST CANCER-TOKYO. 2023;30(6):1085–93. 10.1007/s12282-023-01504-y. [DOI] [PubMed] [Google Scholar]
- 12.Gu J, Deng S, Jiang Z, et al. Modified Naples prognostic score for evaluating the prognosis of patients with obstructive colorectal cancer. BMC Cancer. 2023;23(1):941. 10.1186/s12885-023-11435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokumaru Y, Oshi M, Murthy V, et al. Low intratumoral genetic neutrophil-to-lymphocyte ratio (NLR) is associated with favorable tumor immune microenvironment and with survival in triple negative breast cancer (TNBC). Am J Cancer Res. 2021;11(11):5743–55. PMID: 34873491. [PMC free article] [PubMed] [Google Scholar]
- 14.Liubomirski Y, Lerrer S, Meshel T, et al. Tumor-stroma-inflammation networks promote pro-metastatic chemokines and aggressiveness characteristics in Triple-negative breast Cancer. Front Immunol. 2019;10:757. 10.3389/fimmu.2019.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie H, Ruan G, Wei L, et al. Obesity-associated metabolic inflammation promotes triple-negative breast cancer progression through the interleukin-6/STAT3/pentraxin 3/matrix metalloproteinase 7 axis. INT IMMUNOPHARMACOL. 2024;136:112332. 10.1016/j.intimp.2024.112332. [DOI] [PubMed] [Google Scholar]
- 16.Kumar N, Ehsan S, Banerjee S, et al. The unique risk factor profile of triple-negative breast cancer: a comprehensive meta-analysis. JNCI-J NATL CANCER I. 2024;116(8):1210–9. 10.1093/jnci/djae056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocco S, Piezzo M, Calabrese A, et al. Biomarkers in Triple-negative breast Cancer: state-of-the-art and future perspectives. Int J Mol Sci. 2020;21(13). 10.3390/ijms21134579. [DOI] [PMC free article] [PubMed]
- 18.Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandaliya H, Jones M, Oldmeadow C, et al. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). TRANSL LUNG CANCER R. 2019;8(6):886–94. 10.21037/tlcr.2019.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenpei G, Yuan L, Liangbo L, et al. Predictive value of preoperative inflammatory indexes for postoperative early recurrence of hepatitis B-related hepatocellular carcinoma. Front Oncol. 2023;13:1142168. 10.3389/fonc.2023.1142168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamoto S, Shien T, Itoh M, et al. Systemic immunity markers are associated with clinical outcomes of atezolizumab treatment in patients with triple-negative advanced breast cancer: a retrospective multicenter observational study. CLIN EXP MED. 2023;23(8):5129–38. 10.1007/s10238-023-01230-x. [DOI] [PubMed] [Google Scholar]
- 22.Wang P, Yue W, Li W, et al. Systemic immune-inflammation index and ultrasonographic classification of breast imaging-reporting and data system predict outcomes of triple-negative breast cancer. Cancer Manag Res. 2019;11:813–9. 10.2147/CMAR.S185890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, He M, Wang C, et al. Prognostic value of neutrophil-to-lymphocyte ratio for patients with triple-negative breast cancer: a meta-analysis. Medicine. 2022;101(28):e29887. 10.1097/MD.0000000000029887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lusho S, Durando X, Mouret-Reynier MA, et al. Platelet-to-lymphocyte ratio is Associated with favorable response to Neoadjuvant Chemotherapy in Triple negative breast Cancer: a study on 120 patients. Front Oncol. 2021;11:678315. 10.3389/fonc.2021.678315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pistelli M, De Lisa M, Ballatore Z, et al. Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer. 2015;15:195. 10.1186/s12885-015-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasry A, Nadorp B, Fornerod M, et al. An inflammatory state remodels the immune microenvironment and improves risk stratification in acute myeloid leukemia. Nat Cancer. 2022;4(1):27–42. 10.1038/s43018-022-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei J, Wang Y, Guo X, et al. Low preoperative serum ALB level is independently associated with poor overall survival in endometrial cancer patients. FUTURE ONCOL. 2020;16(8):307–16. 10.2217/fon-2019-0732. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Li L, Duan Y, et al. Prognostic role of pre-treatment serum ALB in patients with oropharyngeal cancer: a retrospective cohort study. Front Oncol. 2022;12:924210. 10.3389/fonc.2022.924210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie H, Wei L, Ruan G, et al. AWGC2023 cachexia consensus as a valuable tool for predicting prognosis and burden in Chinese patients with cancer. J CACHEXIA SARCOPENI. 2024;15(5):2084–93. 10.1002/jcsm.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takagi A, Hawke P, Tokuda S, et al. Serum carnitine as a biomarker of Sarcopenia and nutritional status in preoperative gastrointestinal cancer patients. J CACHEXIA SARCOPENI. 2021;13(1):287–95. 10.1002/jcsm.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinninella E, Cintoni M, Raoul P, et al. Effects of nutritional interventions on nutritional status in patients with gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN. 2020;38:28–42. 10.1016/j.clnesp.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Chen N, Yu Y, Shen W, et al. Nutritional status as prognostic factor of advanced oesophageal cancer patients treated with immune checkpoint inhibitors. CLIN NUTR. 2023;43(1):142–53. 10.1016/j.clnu.2023.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Foecke Munden E, Kemp M, Guth A, et al. Patient-important needs and goals related to Nutrition interventions during Cancer Treatment. NUTR CANCER. 2023;75(4):1143–50. 10.1080/01635581.2023.2178938. [DOI] [PubMed] [Google Scholar]
- 34.Diem S, Schmid S, Krapf M, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–81. 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Cupp MA, Cariolou M, Tzoulaki I, et al. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18(1):360. 10.1186/s12916-020-01817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. NAT REV IMMUNOL. 2021;22(3):173–87. 10.1038/s41577-021-00571-6. [DOI] [PubMed] [Google Scholar]
- 37.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng SP, Bahig H, Jethanandani A, et al. Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio (NLR) in patients with oropharyngeal cancer treated with radiotherapy. BRIT J CANCER. 2020;124(3):628–33. 10.1038/s41416-020-01106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beal EW, Wei L, Ethun CG, et al. Elevated NLR in gallbladder cancer and cholangiocarcinoma - making bad cancers even worse: results from the US extrahepatic biliary Malignancy Consortium. HPB. 2016;18(11):950–7. 10.1016/j.hpb.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Y, Liu X, Wang M, et al. Neutrophil-to-lymphocyte ratio as a predictor of cardiovascular mortality in cancer survivors. Sci Rep. 2024;14(1):20980. 10.1038/s41598-024-72027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan S, Zheng Q, Zhang W, et al. Prognostic value of inflammatory markers NLR, PLR, and LMR in gastric cancer patients treated with immune checkpoint inhibitors: a meta-analysis and systematic review. Front Immunol. 2024;15:1408700. 10.3389/fimmu.2024.1408700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo E, Guizzardi M, Canali L, et al. Preoperative systemic inflammatory markers as prognostic factors in differentiated thyroid cancer: a systematic review and meta-analysis. REV ENDOCR METAB DIS. 2023;24(6):1205–16. 10.1007/s11154-023-09845-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study can be provided by the corresponding author upon reasonable request.