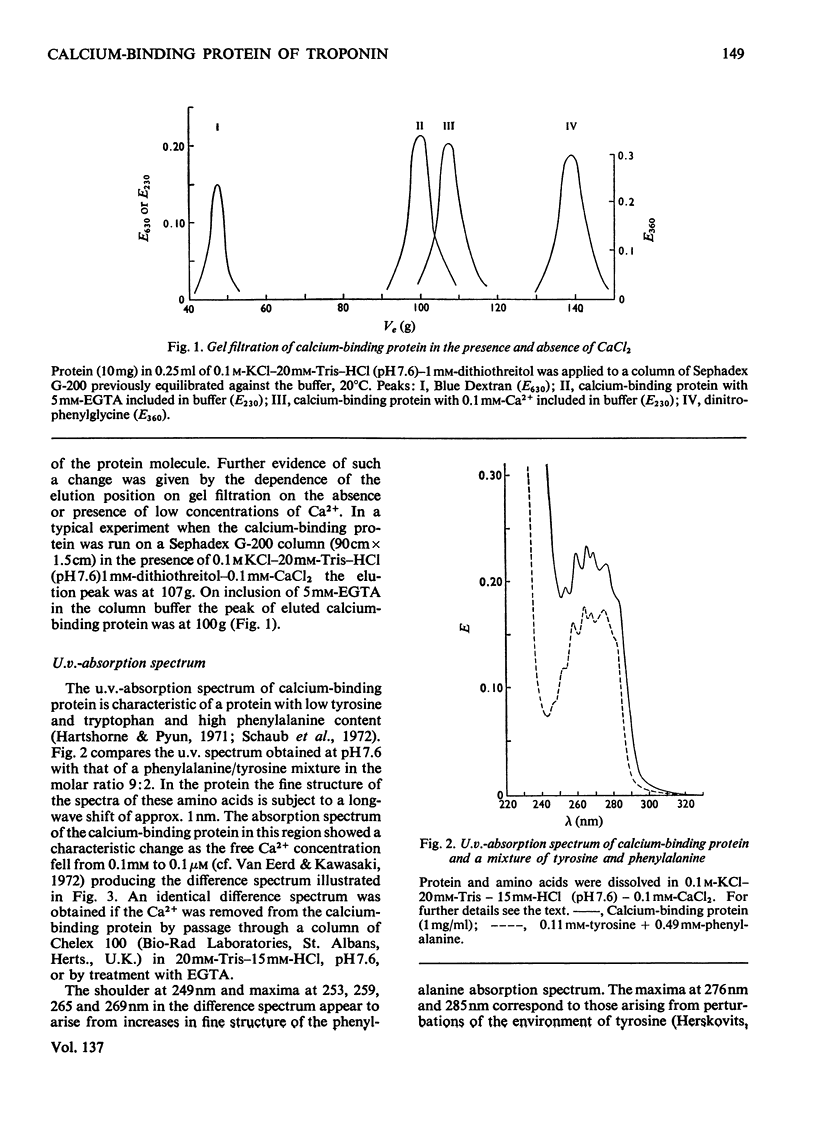
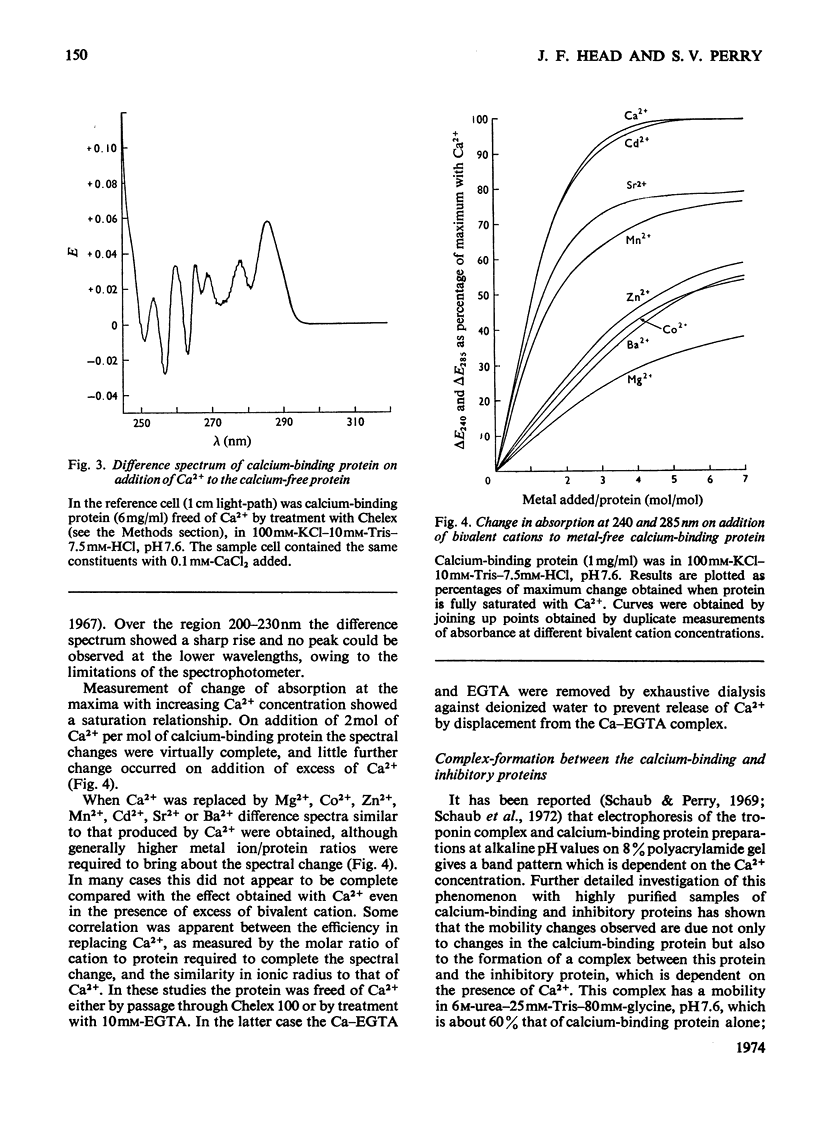
Abstract
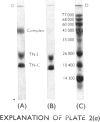
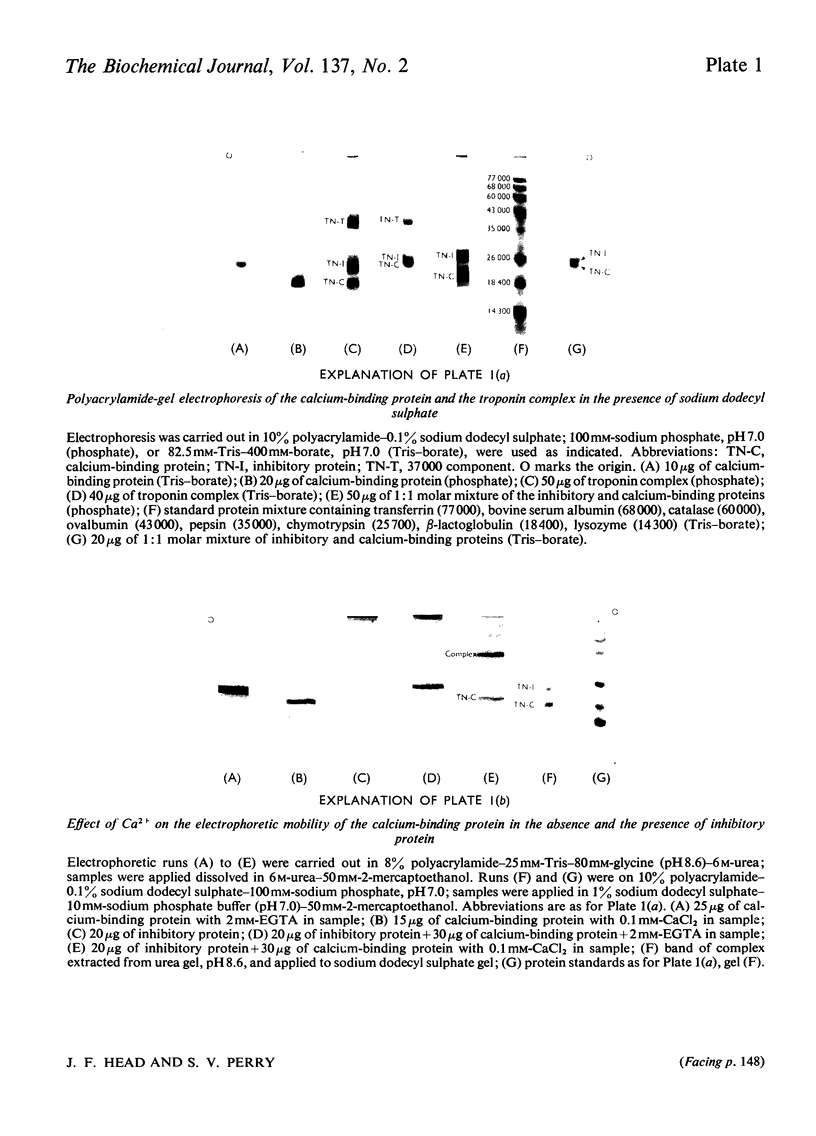
1. The molecular weight of the calcium-binding protein of rabbit white skeletal muscle was estimated to be 18500 by sedimentation equilibrium and electrophoresis in sodium dodecyl sulphate. 2. Addition of 2 Ca2+ ions per molecule produced reversible changes in the u.v.-absorption spectrum that are interpreted as arising from conformational changes in the structure of the protein. 3. Cd2+ was almost as effective as Ca2+ in producing the spectral changes. Other bivalent metal ions, particularly Mg2+, were less effective. 4. Binding of Ca2+ by the calcium-binding protein produced an increase in mobility to the anode on electrophoresis in 6m-urea at pH8.6. The Ca2+-saturated form of the protein was more retarded on gel filtration than the Ca2+-free form. 5. In the presence of Ca2+ the calcium-binding protein formed an equimolar complex with the inhibitory protein. This complex was stable in 8m-urea and in the pH range 7.0–8.6. 6. An isotope-dilution method for the measurement of the content of calcium-binding protein in whole muscle is described. In rabbit psoas muscle the ratio of actin monomers to molecules of calcium-binding protein was approx. 7:1. Similar values were obtained for red skeletal and cardiac muscle. 7. Evidence is presented indicating that in the rabbit the inhibitory protein of the troponin complex of red skeletal and cardiac muscles is different from the inhibitory protein of white skeletal muscle.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chervenka C. H. Long-column meniscus depletion sedimentation equilibrium technique for the analytical ultracentrifuge. Anal Biochem. 1970 Mar;34:24–29. doi: 10.1016/0003-2697(70)90082-5. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabikowski W., Dabrowska R., Barylko B. Separation and characterization of the constituents of troponin. FEBS Lett. 1971 Jan 12;12(3):148–152. doi: 10.1016/0014-5793(71)80055-8. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Ebashi S. Separation of troponin into its three components. J Biochem. 1972 Sep;72(3):787–790. doi: 10.1093/oxfordjournals.jbchem.a129961. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Fuchs F. The effect of Ca2+ on the sulfhydryl reactivity of troponin. Evidence for a Ca2+-induced conformational charge. Biochim Biophys Acta. 1971 Mar 2;226(2):453–458. doi: 10.1016/0005-2728(71)90111-3. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971 Jul 10;246(13):4226–4233. [PubMed] [Google Scholar]
- Han M. H., Benson E. S. Conformational changes in troponin induced by Ca++1. Biochem Biophys Res Commun. 1970 Feb 6;38(3):378–384. doi: 10.1016/0006-291x(70)90724-2. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Mueller H. Fractionation of troponin into two distinct proteins. Biochem Biophys Res Commun. 1968 Jun 10;31(5):647–653. doi: 10.1016/0006-291x(68)90610-4. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Pyun H. Y. Calcium binding by the troponin complex, and the purification and properties of troponin A. Biochim Biophys Acta. 1971 Mar 23;229(3):698–711. doi: 10.1016/0005-2795(71)90286-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murray A. C., Kay C. M. Hydrodynamic and optical properties of troponin A. Demonstration of a conformational change upon binding calcium ion. Biochemistry. 1972 Jul 4;11(14):2622–2627. doi: 10.1021/bi00764a012. [DOI] [PubMed] [Google Scholar]
- Murray A. C., Kay C. M. Separation and characterization of the inhibitory factor of the troponin system. Biochem Biophys Res Commun. 1971 Jul 2;44(1):237–244. doi: 10.1016/s0006-291x(71)80184-5. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome E. M., Hirabayashi T., Perry S. V. X-ray diffraction of muscle labelled with antibody to troponin-C. Nat New Biol. 1973 Aug 1;244(135):154–155. doi: 10.1038/newbio244154a0. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V., Häcker W. The regulatory proteins of the myofibril. Characterization and biological activity of the calcium-sensitizing factor (troponin A). Biochem J. 1972 Jan;126(1):237–249. doi: 10.1042/bj1260237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V. The relaxing protein system of striated muscle. Resolution of the troponin complex into inhibitory and calcium ion-sensitizing factors and their relationship to tropomyosin. Biochem J. 1969 Dec;115(5):993–1004. doi: 10.1042/bj1150993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eerd J. P., Kawasaki Y. Ca ++ induced conformational changes in the Ca ++ binding component of troponin. Biochem Biophys Res Commun. 1972 May 26;47(4):859–865. doi: 10.1016/0006-291x(72)90572-4. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T., Ebashi S. Reversible change in physical state of troponin induced by calcium ion. J Biochem. 1968 Nov;64(5):731–732. doi: 10.1093/oxfordjournals.jbchem.a128955. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilkinson J. M., Perry S. V., Cole H. A., Trayer I. P. The regulatory proteins of the myofibril. Separation and biological activity of the components of inhibitory-factor preparations. Biochem J. 1972 Mar;127(1):215–228. doi: 10.1042/bj1270215. [DOI] [PMC free article] [PubMed] [Google Scholar]