Abstract
Objective
Cerebral air embolism during Cardio-Pulmonary Bypass is a severe complication with significant neurological risks. We present six pediatric cases, detailing their presentation, management, and outcomes. The discussion reviews existing literature and proposes management guidelines for suspected air emboli.
Methods
A Case Series Report. A retrospective review of medical records was conducted to identify pediatric patients who experienced cerebral air embolism during cardiac surgeries with CPB between January 2009 to December 2023.
Main findings
Throughout the study duration, six patients experienced cerebral air emboli as a complication of their cardiac surgeries. Patients’ ages ranged from 8 months to 16 years, with five having single ventricle physiology. Tear-induced cerebral air emboli from right atrium dissection were observed in five cases. Seizures following surgery were the predominant clinical manifestation. Neurological outcomes varied; three patients showed minimal deficits at discharge, while other three cases suffered significant damage leading to substantial deficits.
Conclusions
We present a case series documenting the occurrence of a rare complication—cerebral air emboli—during cardiac surgery in children. Our study highlight a spectrum of neurological outcomes in affected patients and provides guidelines for the identification and treatment of this complication.
Introduction
The occurrence of cerebral air emboli in the context of cardiac surgery represents a multifaceted and potentially perilous phenomenon. This phenomenon stems from the inadvertent introduction of air into the vascular system, a consequence of intricate surgical procedures involving cardiac manipulation and vascular engagement. The subsequent transit of air bubbles within the circulatory system to cerebral vasculature can disrupt of physiological hemodynamics, thereby leading to a spectrum of neurological sequelae.
Methods
We present six cases of cerebral air emboli during cardiac surgery under cardiopulmonary bypass (CPB). The CPB strategy involved cannulation of the inferior and superior vena cava for venous drainage and the aorta for arterial return, ensuring stable hemodynamics throughout the procedure. This study aims to share our experience in managing cerebral air emboli in pediatric cardiac surgeries and the associated neurological outcomes. Our Department of Pediatrics and Congenital Cardiothoracic Surgery is a referral center for children from Israel, the Palestinian Authority, West Asia, and Eastern Europe, performing approximately 350–400 surgeries on CPB each year. The study was approved by the hospital’s Ethics Committee.
Case descriptions
Patient 1
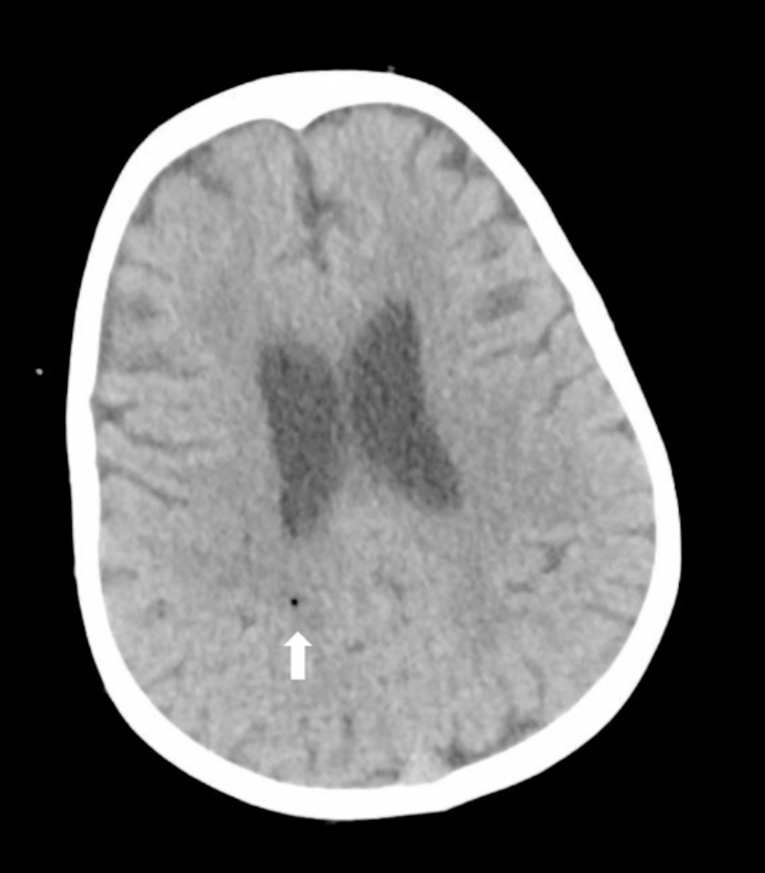
A three-and-a-half-year-old patient with Hypoplastic Left Heart Syndrome (HLHS) and previous Norwood and Sano procedures underwent Fontan procedure. During the procedure, air entry occurred when the atrial clamp slipped during suturing. The patient was positioned in Trendelenburg, and fibrillation with aortic root venting was induced. Postoperatively, due to delayed awakening, an immediate brain CT scan revealed a single air bubble in the parietal area (Fig. 1). The patient was transferred to another medical center for hyperbaric oxygen therapy (HBOT). Neurological improvement was gradual, with extubation on the second postoperative day. An EEG showed a slow electrical pattern without epileptic activity, and the patient was discharged to a rehabilitation center after 18 days. At neurological follow-up at age four, delays in fine and gross motor activities were observed.
Fig. 1.
A small air bubble is visible in the white matter of the right parietal region (indicated by the white arrow)
Patient 2
A 16-year-old patient with Double Outlet Right Ventricle and Pulmonary Atresia underwent a right ventricle to pulmonary artery (RV-PA) conduit replacement. After dissection of extensive adhesions and removal of a calcified conduit, a new conduit was inserted. A Transesophageal Echocardiogram (TEE) revealed air in the left ventricle. The patient was placed in Trendelenburg position, and aortic root venting was performed. Postoperatively, the patient developed seizures, and an immediate head CT scan was performed, revealing no signs of hemorrhage or cerebral emboli. The patient was transferred for hyperbaric therapy due to the intraoperative air entry. Seizures ceased after treatment, but the patient remained unresponsive. On day six, imaging revealed sub-acute infarctions in the right frontal and temporal regions. The patient was discharged with significant hypoxic-ischemic encephalopathy.
Patient 3
A 6-year-old patient with Tetralogy of Fallot and Pulmonary Atresia, previously treated with two BT shunt insertions, was admitted for a modified Blalock–Thomas–Taussig shunt (BTT shunt) and Pulmonary artery plasty. During the procedure, a right atrial tear led to air entry and hemodynamic instability. In the ICU, the patient experienced intractable seizures, prompting an urgent brain CT scan, which showed no signs of bleeding or air emboli. Following HBOT treatment, her clinical condition improved. Upon discharge, she exhibited right hemiparesis, though overall neurological function had improved. Neurological follow-up at age 14 showed significant recovery, with only mild residual weakness.
Patient 4
An 8-month-old patient with HLHS, post Norwood-Sano operation, underwent a Glenn procedure. During adhesion dissection, a right atrial perforation occurred, leading to the initiation of cardiopulmonary support and subsequent repair. Postoperatively, seizures prompted hyperbaric oxygen therapy (HBOT). Initial brain imaging was normal, but an MRI on the fourth postoperative day revealed bilateral hypoxic-ischemic changes. After a rehabilitation phase, the patient was discharged on the 16th day with regained neurological functions. Neurological follow-up at two and a half years revealed mild right-sided weakness.
Patient 5
An 11-year-old patient underwent aortic valve replacement after previous truncus arteriosus repair and conduit to homograft repair. Prior to surgery, the TEE showed a negative bubble test. During surgery, a highly calcified homograft was excised, leading to an aortic perforation. An aortic cross-clamp was applied, left atrium venting was initiated, and selective antegrade cardioplegia was administered. In the ICU, the patient exhibited rhythmic eye movements, facial muscle fasciculations, and did not wake up. A head CT scan, performed immediately after the patient returned from the operating room, showed no abnormalities, but HBOT treatment was initiated. On postoperative day one, a repeat CT scan revealed acute embolic infarcts in the right fronto-parietal region. The patient later developed significant cerebral edema, resulting in extensive brain damage.
Patient 6
A 10-month-old patient with a Double Outlet Right Ventricle and Transposition of the Great Arteries, with a history of multiple surgeries, underwent a Glenn procedure. A sternotomy accident during surgery caused a tear in the right atrium, leading to significant blood loss and air entry as seen on TEE. The patient was positioned in Trendelenburg, and retrograde cerebral perfusion (RCP) was initiated. During RCP, the SVC cannula was secured on the pulmonary artery side, the aortic cross-clamp was removed, and the aortic root was vented via the cardioplegia site, which was left open to the atmosphere as a vent. RCP was conducted for 3 min at 35% of full flow (120 cc/kg) without systemic perfusion. After achieving hemodynamic stability, the patient was transferred for HBOT. A pre-transfer head CT revealed inconspicuous subdural hemorrhages. Despite rehabilitation efforts, the patient was discharged with severe encephalopathy and cortical blindness.
Results
The primary objective of this study was to elucidate our collective experience regarding the management of cerebral air emboli in pediatric patients undergoing cardiac surgery. Throughout the study duration, six patients underwent cardiac surgery that complicated with cerebral air emboli. The patients’ ages ranged from 8 months to 16 years, with five demonstrating single ventricular physiology (Table 1). In five cases, the cause of cerebral air emboli was the result of a tear in the right atrium during the dissection of adhesions.
Table 1.
Case descriptions
| Patient | Age (Years) | Cardiac Lesion | Surgery | Brain CT Finding Post OP | Outcome |
|---|---|---|---|---|---|
| 1 | 3.5 | HLHS | Fontan | Single air bubble | Fine and gross motor activities delay |
| 2 | 16 | DORV/PA | Conduit Replacement | Normal | Hypoxic Ischemic Encephalopathy |
| 3 | 6 | TOF/PA | Modified BT Shunt and PV plasty | Normal | Right hemiparesis, improved neurological condition |
| 4 | 8 months | HLHS | Glenn procedure | Normal | Mild right sided weakness |
| 5 | 11 | Truncus Arteriosus | AV Replacement | Normal | Extensive brain damage |
| 6 | 10 months | DORV/TOF type | Glenn procedure | Subdural hemorrhages | Diffuse neurologic damage, encephalopathy, cortical blindness |
Upon presentation after surgery, all patients displayed unconsciousness along with abnormal movements or seizures. Immediate postoperative CT scans were found to be normal in four cases, with only one CT revealing the presence of air. The neurological outcomes exhibited variability. Three patients displayed favorable neurological examination results at the time of discharge, demonstrating minimal neurological deficits. Conversely, in the other three cases, there was notable and substantial neurological damage resulting in significant deficits.
Discussion
Cerebral air embolism during cardiac surgery on CPB remains an infrequent but serious complication in pediatric patients. Reviewing the existing literature reveals a handful of reports documenting similar cases. Kim et al. [1] reported a case of cerebral air embolism during open-heart surgery with CPB in an adult patient, underscoring the potential risk of this complication across different age groups. Olga et al. [2] documented the effective mitigation of substantial air emboli during cardiopulmonary bypass in a 20-year-old patient. Watkins et al. [3] reported a case of venous air embolism leading to cardiac arrest in an infant, reinforcing the importance of thorough monitoring and timely intervention to prevent complications. Likewise, several other studies have documented instances of arterial air embolism occurring during open-heart surgery, with a minority of these cases involving pediatric patients [4, 5].
Efforts to prevent cerebral air embolism include meticulous surgical planning and adherence to best practices during CPB. Preoperatively, some centers, including our department, perform a chest CT scan on children who have undergone previous surgeries (redo surgery) to assess the distance between the sternum and the heart and blood vessels. During a routine re-sternotomy without cardiopulmonary bypass (CPB), any tear or injury is unlikely to result in an air embolism, particularly in right-sided structures like the right atrium, as the heart remains filled with blood. However, if CPB is initiated and re-sternotomy continues with dissection, there is a heightened risk of embolization, especially in decompressed cardiac chambers, as seen in single ventricle physiology with a septal defect. Intraoperatively, Van der Zee et al. [6] analyzed an incident of fatal air embolism during CPB, emphasizing the significance of air detection systems and vigilant cannulation techniques. The processes of generating, detecting, and preventing gaseous microemboli during cardiopulmonary bypass procedures have been thoroughly discussed in prior literature [7, 8].
Intra-operative management of cerebral air embolism revolves around reducing the volume of air in cerebral vessels and preventing further emboli. The first intervention includes placing the patient in Trendelenburg position. Gravity plays a pivotal role in impeding the ascent of air within the arterial circulation, encompassing the cerebral arteries [9].
An additional procedural approach to consider entails the utilization of retrograde cerebral perfusion. This method encompasses the transient reversal of blood flow within the aorta by introducing oxygenated blood into the ascending aorta via a cannula [9–11]. Additional suggested therapeutic interventions encompass patient cooling, the administration of vasopressors and steroids, carbon dioxide (CO2) flush as well as ventilation using 100% oxygen [12].
hyperbaric oxygen therapy (HBOT) involves administration of 100% oxygen inhalation within a pressurized chamber. In addressing cerebral air emboli, HBOT utilizes elevated pressure to enhance oxygen solubility in blood and tissues, reducing the size of air bubbles and improving oxygen delivery. This technique aids in mitigating the obstructive effects of emboli, promoting oxygen supply, and supporting tissue recovery. HBOT for cerebral air embolism should be initiated as soon as possible, ideally within hours of the event. The initial treatment typically lasts 6 h according to our protocol. There are no strict weight or age restrictions. Intubation is necessary for patients unable to protect their airway or requiring mechanical ventilation, particularly if they are unstable postoperatively. Drains and lines generally do not pose problems if managed properly. Typically, the treatment does not affect the patient’s hemodynamics or respiration. The main challenge arises when patients, unstable post-surgery, are moved from the ICU for HBOT. To ensure safety, we send an ICU physician and nurse with all necessary monitoring equipment, medications, and blood products. Unstable patients are not transferred for treatment. Continuous monitoring of neurological status, vital signs, and oxygen levels is essential during therapy. After therapy, assessing neurological status, alertness, and the cessation of seizures are crucial to evaluate the therapy’s success.
The outcomes of cerebral air embolism vary, with some patients experiencing severe and irreversible neurological damage while others show partial recovery. Fakkert et al. [13] conducted a systematic review and meta-analysis, highlighting the potential favorable outcome associated with early hyperbaric oxygen therapy in patients with iatrogenic cerebral arterial gas embolism. However, it is noteworthy that favorable neurological outcomes have been documented even when the initiation of therapy is deferred for a period surpassing 48 h [14].
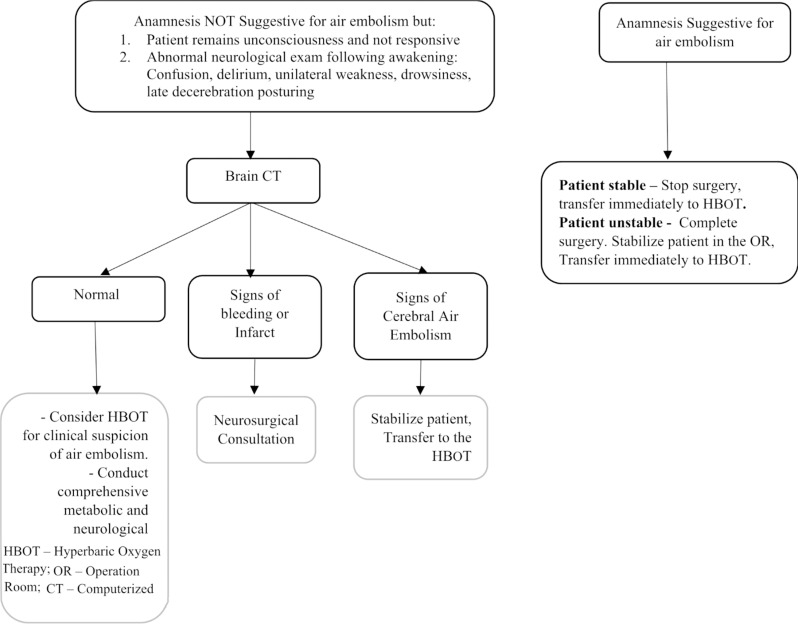
The optimal timing for hyperbaric oxygen therapy (HBOT) remains uncertain. Recognizing the potential detrimental impact of any delay on brain health, early consideration of HBOT is recommended. The initial decision revolves around the potential cessation of the surgical procedure, if deemed possible. In cases when the surgery is completed, a subsequent consideration arises regarding the optimal course of action: whether to promptly transfer the patient to HBOT immediately after surgery or assessing the patient’s wakefulness and neurological status before commencing therapy. The possibility of promptly transferring a patient to HBOT immediately after surgery, rather than providing post-operative care in the ICU, raises safety concerns. We developed a protocol for managing suspected cerebral air embolism during pediatric cardiac surgery, utilizing intraoperative anamnestic information and assessing the patient’s neurological status postoperatively (Fig. 2).
Fig. 2.
Protocol for the Treatment of Suspected Cerebral Air Embolism During Pediatric Cardiac Surgery
Evaluating the neurological outcomes of the affected patients in our study revealed a diverse spectrum of results. Among these, three patients exhibited favorable neurological examination outcomes upon their discharge, indicative of minimal neurological impairments. In contrast, in three separate cases, a more concerning scenario unfolded, manifesting as conspicuous and substantial neurological impairment that resulted in notable deficits. This contrast of outcomes underscores the variable nature of neurological consequences following cerebral air emboli, thereby emphasizing the complexity of predicting and addressing the aftermath of such occurrences.
The primary limitation in our study lies in its descriptive approach and limited sample size. This limitation restricts the ability to draw definitive conclusions or identify significant trends within the data. The absence of a control group or a comparator arm diminishes the ability to distinguish the true effects of the intervention.
Conclusion
Cerebral air embolism during cardiac surgery with CPB remains a rare but serious complication in pediatric patients. Our study highlights the rare complication that can lead to abnormal neurological findings following pediatric cardiac surgery and provides guidelines for the management of these patients.
Author contributions
E.H., I.M.F., and Y.S. wrote the main manuscript text. A.V., D.M., and U.K. prepared Figs. 1, 2 and 3. O.B.Y. and R.K.L reviewed and edited the manuscript for critical content. All authors reviewed the manuscript.Evyatar Hubara is the corresponding author.
Funding
The authors declare that no funding was received for the conduct of this research or the preparation of this manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim DY, Park CI, Yu SJ. Cerebral Air Embolism during Open Heart surgery with cardiopulmonary bypass. Korean Circ J. 2012;42(9):647–50. [Google Scholar]
- 2.Olga L, Quintero MD, Juan C, Giraldo MD, Néstor F, Sandoval MD. Successful management of massive air Embolism during Cardiopulmonary Bypass using Multimodal Neuroprotection Strategies. Semin Cardiothorac Vasc Anesth 1–9. 2018. [DOI] [PubMed]
- 3.Watkins SC, McCarver L, VanBebber A, Bichell DP. Venous air Embolism leading to Cardiac arrest in an infant with cyanotic congenital heart disease. Case Rep. 2020;15(11):e01430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber S, Rigler B, Ma¨chler HE, Metzler H, Smolle-Ju¨ttner FM. Successful treatment of massive arterial air Embolism during Open Heart surgery. Ann Thorac Surg. 2001;71(5):1664–6. [DOI] [PubMed] [Google Scholar]
- 5.Scott C, Watkins L, McCarver A, VanBebber. and David P. Bichell. Venous Air Embolism Leading to Cardiac Arrest in an Infant with Cyanotic Congenital Heart Disease. Case Reports in Anesthesiology. Volume 2012, Article ID 208430. [DOI] [PMC free article] [PubMed]
- 6.Van der Zee MP, Koene BM, Mariani MA. Fatal air embolism during cardiopulmonary bypass: analysis of an incident and prevention measures. Interact Cardiovasc Thorac Surg. 2014;19(2):232–6. [DOI] [PubMed] [Google Scholar]
- 7.Lou S, Ji B, Liu J, Yu K, Long C. Generation, detection and prevention of gaseous microemboli during cardiopulmonary bypass procedure. Int J Artif Organs. 2011;34(11):1039–51. [DOI] [PubMed] [Google Scholar]
- 8.Nicks R. Air Embolism in Cardiac surgery: incidence and Prophylaxis. Aust N Z J Surg. 1972;38(4):328–32. [PubMed] [Google Scholar]
- 9.Quintero OL, Giraldo JC, Sandoval NF. Successful management of massive air Embolism during Cardiopulmonary Bypass using Multimodal Neuroprotection Strategies. Neurohospitalist. 2015;5(3):159–63. [DOI] [PubMed] [Google Scholar]
- 10.Guy TS, Kelly MP, Cason B, Tseng E. Retrograde cerebral perfusion and delayed hyperbaric oxygen for massive air embolism during cardiac surgery. Perfusion. 2008;23(6):383–6. [DOI] [PubMed] [Google Scholar]
- 11.Gadhinglajkar SV, Sankarkumar R, Rupa S. Retrograde cerebral perfusion for treatment of air embolism after valve surgery. Asian Cardiovasc Thorac Ann. 2004;12(1):81–2. [DOI] [PubMed] [Google Scholar]
- 12.Noel L, Mills MD, Ochsner JL. Massive air embolism during cardiopulmonary bypass: causes, prevention, and management. J THoRAc CARDIOVASC SURG. 1980;80:708–17. [PubMed] [Google Scholar]
- 13.Fakkert RA, Karlas N, Schober P. Early hyperbaric oxygen therapy is associated with favorable outcome in patients with iatrogenic cerebral arterial gas embolism: systematic review and individual patient data meta-analysis of observational studies. BMC Neurol. 2021;21(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niyibizi E, Kembi GE, Lae C, Pignel R, Sologashvili T. Delayed hyperbaric oxygen therapy for air emboli after open heart surgery: case report and review of a success story. J Cardiothorac Surg. 2016;11(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.