Abstract
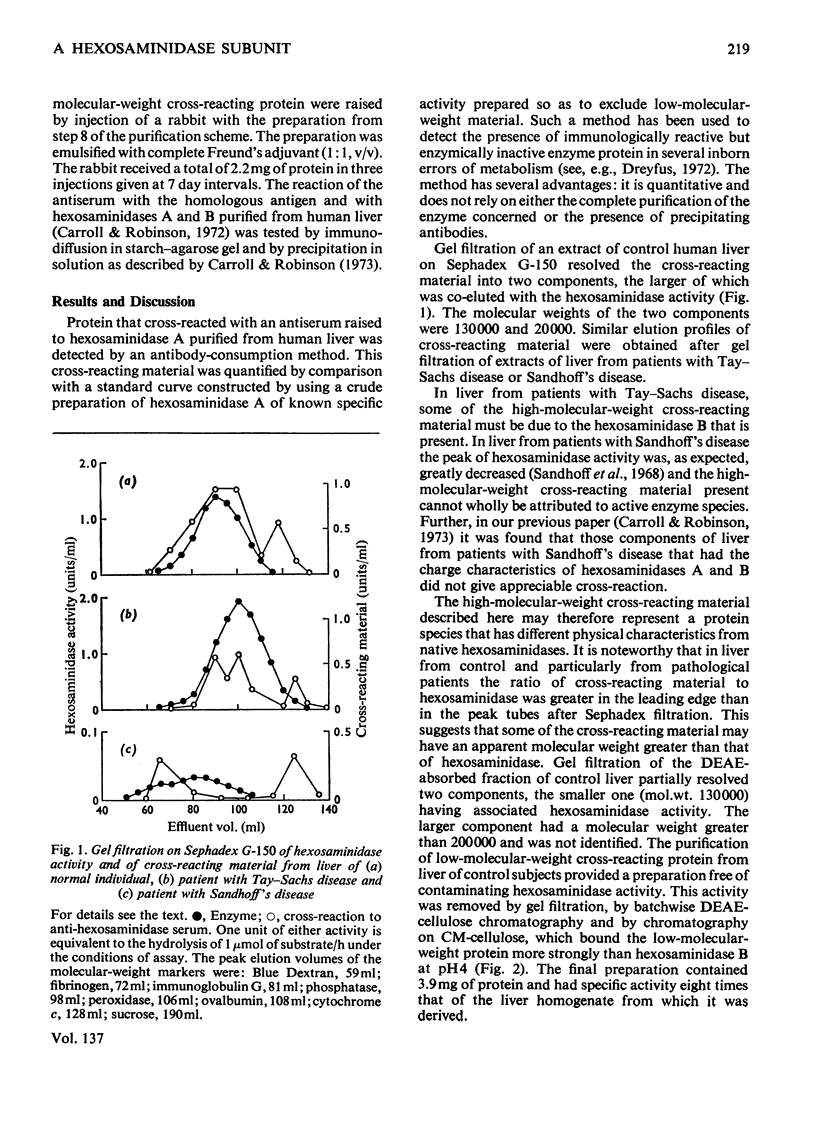
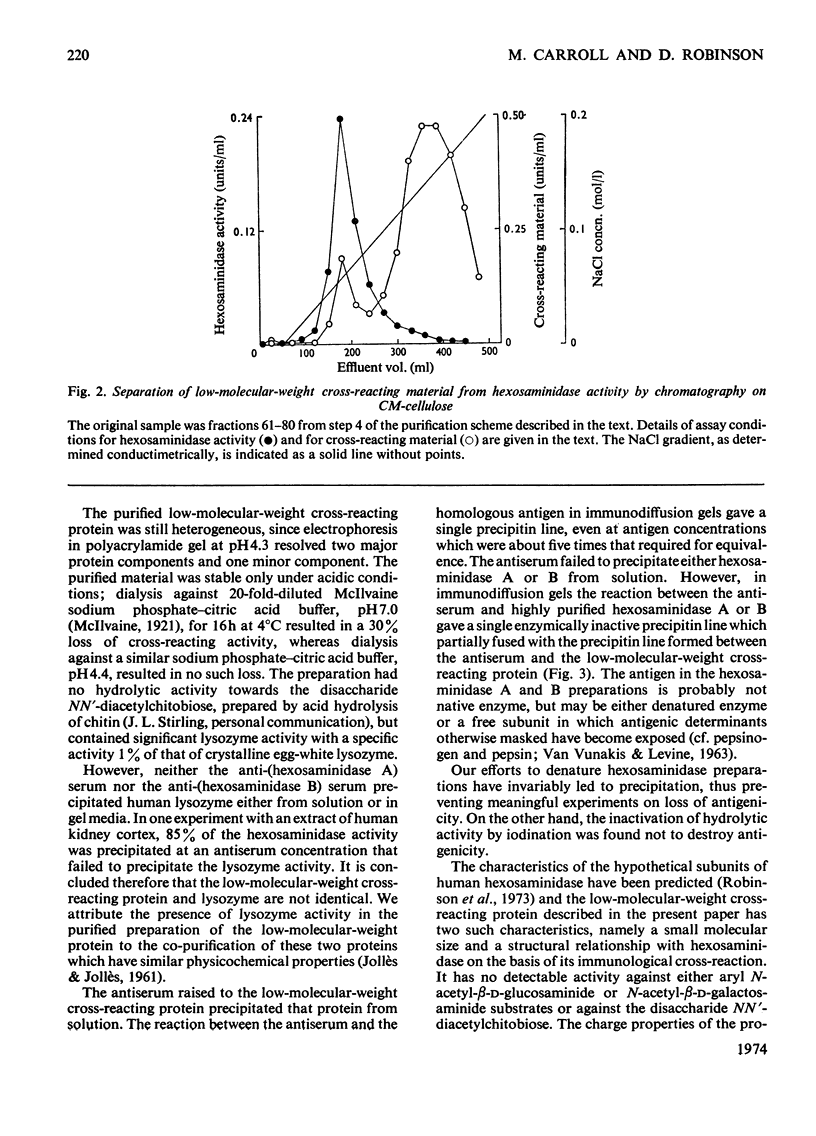
Antisera were raised to preparations of hexosaminidase isoenzymes A and B purified from human liver. Protein that cross-reacted with the liver hexosaminidase was detected by an antibody-consumption method. A cross-reacting protein with a low molecular weight (20000) was partially characterized and purified from control human liver. This protein is also present in the liver of patients with Tay–Sachs disease or with Sandhoff's disease. Hexosaminidases A and B gave an immunological reaction of partial identity with the low-molecular-weight protein. The possible identity of the low-molecular-weight cross-reacting protein as a subunit of hexosaminidase is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carroll M., Robinson D. Immunological properties of N-acetyl-beta-D-glucosaminidase of normal human liver and of GM2-gangliosidosis liver. Biochem J. 1973 Jan;131(1):91–96. doi: 10.1042/bj1310091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus J. C. Bases moléculaires des maladies enzymatiques génétiques. Biochimie. 1972;54(5):559–571. [PubMed] [Google Scholar]
- FERRARI R., MATRACIA S. Interaction between lysozyme and a phospholipid of neoplastic tissue (oncolipin). Nature. 1961 Dec 23;192:1187–1187. doi: 10.1038/1921187a0. [DOI] [PubMed] [Google Scholar]
- Harrison J. F., Lunt G. S., Scott P., Blainey J. D. Urinary lysozyme, ribonuclease, and low-molecular-weight protein in renal disease. Lancet. 1968 Feb 24;1(7539):371–375. doi: 10.1016/s0140-6736(68)91350-0. [DOI] [PubMed] [Google Scholar]
- Harzer K., Sandhoff K. Age-dependent variations of the human N-acetyl- -D-hexosaminidases. J Neurochem. 1971 Nov;18(11):2041–2050. doi: 10.1111/j.1471-4159.1971.tb05063.x. [DOI] [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Robinson D., Carrol M., Stirling J. L. Identification of a possible subunit of hexosaminidase A and B. Nature. 1973 Jun 15;243(5407):415–416. doi: 10.1038/243415a0. [DOI] [PubMed] [Google Scholar]
- Robinson D., Carroll M. Tay-Sachs disease: interrelation of hexosaminidases A and B. Lancet. 1972 Feb 5;1(7745):322–323. doi: 10.1016/s0140-6736(72)90332-7. [DOI] [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff K., Andreae U., Jatzkewitz H. Deficient hexozaminidase activity in an exceptional case of Tay-Sachs disease with additional storage of kidney globoside in visceral organs. Life Sci. 1968 Mar 15;7(6):283–288. doi: 10.1016/0024-3205(68)90024-6. [DOI] [PubMed] [Google Scholar]
- Sandhoff K. Variation of beta-N-acetylhexosaminidase-pattern in Tay-Sachs disease. FEBS Lett. 1969 Aug;4(4):351–354. doi: 10.1016/0014-5793(69)80274-7. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Wässle W. Anreicherung und Charakterisierung zweier Formen der menschlichen N-acetyl- -D-hexosaminidase. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1119–1133. [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Antibody against purified human hexosaminidase B cross-reacting with human hexosaminidase A. Biochem Biophys Res Commun. 1972 May 26;47(4):753–759. doi: 10.1016/0006-291x(72)90556-6. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Hexosaminidase-A and hexosaminidase-B: studies in Tay-Sachs' and Sandhoff's disease. Nature. 1973 Feb 16;241(5390):463–463. doi: 10.1038/241463a0. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O. The catabolism of Tay-Sachs ganglioside in rat brain lysosomes. J Biol Chem. 1972 Dec 10;247(23):7570–7575. [PubMed] [Google Scholar]
- Tallman J. F., Johnson W. G., Brady R. O. The metabolism of Tay-Sachs ganglioside: catabolic studies with lysosomal enzymes from normal and Tay-Sachs brain tissue. J Clin Invest. 1972 Sep;51(9):2339–2345. doi: 10.1172/JCI107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN VUNAKIS H., LEVINE L. Structural studies on pepsinogen and pepsin: an immunological approach. Ann N Y Acad Sci. 1963 May 8;103:735–743. doi: 10.1111/j.1749-6632.1963.tb53730.x. [DOI] [PubMed] [Google Scholar]
- Wenger D. A., Okada S., O'Brien J. S. Studies on the substrate specificity of hexosaminidase A and B from liver. Arch Biochem Biophys. 1972 Nov;153(1):116–129. doi: 10.1016/0003-9861(72)90427-4. [DOI] [PubMed] [Google Scholar]


