Abstract
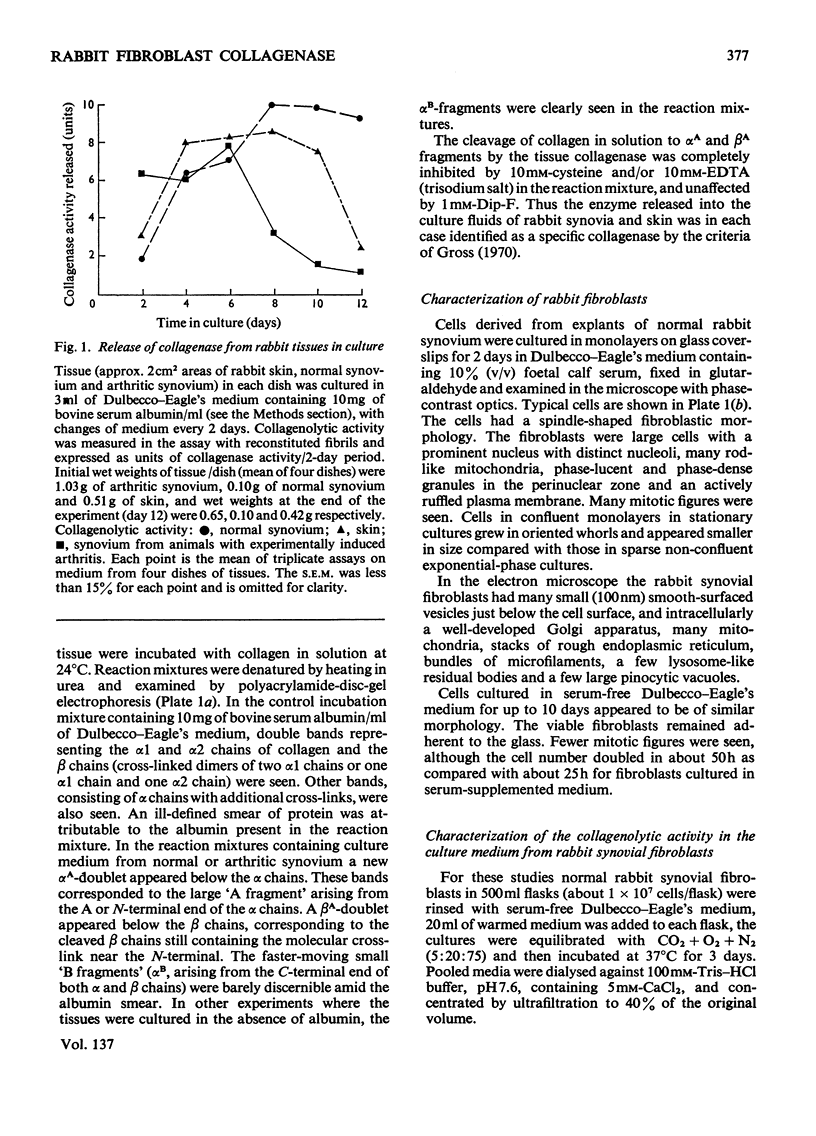
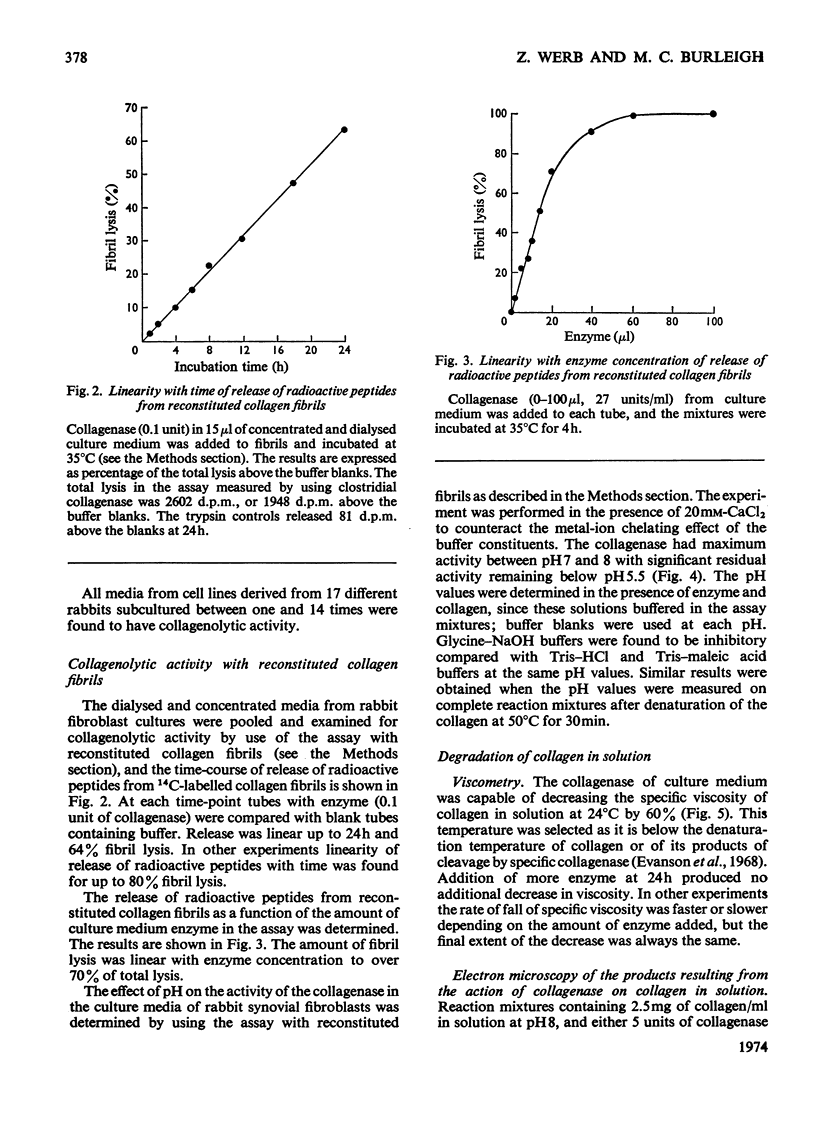
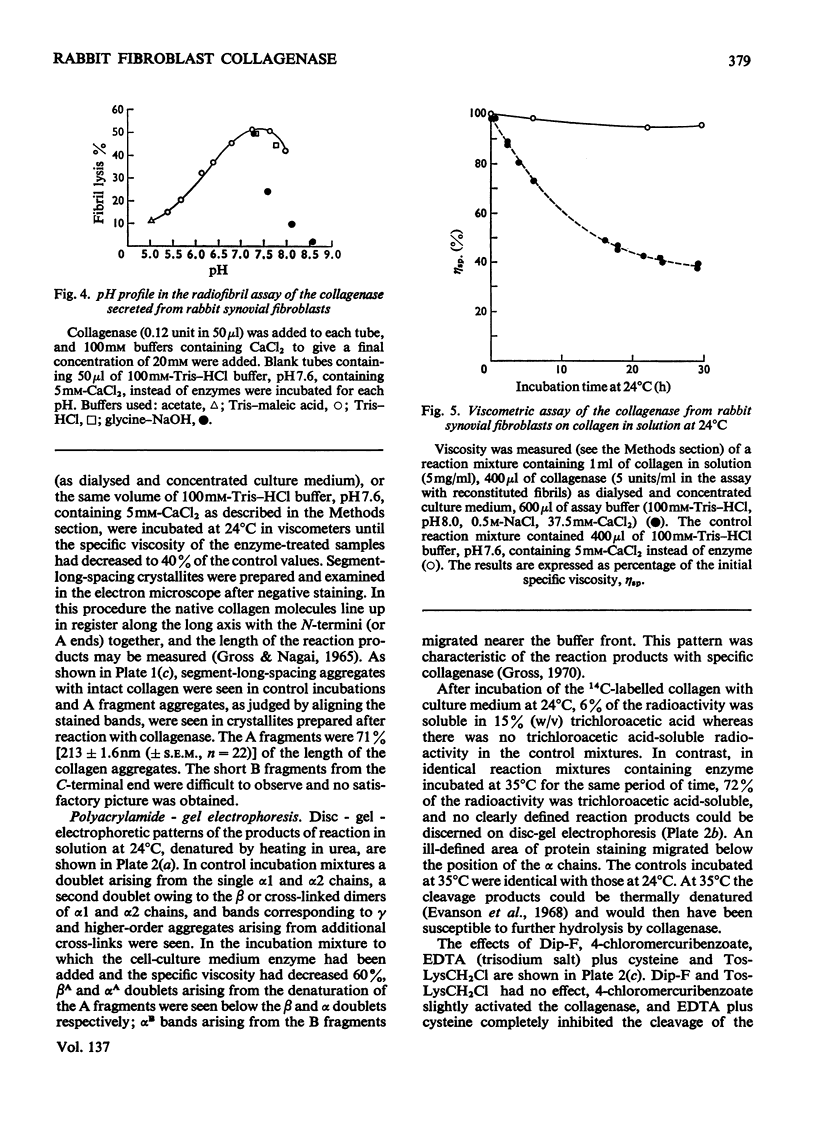
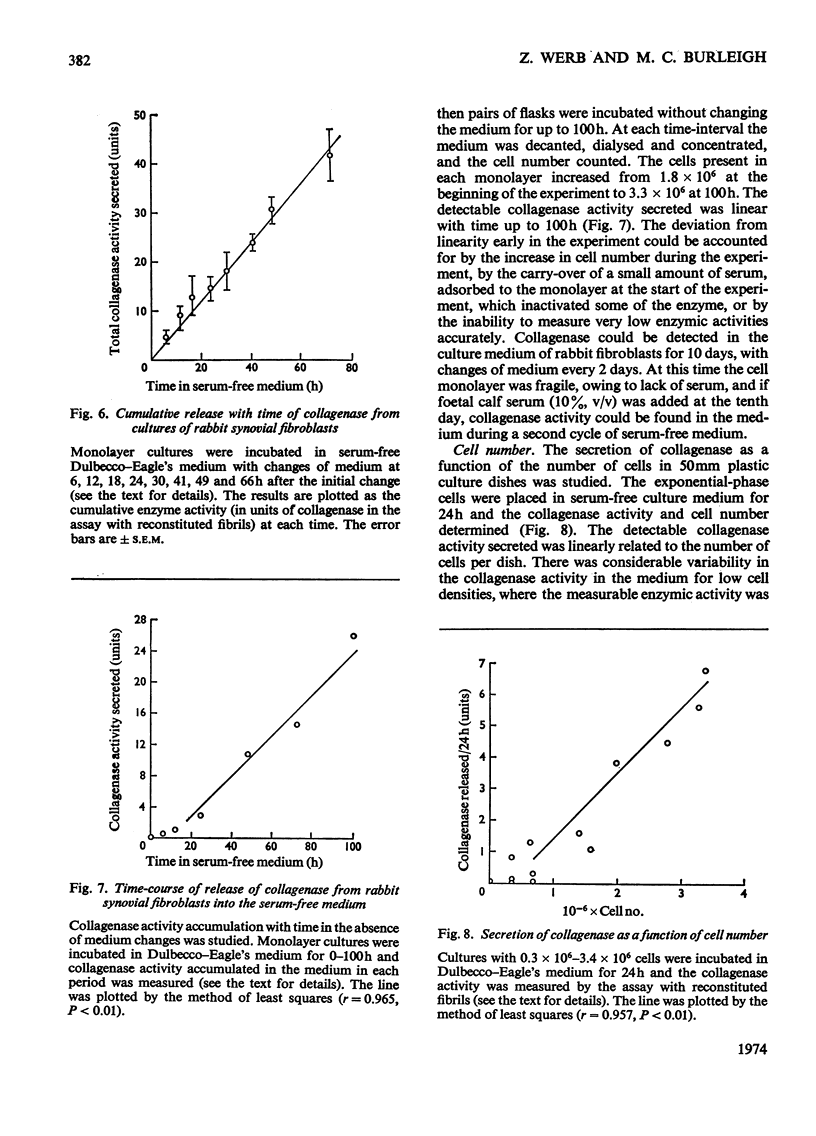
1. Explants of rabbit skin and synovium in tissue culture secreted a specific collagenase into their culture media. Primary cultures of fibroblast-like cells, which were obtained from these tissues and maintained in culture for up to 14 subculture passages, also secreted high activities of a specific collagenase into serum-free culture medium. Secretion of enzyme activity from the cell monolayer was at constant rate for over 100h and continued for up to 8 days in serum-free culture medium. The enzymic activity released was proportional to the number of cells in the monolayer. 2. The fibroblast collagenase was maximally active between pH7 and 8. At 24°C the collagenase decreased the viscosity of collagen in solution by 60%. The collagen molecule was cleaved into three-quarters and one-quarter length fragments as demonstrated by electron microscopy of segment-long-spacing crystallites (measured as native collagen molecules aligned with N-termini together along the long axis), and by polyacrylamide-gel electrophoresis of the denatured products. The collagenase hydrolysed insoluble collagen, reconstituted collagen fibrils and gelatin, but had no effect on haemoglobin or Pz-Pro-Leu-Gly-Pro-d-Arg (where Pz=4-phenylazobenzyloxycarbonyl). 3. The fibroblast collagenase was partially purified by gel filtration and the molecular weight was estimated as 38000. The activity of the partially purified enzyme was stimulated by 4-chloromercuribenzoate, inhibited by EDTA, cysteine, 1,10-phenanthroline and serum, but was unaffected by di-isopropyl phosphorofluoridate, Tos-LysCH2Cl and pepstatin. 4. Long-term cell cultures originating from rabbit skin or synovium from rabbits with experimentally induced arthritis also secreted specific collagenase. Human fibroblasts released only very small amounts of collagenase.
Full text
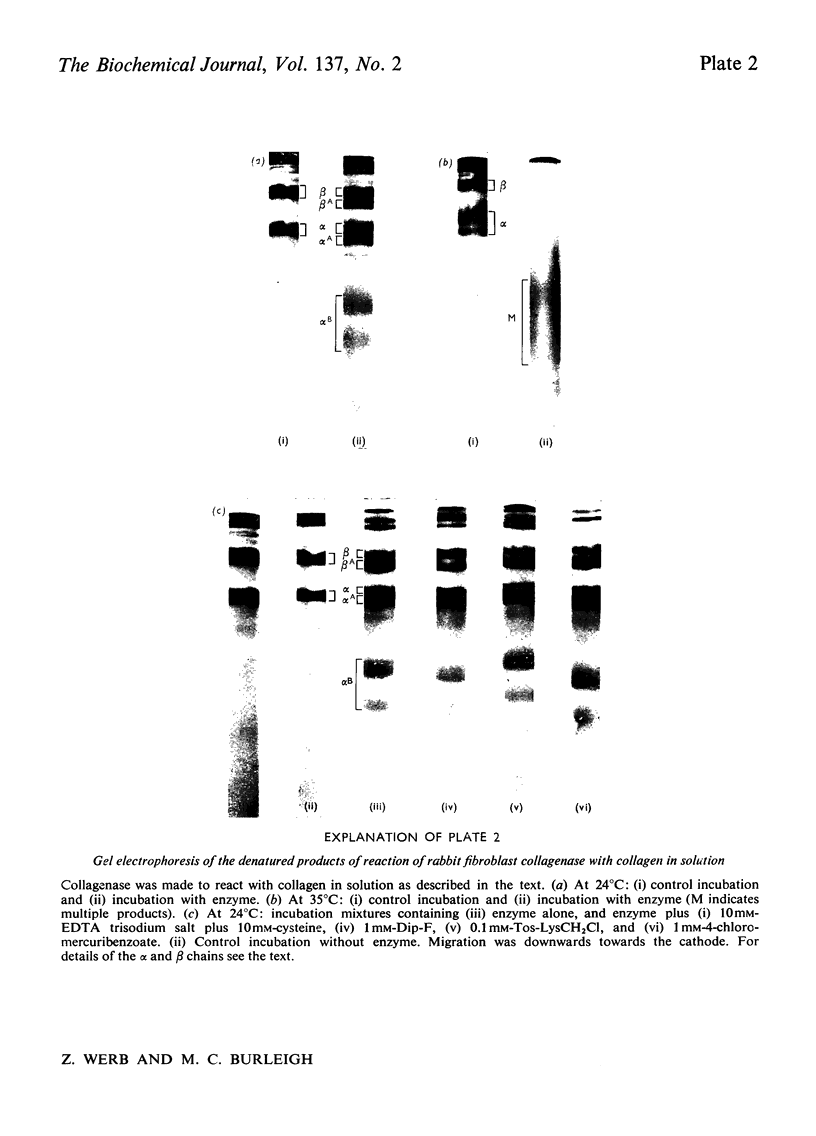
PDF



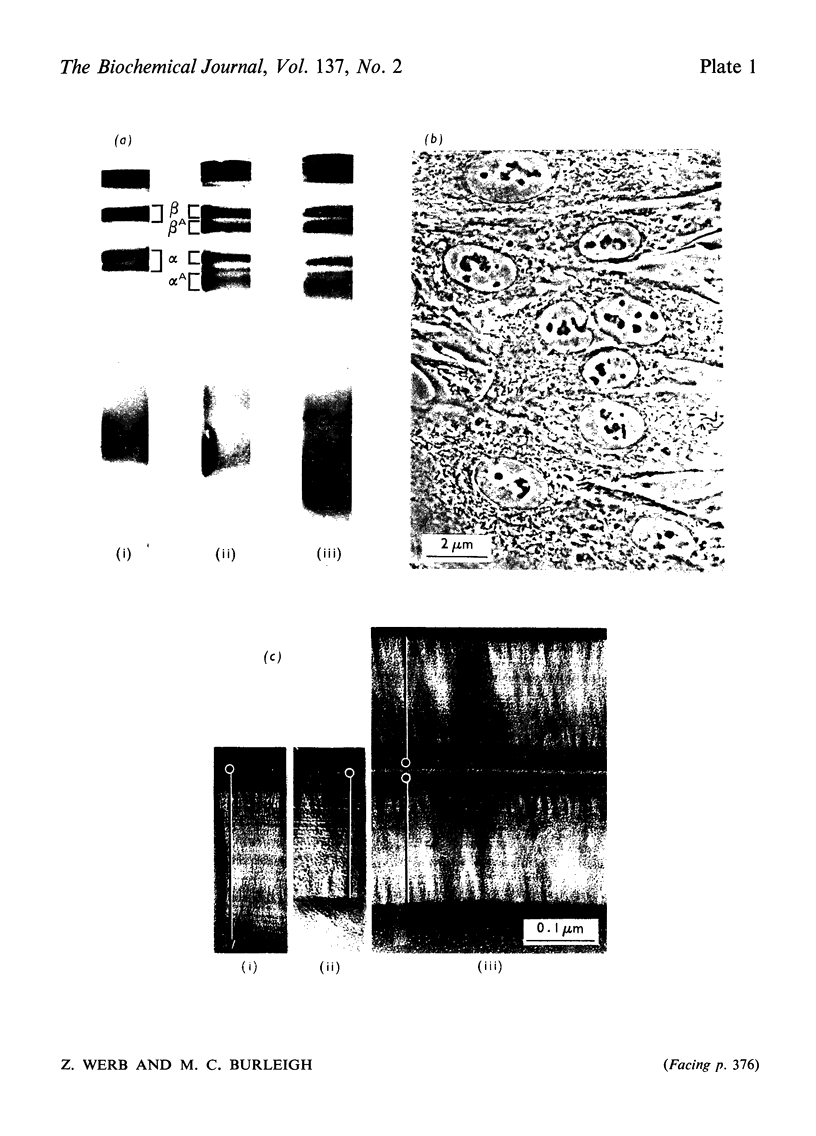





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F. Sequential degranulation of the two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J Cell Biol. 1973 Aug;58(2):249–264. doi: 10.1083/jcb.58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Dingle J. T. The inhibition of tissue acid proteinases by pepstatin. Biochem J. 1972 Apr;127(2):439–441. doi: 10.1042/bj1270439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh M. C., Barrett A. J., Lazarus G. S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J. 1974 Feb;137(2):387–398. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoff R. B., McLennan J. E., Grillo H. C. Preparation and properties of collagenases from epithelium and mesenchyme of healing mammalian wounds. Biochim Biophys Acta. 1971 Mar 10;227(3):639–653. doi: 10.1016/0005-2744(71)90014-3. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z., Bauer E. A., Jeffrey J. J. Animal and human collagenases. J Invest Dermatol. 1970 Dec;55(6):359–373. doi: 10.1111/1523-1747.ep12260483. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z., Jeffrey J. J., Gross J. Human skin collagenase. Isolation and mechanism of attack on the collagen molecule. Biochim Biophys Acta. 1968 Mar 25;151(3):637–645. doi: 10.1016/0005-2744(68)90010-7. [DOI] [PubMed] [Google Scholar]
- Evanson J. M., Jeffrey J. J., Krane S. M. Studies on collagenase from rheumatoid synovium in tissue culture. J Clin Invest. 1968 Dec;47(12):2639–2651. doi: 10.1172/JCI105947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., LAPIERE C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY B. S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Janis R., Smith C. Cartilage matrix depletion by rheumatoid synovial cells in tissue culture. J Exp Med. 1967 Dec 1;126(6):1005–1012. doi: 10.1084/jem.126.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E., Bloch K. J., Gross J. The zymogen of tadpole collagenase. Biochemistry. 1971 Aug 3;10(16):3035–3041. doi: 10.1021/bi00792a008. [DOI] [PubMed] [Google Scholar]
- Harper E., Gross J. Collagenase, procollagenase and activator relationships in tadpole tissue cultures. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1147–1152. doi: 10.1016/0006-291x(72)90830-3. [DOI] [PubMed] [Google Scholar]
- Harper E., Gross J. Separation of collagenase and peptidase activities of tadpole tissues in culture. Biochim Biophys Acta. 1970 Feb 11;198(2):286–292. doi: 10.1016/0005-2744(70)90061-6. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr A collagenolytic system produced by primary cultures of rheumatoid nodule tissue. J Clin Invest. 1972 Nov;51(11):2973–2976. doi: 10.1172/JCI107122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, DiBona D. R., Krane S. M. Collagenases in human synovial fluid. J Clin Invest. 1969 Nov;48(11):2104–2113. doi: 10.1172/JCI106177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Faulkner C. S., 2nd, Wood S., Jr Collagenase in carcinoma cells. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1247–1253. doi: 10.1016/0006-291x(72)90845-5. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. An endopeptidase from rheumatoid synovial tissue culture. Biochim Biophys Acta. 1972 Feb 28;258(2):566–576. doi: 10.1016/0005-2744(72)90249-5. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Effects of colchicine on collagenase in cultures of rheumatoid synovium. Arthritis Rheum. 1971 Nov-Dec;14(6):669–684. doi: 10.1002/art.1780140602. [DOI] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- Hook C. W., Brown S. I., Iwanij W., Nakanishi I. Characterization and inhibition of corneal collagenase. Invest Ophthalmol. 1971 Jul;10(7):496–503. [PubMed] [Google Scholar]
- Jeffrey J. J., Gross J. Collagenase from rat uterus. Isolation and partial characterization. Biochemistry. 1970 Jan 20;9(2):268–273. doi: 10.1021/bi00804a012. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Nagai Y., Piez K. A., Gross J. Studies on the structure of collagen utilizing a collagenolytic enzyme from tadpole. Biochemistry. 1966 Feb;5(2):509–515. doi: 10.1021/bi00866a016. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Brown R. S., Daniels J. R., Fullmer H. M. Human granulocyte collagenase. Science. 1968 Mar 29;159(3822):1483–1485. doi: 10.1126/science.159.3822.1483. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Lian J., Burleigh M. C. Role of granulocyte collagenase in collagen degradation. Am J Pathol. 1972 Sep;68(3):565–578. [PMC free article] [PubMed] [Google Scholar]
- Lazarus G. S., Fullmer H. M. Collagenase production by human dermis in vitro. J Invest Dermatol. 1969 Jun;52(6):545–547. doi: 10.1038/jid.1969.94. [DOI] [PubMed] [Google Scholar]
- NAGAI Y., GROSS J., PIEZ K. A. DISC ELECTROPHORESIS OF COLLAGEN COMPONENTS. Ann N Y Acad Sci. 1964 Dec 28;121:494–500. doi: 10.1111/j.1749-6632.1964.tb14221.x. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Hori H. Vertebrate collagenase: direct extraction from animal skin and human synovial membrane. J Biochem. 1972 Nov;72(5):1147–1153. doi: 10.1093/oxfordjournals.jbchem.a130002. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Dingle J. T., Barrett A. J. The immunocytochemical demonstration of cathepsin D. J Histochem Cytochem. 1972 Apr;20(4):261–265. doi: 10.1177/20.4.261. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Tuhy P. M. Active-site specific inhibitors of elastase. J Am Chem Soc. 1972 Sep 6;94(8):6544–6545. doi: 10.1021/ja00773a049. [DOI] [PubMed] [Google Scholar]
- Pérez-Tamayo R. Collagen resorption in carrageenin granulomas. I. Collagenolytic activity in in vitro explants. Lab Invest. 1970 Feb;22(2):137–141. [PubMed] [Google Scholar]
- Reynolds J. M. Motivating office personnel for increased efficiency. Trans Eur Orthod Soc. 1973:379–385. [PubMed] [Google Scholar]
- Robertson P. B., Ryel R. B., Taylor R. E., Shyu K. W., Fullmer H. M. Collagenase: localization in polymorphonuclear leukocyte granules in the rabbit. Science. 1972 Jul 7;177(4043):64–65. doi: 10.1126/science.177.4043.64. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Glimcher M. J., Travis D., Goldhaber P. Mouse bone collagenase: isolation, partial purification, and mechanism of action. Proc Soc Exp Biol Med. 1969 Apr;130(4):1175–1180. doi: 10.3181/00379727-130-33747. [DOI] [PubMed] [Google Scholar]
- Tokoro Y., Eisen A. Z., Jeffrey J. J. Characterization of a collagenase from rat skin. Biochim Biophys Acta. 1972 Jan 20;258(1):289–302. doi: 10.1016/0005-2744(72)90986-2. [DOI] [PubMed] [Google Scholar]
- Vaes G. The release of collagenase as an inactive proenzyme by bone explants in culture. Biochem J. 1972 Jan;126(2):275–289. doi: 10.1042/bj1260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WUENSCH E., HEIDRICH H. G. ZUR QUANTITATIVEN BESTIMMUNG DER KOLLAGENASE. Hoppe Seylers Z Physiol Chem. 1963;333:149–151. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]
- ZUCKER-FRANKLIN D., HIRSCH J. G. ELECTRON MICROSCOPE STUDIES ON THE DEGRANULATION OF RABBIT PERITONEAL LEUKOCYTES DURING PHAGOCYTOSIS. J Exp Med. 1964 Oct 1;120:569–576. doi: 10.1084/jem.120.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]




