Abstract
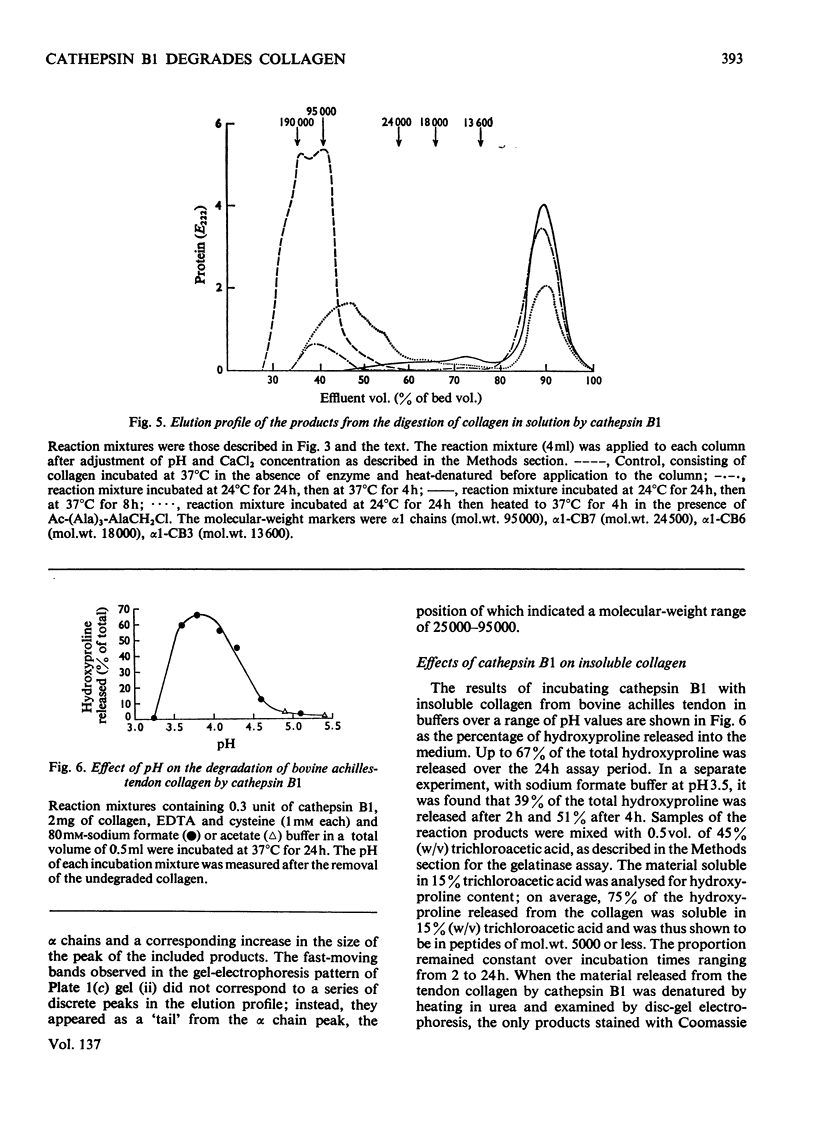
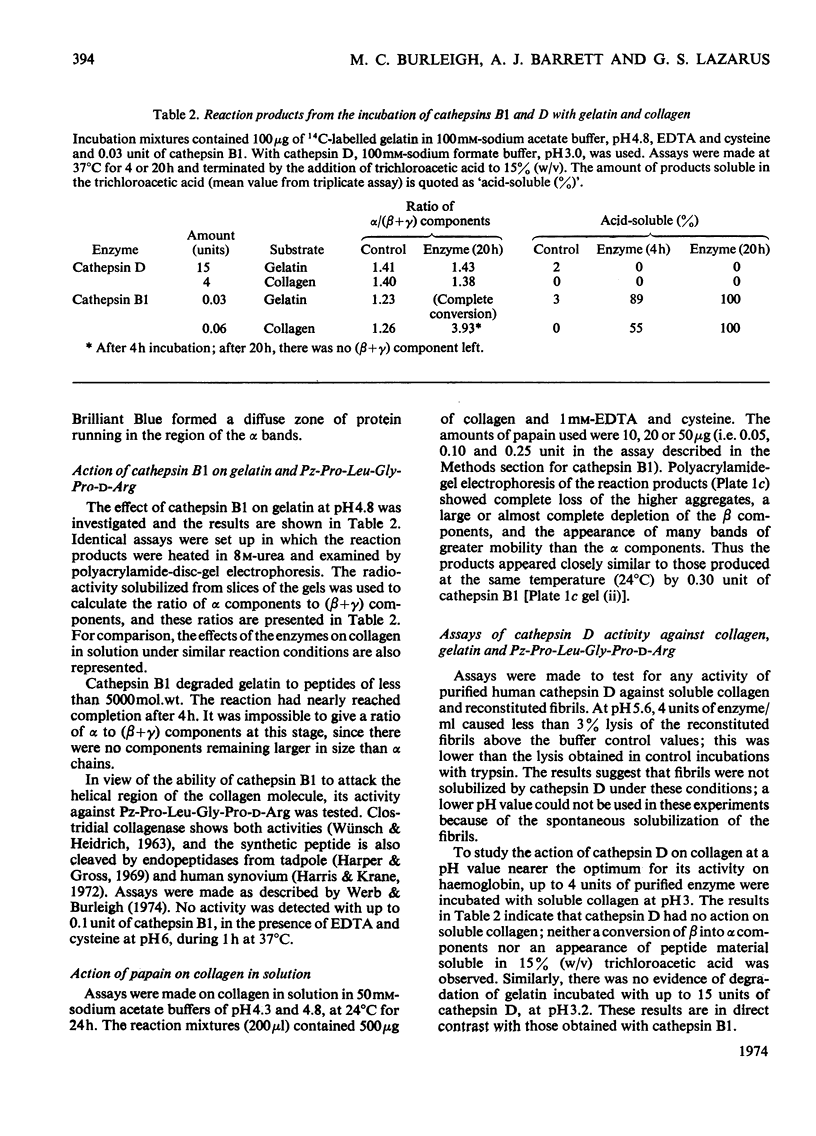
1. Experiments were made to determine whether the purified lysosomal proteinases, cathepsins B1 and D, degrade acid-soluble collagen in solution, reconstituted collagen fibrils, insoluble collagen or gelatin. 2. At acid pH values cathepsin B1 released 14C-labelled peptides from collagen fibrils reconstituted at neutral pH from soluble collagen. The purified enzyme required activation by cysteine and EDTA and was inhibited by 4-chloromercuribenzoate, by the chloromethyl ketones derived from tosyl-lysine and acetyltetra-alanine and by human α2-macroglobulin. 3. Cathepsin B1 degraded collagen in solution, the pH optimum being pH4.5–5.0. The initial action was cleavage of the non-helical region containing the cross-link; this was seen as a decrease in viscosity with no change in optical rotation. The enzyme also attacked the helical region of collagen by a mechanism different from that of mammalian neutral collagenase. No discrete intermediate products of a specific size were observed in segment-long-spacing crystalloids (measured as native collagen molecules aligned with N-termini together along the long axis) or as separate peaks on gel filtration chromatography. This suggests that once an α-chain was attacked it was rapidly degraded to low-molecular-weight peptides. 4. Cathepsin B1 degraded insoluble collagen with a pH optimum below 4; this value is lower than that found for the soluble substrate, and a possible explanation is given. 5. The lysosomal carboxyl proteinase, cathepsin D, had no action on collagen or gelatin at pH3.0. Neither cathepsin B1 nor D cleaved Pz-Pro-Leu-Gly-Pro-d-Arg. 6. Cathepsin B1 activity was shown to be essential for the degradation of collagen by lysosomal extracts. 7. Cathepsin B1 may provide an alternative route for collagen breakdown in physiological and pathological situations.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J. Effects of lysosomal collagenolytic enzymes, anti-inflammatory drugs and other substances on some properties of insoluble collagen. Biochem J. 1969 Jul;113(3):457–463. doi: 10.1042/bj1130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAZIN S., DELAUNAY A. ACTIVIT'E COLLAG'ENASIQUE DE CERTAINS EXTRAITS H'EPATIQUES. SES VARIATIONS CHEZ LE RAT INTOXIQU'E PAR LE T'ETRACHLORURE DE CARBONE. Ann Inst Pasteur (Paris) 1964 Apr;106:543–552. [PubMed] [Google Scholar]
- Barrett A. J. A new assay for cathepsin B1 and other thiol proteinases. Anal Biochem. 1972 May;47(1):280–293. doi: 10.1016/0003-2697(72)90302-8. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Dingle J. T. The inhibition of tissue acid proteinases by pepstatin. Biochem J. 1972 Apr;127(2):439–441. doi: 10.1042/bj1270439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin S., Delaunay A. Caractères de cathepsines collagénolytiques présentes dans les tissus enflammés du rat. Ann Inst Pasteur (Paris) 1966 Feb;110(2):192–204. [PubMed] [Google Scholar]
- Becker U., Timpl R., Kühn K. Carboxyterminal antigenic determinants of collagen from calf skin. Localization within discrete regions of the nonhelical sequence. Eur J Biochem. 1972 Jul 13;28(2):221–231. doi: 10.1111/j.1432-1033.1972.tb01905.x. [DOI] [PubMed] [Google Scholar]
- Brandes D., Anton E. Lysosomes in uterine involution: intracytoplasmic degradation of myofilaments and collagen. J Gerontol. 1969 Jan;24(1):55–69. doi: 10.1093/geronj/24.1.55. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Piez K. A., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha-1 chain of rat skin collagen. Biochemistry. 1967 Dec;6(12):3771–3780. doi: 10.1021/bi00864a022. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison P. F., Drake M. P. The physical characterization of monomeric tropocollagen. Biochemistry. 1966 Jan;5(1):313–321. doi: 10.1021/bi00865a040. [DOI] [PubMed] [Google Scholar]
- Drake M. P., Davison P. F., Bump S., Schmitt F. O. Action of proteolytic enzymes on tropocollagen and insoluble collagen. Biochemistry. 1966 Jan;5(1):301–312. doi: 10.1021/bi00865a039. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z., Bauer E. A., Jeffrey J. J. Animal and human collagenases. J Invest Dermatol. 1970 Dec;55(6):359–373. doi: 10.1111/1523-1747.ep12260483. [DOI] [PubMed] [Google Scholar]
- Etherington D. J. Collagenolytic-cathepsin and acid-proteinase activities in the rat uterus during post partum involution. Eur J Biochem. 1973 Jan 3;32(1):126–128. doi: 10.1111/j.1432-1033.1973.tb02587.x. [DOI] [PubMed] [Google Scholar]
- Etherington D. J. The nature of the collagenolytic cathepsin of rat liver and its distribution in other rat tissues. Biochem J. 1972 May;127(4):685–692. doi: 10.1042/bj1270685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson J. M., Jeffrey J. J., Krane S. M. Studies on collagenase from rheumatoid synovium in tissue culture. J Clin Invest. 1968 Dec;47(12):2639–2651. doi: 10.1172/JCI105947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLAND D. M., WYNN C. H. The degradation of acidsoluble collagen by rat-liver preparations. Biochem J. 1962 Nov;85:276–282. doi: 10.1042/bj0850276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Fell H. B., Dingle J. T. Endocytosis of sugars in embryonic skeletal tissues in organ culture. II. Effect of sucrose on cellular fine structure. J Cell Sci. 1969 Jan;4(1):105–131. doi: 10.1242/jcs.4.1.105. [DOI] [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E., Gross J. Separation of collagenase and peptidase activities of tadpole tissues in culture. Biochim Biophys Acta. 1970 Feb 11;198(2):286–292. doi: 10.1016/0005-2744(70)90061-6. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, DiBona D. R., Krane S. M. Collagenases in human synovial fluid. J Clin Invest. 1969 Nov;48(11):2104–2113. doi: 10.1172/JCI106177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. An endopeptidase from rheumatoid synovial tissue culture. Biochim Biophys Acta. 1972 Feb 28;258(2):566–576. doi: 10.1016/0005-2744(72)90249-5. [DOI] [PubMed] [Google Scholar]
- Huisman W., Bouma J. M., Gruber M. Involvement of thiol enzymes in the lysosomal breakdown of native and denatured proteins. Biochim Biophys Acta. 1973 Jan 24;297(1):98–109. doi: 10.1016/0304-4165(73)90053-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
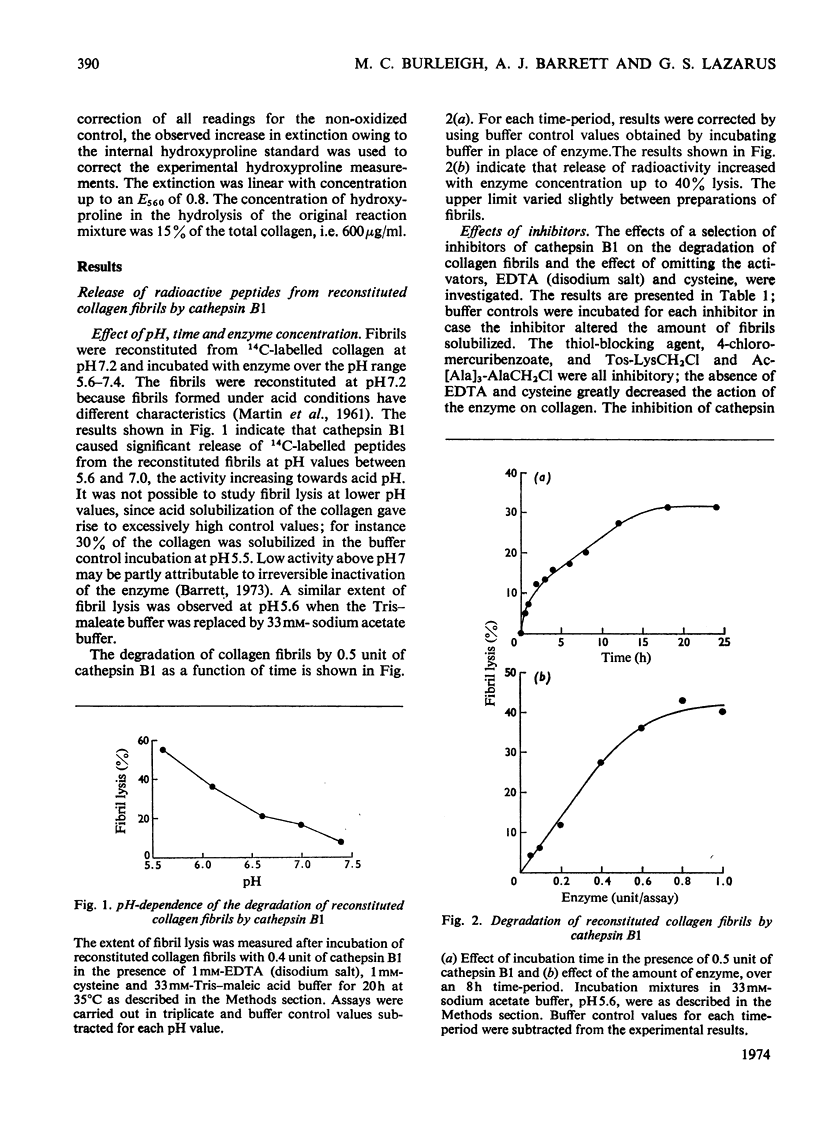
- MARTIN G. R., MERGENHAGEN S. E., SCOTT D. B. Relation of ionizing groups to the structure of the collagen fibril. Biochim Biophys Acta. 1961 May 13;49:245–250. doi: 10.1016/0006-3002(61)90123-8. [DOI] [PubMed] [Google Scholar]
- MORRIONE T. G., SEIFTER S. Alteration in the collagen content of the human uterus during pregnancy and post partum involution. J Exp Med. 1962 Feb 1;115:357–365. doi: 10.1084/jem.115.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. I., Barrett A. J., Dingle J. T., Prior D. Cathepsins BI and D. Action on human cartilage proteoglycans. Biochim Biophys Acta. 1973 Apr 12;302(2):411–419. doi: 10.1016/0005-2744(73)90170-8. [DOI] [PubMed] [Google Scholar]
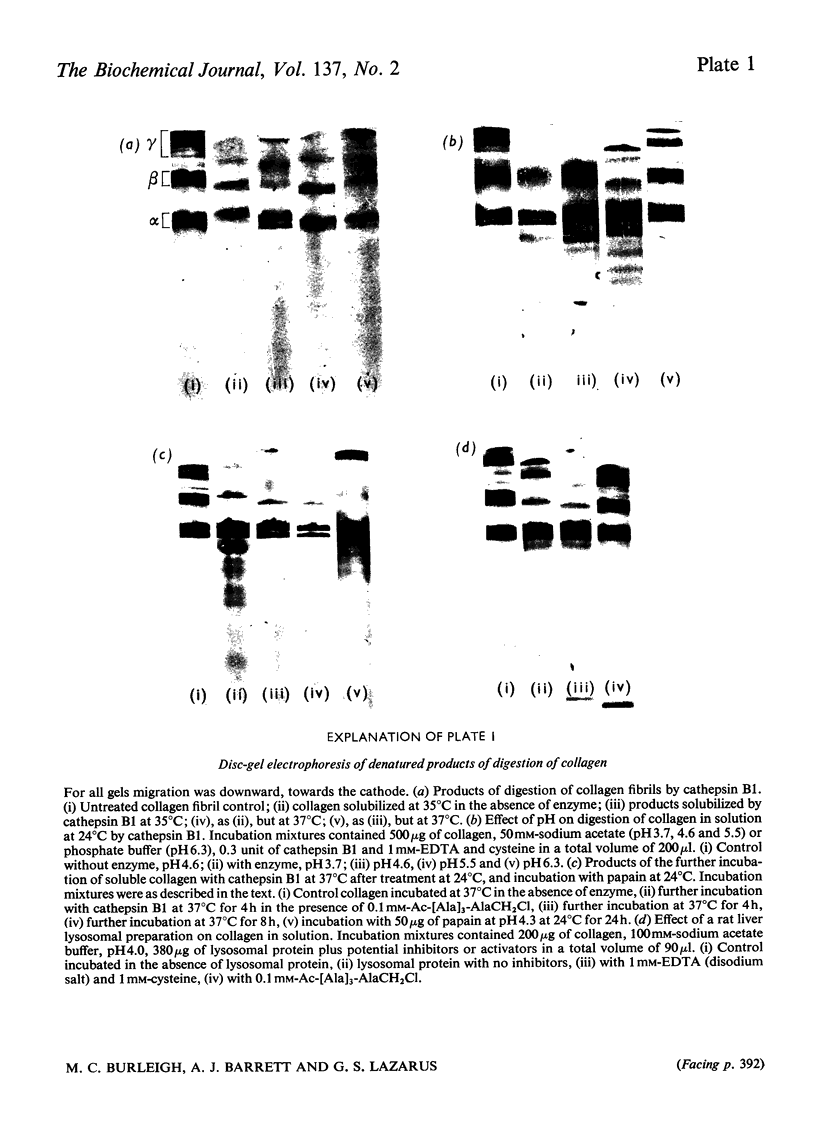
- NAGAI Y., GROSS J., PIEZ K. A. DISC ELECTROPHORESIS OF COLLAGEN COMPONENTS. Ann N Y Acad Sci. 1964 Dec 28;121:494–500. doi: 10.1111/j.1749-6632.1964.tb14221.x. [DOI] [PubMed] [Google Scholar]
- Parakkal P. F. Involvement of macrophages in collagen resorption. J Cell Biol. 1969 Apr;41(1):345–354. doi: 10.1083/jcb.41.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parakkal P. F. Macrophages: the time course and sequence of their distribution in the postpartum uterus. J Ultrastruct Res. 1972 Aug;40(3):284–291. doi: 10.1016/s0022-5320(72)90101-3. [DOI] [PubMed] [Google Scholar]
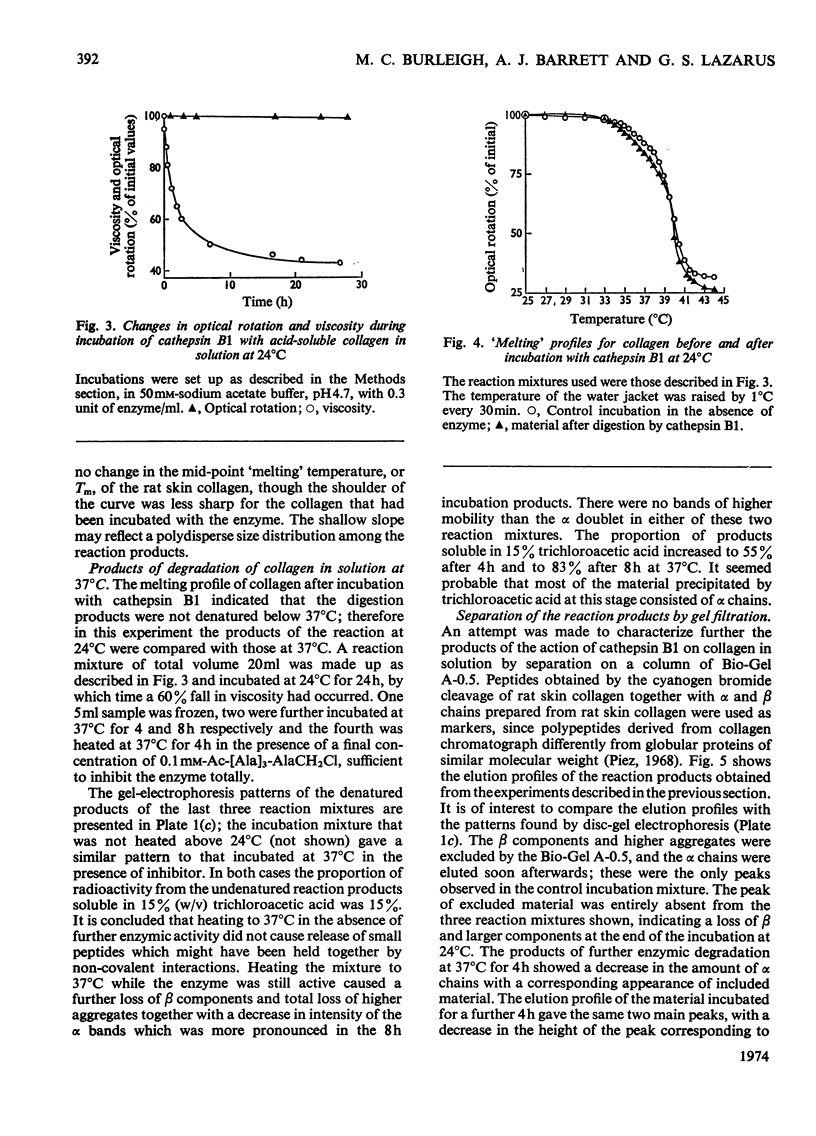
- Parakkal P. F. Role of macrophages in collagen resorption during hair growth cycle. J Ultrastruct Res. 1969 Nov;29(3):210–217. doi: 10.1016/s0022-5320(69)90101-4. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Molecular weight determination of random coil polypeptides from collagen by molecular sieve chromatography. Anal Biochem. 1968 Nov;26(2):305–312. doi: 10.1016/0003-2697(68)90342-4. [DOI] [PubMed] [Google Scholar]
- Pérez-Tamayo R. Collagen resorption in carrageenin granulomas. II. Ultrastructure of collagen resorption. Lab Invest. 1970 Feb;22(2):142–159. [PubMed] [Google Scholar]
- RUBIN A. L., PFAHL D., SPEAKMAN P. T., DAVISON P. F., SCHMITT F. O. Tropocollagen: significance of protease-induced alterations. Science. 1963 Jan 4;139(3549):37–39. doi: 10.1126/science.139.3549.37. [DOI] [PubMed] [Google Scholar]
- SHELDON H., KIMBALL F. B. Studies on cartilage. III. The occurrence of collagen within vacuoles of the golgi apparatus. J Cell Biol. 1962 Mar;12:599–613. doi: 10.1083/jcb.12.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVEN F. S. THE CLEAVAGE OF TYROSYL PEPTIDES BY PEPSIN FROM COLLAGEN SOLUBILISED BY THE NISHIHARA TECHNIQUE. Biochim Biophys Acta. 1965 Mar 8;97:465–471. doi: 10.1016/0304-4165(65)90158-3. [DOI] [PubMed] [Google Scholar]
- Sakai T., Gross J. Some properties of the products of reaction of tadpole collagenase with collagen. Biochemistry. 1967 Feb;6(2):518–528. doi: 10.1021/bi00854a021. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Inhibition by alpha-macroglobulin and other serum proteins. Biochem J. 1973 Apr;131(4):823–831. doi: 10.1042/bj1310823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz M., Timpl R., Kühn K. Non-helical regions in rat collagen 1-chain. FEBS Lett. 1972 Oct 1;26(1):61–65. doi: 10.1016/0014-5793(72)80542-8. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., BREWER T. H. FORMATION AND BREAKDOWN OF COLLAGEN AND ELASTIN IN THE HUMAN UTERUS DURING PREGNANCY AND POST-PARTUM INVOLUTION. Biochem J. 1963 Oct;89:75–82. doi: 10.1042/bj0890075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr Catabolism of collagen and non-collagen protein in the rat uterus during post-partum involution. Biochem J. 1962 May;83:304–314. doi: 10.1042/bj0830304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- WUENSCH E., HEIDRICH H. G. ZUR QUANTITATIVEN BESTIMMUNG DER KOLLAGENASE. Hoppe Seylers Z Physiol Chem. 1963;333:149–151. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C. A specific collagenase from rabbit fibroblasts in monolayer culture. Biochem J. 1974 Feb;137(2):373–385. doi: 10.1042/bj1370373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn C. H. Solubilization of insoluble collagens by rat liver lysosomes. Nature. 1967 Sep 9;215(5106):1191–1192. doi: 10.1038/2151191a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann B. K., Pikkarainen J., Fietzek P. P., Kühn K. Cross-linkages in collagen. Demonstration of three different intermolecular bonds. Eur J Biochem. 1970 Oct;16(2):217–225. doi: 10.1111/j.1432-1033.1970.tb01074.x. [DOI] [PubMed] [Google Scholar]