Abstract
Background
Magnetic resonance imaging (MRI) yields important information on the development and current status of many different diseases. Whole-body MRI was accordingly made a part of the multicenter, population-based NAKO Health Study. The present analysis concerns the feasibility of the baseline MRI examination and various aspects of quality assurance over the period 2014–2019.
Methods
32 252 participants in the NAKO Health Study, aged 20 to 74, who had no contraindication to MRI were invited to undergo scanning in one of five MRI study centers across Germany. The whole-body MRI scan took about one hour and consisted of sequences for the visualization of structural and functional features of the brain, musculoskeletal system, cardiovascular system, and thoracoabdominal system. A comprehensive quality-assurance assessment was carried out, with evaluation of adverse events, the completeness of the MRI protocols, the participants’ subjective perceptions, and image quality.
Results
31 578 participants (97.9%) were successfully included in the MRI study. They reported a high level of comfort and suffered no severe adverse events (mild adverse events occurred in only four participants). Depending on the imaging sequence, the image quality was rated as excellent in 80.2% to 96.8% of cases. Quality assessment with respect to structural features of the brain revealed high consistency across study centers, as well as with regard to age- and sex-based differences in brain volume (men, 1203.81 ± 102.06 cm³; women, 1068.10 ± 86.69 cm³).
Conclusion
Whole-body MRI was successfully implemented in the NAKO baseline examination and was associated with high patient comfort and very good image quality. The imaging biomarkers of the brain confirmed previously observed differences based on age and sex, underscoring the feasibility of data pooling.
The technological advances in magnetic resonance imaging (MRI) over the past few decades have given it a central role in modern medicine (1). Although specialized training, expertise, and a professional framework ere required (2, 3), this investment of resources is well justified in the age of personalized medicine. The excellent images obtained by MRI provide high-resolution delineation of tissue morphology and function and of potential disease states (4). Given these features, MRI is increasingly being used in research settings to throw more light on the development of common diseases (5, 6).
One of the largest population-based cohort studies in the European Union is the NAKO Health Study. As part of the NAKO baseline examination, a total of 205 415 participants were examined in 18 study centers across Germany between 2014 and 2019 (7, 8). They all underwent various examinations and were followed up prospectively over time. One of the most innovative aspects NAKO Health Study was the whole-body MRI examination, which was performed at five MRI study centers in a subset of over 30 000 participants (9). In order to maximize internal validity and also minimize potential bias between imaging and non-imaging parameters, the employed MR techniques and protocols were state-of-the-art and followed strict standard operating procedures. Various measures and checks were applied for quality assurance. Based on comprehensive ethical considerations (10), all images were read by board-certified radiologists for incidental findings in a standardized fashion (11–13).
While MRI is solidly established in the clinical context, it has not previously been used in a long-term multicenter population study has not been accomplished in Germany, and thus, the performance quality in this setting remains unknown. The aim of the present study was therefore to analyze the feasibility of whole-body MRI as part of the NAKO baseline examination, including participation and dropout rates, as well as participant comfort and image data quality.
Methods
Study design and population
The NAKO Health Study was designed as a prospective cohort study. A total of 205 415 participants aged 20–69 years, selected randomly from compulsory registries of residents within the study areas (8), underwent a highly standardized examination program (labeled L1) of 4 hours’ duration (7). Approximately 20% of all individuals, randomly selected prior to invitation, spent about an additional 1 hour undergoing further examinations, including more in-depth medical tests (labeled L2) (8).
As part of the MRI baseline examination, L2 participants from 11 of the 18 NAKO study centers were also invited to participate in a whole-body MRI examination. They were recruited at five imaging study centers (Augsburg, Berlin, Essen, Mannheim, Neubrandenburg) and 6 adjacent study centers (Berlin Central, Berlin South, Düsseldorf, Freiburg, Münster, Saarbrücken), from where they were sent to the nearest MRI study centers.
Persons were considered eligible if no contraindications were present and they were willing to participate in the MRI examination taking approximately 1 hour (for the exclusion criteria see eBox 1). The MRI study was approved by the Bavarian State Medical Association and the local ethics committees. Written informed consent was obtained from all participants prior to the examination.
eBox 1. Exclusion criteria for participation in whole-body MRI examination as part of the NAKO Health Study.
Cardiac pacemaker or intracardiac defibrillator
Medical foreign bodies not considered safe for 3-T MRI, e.g., cerebral aneurysm clip, cochlear implant, insulin pump, prosthetic cardiac valve
Other orthopedic implants not considered safe for 3-T MRI, including screws, plates, and joint prostheses, and/or metallic foreign bodies such as shrapnel or bullets
Surgical procedures in the head, abdomen, or back within 3 months prior to the MRI examination
Non-removable metallic body art
Tattoos (larger than the size of the participant’s palm or applied more than 20 years earlier) or make-up not considered safe for 3-T MRI (permanent/glossy make-up)
A possible ferromagnetic intrauterine pessary (e.g., one containing copper)
(Possible) pregnancy
Claustrophobia
Deafness
Inability to hold breath (for approximately 10 seconds) or lie supine (for approximately 1 hour)
No consent to be informed of potential incidental findings
Medical Resonance imaging program
MRI examinations were performed on five study-dedicated 3-T MR systems (MAGNETOM Skyra, Siemens Healthineers, Erlangen, Germany) with identical hardware and software components. The applied MRI program was also identical at all sites and was overseen by local board-certified radiologists. It comprised four organ areas: brain, musculoskeletal system, cardiovascular system, and thoracoabdominal system (9). Following the examination, participants were discharged without feedback (blinded) and the MR images were reviewed by board-certified radiologists for the presence of incidental findings according to a predefined list (14).
In order to assure high study and image quality, a so-called MRI Core of four centers was established to take responsibility for the planning, conduct, monitoring, and completion of all MRI-related study procedures. These centers were: University Hospital Freiburg (coordination and training), University Hospital Heidelberg (incidental findings), MEVIS Bremen (data management), and University Medical Center Greifswald (quality assurance).
Side effects, safety, and comfort
At each of the five MRI study centers, one dedicated radiologist monitored the various aspects of the MRI study. This included the clarification of exclusion criteria, dealing with questions that raised by participants, and providing any support necessary during the image acquisition process. Self-reported side effects and adverse events were documented prospectively by the MRI study centers.
Five surveys of satisfaction were carried out at 6-month intervals between fall 2016 and fall 2018. Each time, 100 questionnaires were distributed at each of the five study centers. Altogether, therefore, a subgroup of 2500 participants were asked about their satisfaction with the study program on a voluntary basis. The topics concerned were satisfaction with the consent process, the overall procedure at the MRI study center, and the duration of the MRI examination. Responses were given on a five- or three-point Likert scale.
MRI image quality
MR image quality was assessed subjectively by certified radiologists on a three-tier scale according to predefined quality criteria. Furthermore, a series of image-based quality measures were derived fully automatically (15), e.g., common values (signal-to-noise ratio, sharpness, etc.) and artifact- and protocol-specific parameters (16, 17). More details are provided in the eSupplement
Statistics
For the present analyses we used an exploratory approach without formal testing of hypotheses and without defining a formal level of statistical significance.
For further evaluation of image quality a state-of-the-art imaging pipeline was used that enabled estimation of morphometric parameters (total brain volume, white matter and gray matter volume, and cerebrospinal fluid volume) from T1w images. The images were processed using FreeSurfer (v7.1.1, recon-all pipeline for surface reconstruction [18, 19]) and CAT12.8 (20). The extracted variables were additionally corrected for height and weight and were classified by age, MRI study center, and biological sex.
We used SAS (Version 9.5) and NIST DataPlot (National Institute of Standards and Technology, Gaithersburg, MD, USA) for statistical analyses.
Results
A total of 32 252 participants were invited to attend one of the five MRI study centers for whole-body MRI examination. Of these, 641 participants (2.0%) were excluded from the study due to contraindications and 33 participants (0.1%) decided not to take part. Ultimately, 31 578 participants (97.9%) gave their written informed consent and were included for MRI examination. Table 1 shows the sample characteristics stratified by study site. Overall, a slight majority of participants were male (56.0%). The participants’ age on the day of the examination ranged from 20 to 74 years (49.0 ± 12.3 years).
Table 1. Demographic data of the participants in the whole-body MRI study in the NAKO Health Study.
| Variable | Total | Augsburg | Berlin | Essen | Mannheim | Neubrandenburg |
| n | 31 578 | 6417 | 5980 | 6155 | 6021 | 7005 |
| Women, n (%) | 13 885 (44.0%) | 2820 (43.9%) | 2574 (43.0%) | 2640 (42.9%) | 2543 (42.2%) | 3308 (47.2%) |
| Men, n (%) | 17 693 (56.0%) | 3597 (56.1%) | 3406 (57.0%) | 3515 (57.1%) | 3478 (57.8%) | 3697 (52.8%) |
| Age*1, mean (SD) | 49.0 (12.3) | 49.4 (12.2) | 47.8 (12.1) | 48.1 (12.6) | 48.4 (12.6) | 50.8 (11.5) |
| BMI*2, mean (SD) | 26.6 (4.79) | 26.7 (4.75) | 25.7 (4.43) | 26.6 (4.95) | 26.4 (4.71) | 27.5 (4.89) |
*1 In years; *2 in kg/m2; BMI, body mass index; SD, standard deviation
Completeness of the MRI data set
Of the participants included for MRI, 710 withdrew their participation during the first MRI sequence, resulting in a dropout rate of 2.2% and a total of n = 30 868 participants (95.7%) with at least one complete MR scan. The full MR protocol with all 16 sequences was completed in 29 757 participants (92.3%).
Side effects, safety, and comfort
During the entire baseline study period, no severe adverse events were recorded. Four mild adverse events (0.013%) were reported, with occurrence of nausea/ vertigo/ vomiting in three cases and temporary tinnitus in one case.
Of the 2500 questionnaires on satisfaction, 2484 were completed and analyzed (Table 2). Altogether, 98.5% of the participants were satisfied with the consenting procedure (82.3% very satisfied) and 99.5% of the participants were satisfied with the overall course of events at the MRI examination centers (83.8% very satisfied). The majority rated the duration of the MRI examination as “alright” (93.2%), while a minority of 5.9% rated the examination as “too long.”
Table 2. Results of the satisfaction survey conducted in connection with the baseline MRI examination at the five NAKO study centers at intervals of 6 months between fall 2016 and fall 2018 (100 questionnaires per site and time point).
| N | Not specified | Very satisfied | Satisfied | Partially satisfied | Dissatisfied | Very dissatisfied | |
| Consenting procedure | 2484 | 0.8% | 82.3% | 16.2% | 0.2% | < 0.1% | 0.4% |
| Overall procedure at MRI study center | 2484 | 0.1% | 83.8% | 15.7% | 0.3% | 0.1% | < 0.1% |
| N | Not specified | Too long | Alright | Too short | |||
| Duration of MRI examination | 2484 | 0.5% | 5.9% | 93.2% | 0.4% | ||
MRI, Magnetic resonance imaging
MRI image quality
On average, the radiologists rated the image quality as good in 9.8% of cases (range 3.0–18.7%) and as excellent in 89.0% (range 80.2–96.8%). In contrast, across all sequences, an average of only 1.2% (range 0.2–2.3%) of the image data acquired were rated as inadequate for postprocessing. The automated image quality assessment also showed excellent results. Detailed information is provided in the eSupplement, which also contains an overview of the derived imaging parameters (eSupplement, Table 1, eFigure).
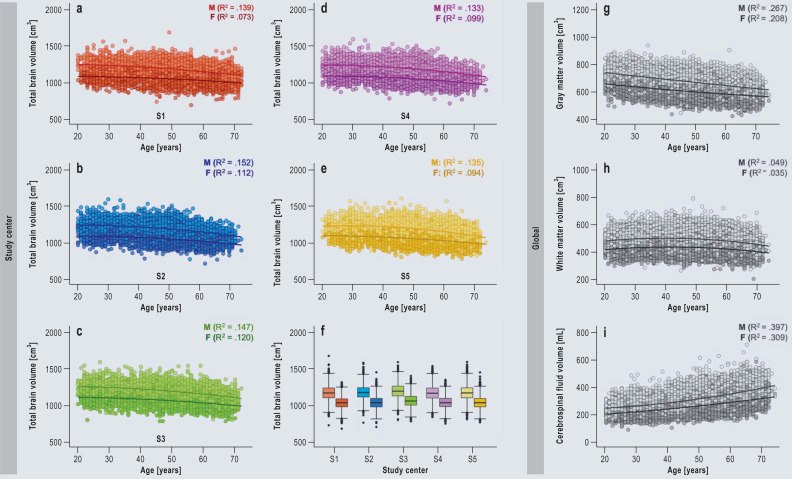
eFigure.
Brain morphological image quality examination
a–f) Age-related differences in total brain volume per MRI study center (Augsburg = S1 = red [n = 6074], Berlin = S2 = blue [n = 5708], Essen = S3 = green [n = 5743], Mannheim = S4 = pink [n = 5609], Neubrandenburg = S5 = yellow [n = 6664]); lighter colors = men, darker colors = women. f) Box plots showing the average total brain volume for the two sexes separately (lighter colors = men [n = 16 647], darker colors = women [n = 13 151]).
g–i) Age-related differences in gray matter volume, white matter volume, and cerebrospinal fluid volume for the two sexes separately (light gray = men, dark gray = women).
Examination of total brain volume, white and gray matter volume, and cerebrospinal fluid volume from the T1w imaging data revealed highly consistent results across all MRI study centers (Table 3). Men showed slightly higher brain volumes than women (1203.81 ± 102.06 cm3 vs. 1,068.10 ± 86.69 cm3; Figure a), even after correction for body size and weight (Figure b). Higher age was associated with lower total brain and gray matter volumes, while white matter volume remained constant and cerebrospinal fluid volume increased slightly (eFigure). Age-related differences with regard to gray matter and cerebrospinal fluid were slightly greater in men than in women. Nevertheless, the associations were very similar across MR sites and were independent of biological sex.
Table 3. Means and standard deviations for the volumes of gray matter, white matter, cerebrospinal fluid, and total brain (cm3) for the whole magnetic resonance imaging sample and separately for men and women*.
| Whole sample | Men | Women | |||||||||||||
| Age group | N | GMV | WMV | CSF | TBV | N | GMV | WMV | CSF | TBV | N | GMV | WMV | CSF | TBV |
| Total | 29 798 | 648.15 (62.95) |
468.32 (58.28) |
304.12 (62.20) |
1143.92 (116.95) |
16 647 | 679.57 (55.67) |
495.84 (52.73) |
327.03 (60.14) |
1203.82 (102.06) |
13 151 | 608.38 .(47.17) |
433.49 (44.78) |
275.11 (51.80) |
1068.10 (86.69) |
| 20–29 | 3109 | 699.07 (63.45) |
468.79 (58.09) |
249.41 (44.15) |
1195.67 (118.44) |
185 | 732.92 (52.61) |
496.18 (51.66) |
267.55 (41.35) |
1258.04 (99.43) |
1324 | 653.42 (45.76) |
431.86 (44.18) |
224.95 (35.09) |
1111.58 (85.44) |
| 30–39 | 3553 | 677.67 (61.25) |
477.81 (58.21) |
266.94 (46.30) |
1183.62 (116.32) |
2102 | 709.93 (49.22) |
504.64 (51.70) |
286.70 (40.69) |
1243.79 (95.78) |
1451 | 630.94 (44.57) |
438.95 (43.26) |
238.33 (38.29) |
1096.45 (83.66) |
| 40–49 | 8780 | 653.04 (58.44) |
475.94 (58.11) |
293.45 (49.25) |
1156.92 (113.90) |
4930 | 685.70 (47.88) |
504.35 (51.89) |
313.91 (44.27) |
1219.10 (95.32) |
3850 | 611.23 (41.70) |
439.55 (43.53) |
267.24 (42.43) |
1077.30 (81.62) |
| 50–59 | 8240 | 636.61 (55.23) |
469.65 (57.29) |
316.35 (55.38) |
1133.73 (109.71) |
4526 | 665.60 (47.47) |
496.84 (51.74) |
341.61 (50.06) |
1190.78 (94.77) |
3714 | 601.28 (41.82) |
436.52 (44.95) |
285.55 (45.00) |
1064.20 (83.43) |
| 60–69 | 5704 | 614.37 (53.53) |
450.65 (55.80) |
350.12 (62.31) |
1091.22 (105.98) |
3061 | 642.79 (46.46) |
477.30 (50.93) |
382.10 (54.81) |
1146.94 (92.69) |
2643 | 581.46 (40.82) |
419.79 (44.05) |
313.07 (48.41) |
1026.69 (80.83) |
| 70–74 | 412 | 603.77 (49.57) |
438.77 (55.52) |
383.48 (66.28) |
1067.72 (101.81) |
243 | 624.45 (45.22) |
459.33 (53.06) |
413.91 (58.15) |
1109.43 (94.48) |
169 | 574.04 (39.47) |
409.22 (44.68) |
339.71 (51.09) |
1007.74 (79.78) |
* The overall mean values across all age groups (20–74 years) and the means for the individual decades
CSF, Cerebrospinal fluid volume; GMV, gray matter volume; TBV, total brain volume; WMV, white matter volume
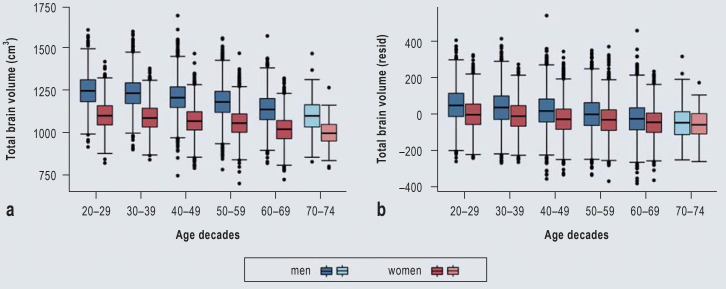
Figure.
Box plots for total brain volume by age decade and by sex
a) Raw data for total brain volume (cm3). b) Residuals of total brain volume, corrected for height and weight.
Note that the oldest group includes only participants ranging in age from 70 to 74 years (light blue and light red).
Discussion
This article presents results of the analysis of quality indicators for the NAKO baseline MRI examination. The participation rate was exceptionally high and the dropout rate very low. Furthermore, the participants reported high levels of safety and comfort. Image quality was subjectively and objectively rated as good to excellent. Moreover, the brain volume findings were extremely consistent across all study centers. This lays the foundation for a qualitatively and quantitatively top-class image database and represents an excellent jumping-off point for epidemiological and radiological research using the NAKO MRI data.
The sample comprised a total of 31 578 women and men from five MRI study centers and 6 neighboring NAKO study centers, randomly selected from the regional resident registration offices, that supplied participants. The MRI participants represent only a subsample of the NAKO cohort, because MRI is a time-consuming and complex procedure that requires specific expertise and workflows. This corresponds to the approach taken in other large-scale population studies (6, 21, 22).
Those who participated in the NAKO MRI examination were middle-aged (mean age 49.0 ± 12.3 years) and more often male (56.0%), whereas the total NAKO sample had a slight preponderance of female participants (50.4%) (7). Possible explanations are that women perceive a greater risk of harm from complex imaging examinations (23) and/or are more averse to unknown scenarios (24). Furthermore, possible or actual pregnancy could also play a role. Similar observations on participation rates in population-based MRI studies have been made in KORA-MRI (6), the UK Biobank (4, 25), and other large clinical trials despite considerable efforts to increase the proportion of women among the participants (26).
The average BMI (26.6 ± 4.79 kg/m²) and other major characteristics of the NAKO MRI subpopulation (including age and gender distribution) are also comparable with other larger cohorts. In the UK Biobank study, for instance, the mean BMI in the MRI sample was 26.6 ± 4.4 kg/m² (27). Therefore, our sample—although slightly different from the overall NAKO cohort (mean BMI 27.4 ± 4.4 kg/m² for men and 26.3 ± 5.5 kg/m² for women) (28)—may serve as a valid source for comparing or merging results of different cohort studies (29).
Notably, an extraordinary level of safety and comfort was achieved. Not a single severe adverse event was reported over the whole study period, and the prevalence of mild adverse events (e.g., nausea) was negligibly low. This was by no means unexpected, as in clinical scenarios a similarly safe environment can be assured by the radiology staff and the specific equipment (30). Ultimately, these conditions may also have contributed to the very low dropout rates.
Image quality was almost exclusively rated as good to excellent, and only a small proportion of data sets were judged unsuitable for image analysis. For the T2-weighted HASTE sequences this was 2.3%, opposed to only 0.2% for the T1-weighted MPRAGE sequences (see eSupplement, Table 1). In the NAKO MRI study, image quality was ensured by comprehensive quality management, including identical software and hardware as well as training, certification, and implementation of quality assurance procedures throughout the study period.
As a kind of proof of concept, we have conducted analyses of commonly examined imaging biomarkers (brain volumes), which replicate previously reported associations between increasing age and decreasing total brain and gray matter volumes (31). Furthermore, the known differences between the sexes, whereby men show higher volumes than women, were also observed in our data sets (Figure a). The most powerful explanatory factor for these differences seems to be the greater height of men (32, 33). Correction of our data for height and weight considerably diminishes the differences in brain volumes between the sexes (Figure b). Some of the remaining sex-specific differences in brain volumes may result from limitations of the statistical correction methods. However, cognitive function depends more on factors such as efficiency, connectivity, and specific regional volumes than on a larger total brain volume (34). These observations showed a high degree of similarity across all MR study centers, indicating high comparability among the sites (eFigure).
In conclusion, the NAKO baseline MRI examination achieved an extremely high participation rate and provided a high level of participant safety and comfort throughout the study period. Among the 31 578 persons who participated in the baseline MRI examination, the rate of complete data sets was high and very good subjective and objective image quality was attained. The MRI database now provides an ideal source for complex image analysis projects and will make a decisive contribution to generation of novel insights into multiple disease entities in future research.
Acknowledgments
Collaborators
The members of the NAKO Investigators Consortium acted as Collaborators (see eBox 2)
eBox 2. The members of the NAKO Investigators Consortium (collaborators).
Prof. Dr. med. Hermann Brenner, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Heidelberg, Germany
PD Dr. med. Robin Bülow, Institute of Diagnostic Radiology and Neuroradiology, University Medicine Greifswald, Germany
Dr. rer. nat. Margit Ecker,Department of Radiology, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Germany
Dr. med. Sylvia Gastell, Institute for Epidemiology, Helmholtz Zentrum München – German Research Center for Environmental Health (GmbH), Neuherberg, Germany
Dr. rer. nat. Thomas Hendel, Institute for Epidemiology, Helmholtz Zentrum München – German Research Center for Environmental Health (GmbH), Neuherberg, Institute for Medical Information Processing, Biometry and Epidemiology, Medical Faculty, Ludwig-Maximilians-Universität München, Munich, Germany
Dr. rer. nat. Daniel Hoinkiss, Fraunhofer Institute for Digital Medicine MEVIS, Bremen, Germany
Dr. sc. hum. Bernd Holleczek, Krebsregister Saarland, Saarbrücken, Germany
Prof. Dr. med. Thomas Keil, Institute of Social Medicine, Epidemiology and Health Economics, Charité-Universitätsmedizin Berlin; Institute of Clinical Epidemiology and Biometry, University of Würzburg; State Institute of Health I, Bavarian Health and Food Safety Authority, Erlangen, Germany
Lisa Kretz, Department of Radiology, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Germany
Dr. med. Lilian Krist, Institute of Social Medicine, Epidemiology and Health Economics, Charité-Universitätsmedizin Berlin, Germany
Prof. Prof. Sc.D. Ph.D. Karin B. Michels, Institute for Prevention and Cancer Epidemiology, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Germany
Stefan Ostrzinski, Institute for Community Medicine, University Medicine Greifswald, Germany
Dr. rer. nat. Markus Otto, Institute for Community Medicine, University Medicine Greifswald; Institute of Diagnostic Radiology and Neuroradiology, University Medicine Greifswald, Germany
Dr. rer. biol. hum. Susanne Rospleszcz, Institute for Epidemiology, Helmholtz Zentrum München – German Research Center for Environmental Health (GmbH), Neuherberg; Department of Diagnostic and Interventional Radiology, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Germany
Dr. rer. med. Sabine Schipf, Institute for Community Medicine, University Medicine Greifswald, Germany
Prof. Dr. med. Börge Schmidt, Institute for Medical Informatics, Biometry and Epidemiology, University Hospital Essen, University of Duisburg-Essen
Prof. Dr. rer. med. Dr. phil. Carsten O. Schmidt, Institute for Community Medicine, University Medicine Greifswald, Germany
Prof. Dr. med. Matthias B. Schulze, Department of Molecular Epidemiology, German Institute of Human Nutrition Potsdam-Rehbruecke, Institute of Nutritional Science, University of Potsdam, Nuthetal, Germany
Prof. Dr. med. Jeanette Schulz-Menger, Charité – Universitätsmedizin Berlin, Berlin, Germany
Dr. rer. nat. Oyunbileg von Stackelberg, Department of Diagnostic and Interventional Radiology, University Hospital Heidelberg, Germany
Dr. rer. nat. Stephan Struckmann, Institute for Community Medicine, University Medicine Greifswald, Germany
Prof. Dr. med. Sabine Weckbach, Department of Diagnostic and Interventional Radiology, University Hospital Heidelberg, Germany
Dr. rer nat. Nicole Werner, Institute for Community Medicine, University Medicine Greifswald, Germany
Acknowledgments
We thank all participants who took part in the NAKO study and the staff of this research initiative.
Ethics
The NAKO Health Study was performed with the approval of the relevant ethics committees and is in accordance with national law and with the Declaration of Helsinki of 1975 (in the current, revised version).
Footnotes
Funding
This project was conducted with data (Application No. NAKO-796) from the German National Cohort (NAKO) (www.nako.de). The NAKO is funded by the Federal Ministry of Education and Research (BMBF) [project funding reference numbers: 01ER1301A/B/C, 01ER1511D, 01ER1801A/B/C/D and 01ER2301A/B/C], federal states of Germany and the Helmholtz Association, the participating universities and the institutes of the Leibniz Association.
Conflict of interest statement
FB holds shares in Siemens Healthineers. He receives financial support from Siemens, Philips, Sanofi, and Bayer.
KB is a member of the Editorial Board of Deutsches Ärzteblatt.
MF receives financial support from Siemens, Philips, Boehringer, and Sanofi.
HUK has received financial support from Siemens and Philips.
The remaining authors declare that no conflict of interest exists.
References
- 1.Schlett CL, Hendel T, Weckbach S, et al. Population-based imaging and radiomics: rationale and perspective of the German National Cohort MRI study. Rofo. 2016;188:652–661. doi: 10.1055/s-0042-104510. [DOI] [PubMed] [Google Scholar]
- 2.Hunold P, Bucher AM, Sandstede J, et al. Statement of the German Roentgen Society, German Society of Neuroradiology, and Society of German-speaking Pediatric Radiologists on Requirements for the performance and reporting of MR imaging examinations outside of radiology. Rofo. 2021;193:1050–1061. doi: 10.1055/a-1463-3626. [DOI] [PubMed] [Google Scholar]
- 3.Schlett CL, Hendel T, Hirsch J, et al. Quantitative, organ-specific interscanner and intrascanner variability for 3 T whole-body magnetic resonance imaging in a multicenter, multivendor study. Invest Radiol. 2016;51:255–265. doi: 10.1097/RLI.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 4.Völzke H, Schmidt CO, Hegenscheid K, et al. Population imaging as valuable tool for personalized medicine. Clin Pharmacol Ther. 2012;92:422–424. doi: 10.1038/clpt.2012.100. [DOI] [PubMed] [Google Scholar]
- 5.Hegenscheid K, Kühn JP, Völzke H, Biffar R, Hosten N, Puls R. Whole-body magnetic resonance imaging of healthy volunteers: pilot study results from the population-based SHIP study. Röfo. 2009;181:748–759. doi: 10.1055/s-0028-1109510. [DOI] [PubMed] [Google Scholar]
- 6.Bamberg F, Hetterich H, Rospleszcz S, et al. Subclinical disease burden as assessed by whole-body MRI in subjects with prediabetes, subjects with diabetes, and normal control subjects from the general population: the KORA-MRI study. Diabetes. 2017;66:158–169. doi: 10.2337/db16-0630. [DOI] [PubMed] [Google Scholar]
- 7.Peters A, Peters A, Greiser KH, et al. Framework and baseline examination of the German National Cohort (NAKO) Eur J Epidemiol. 2022;37:1107–1124. doi: 10.1007/s10654-022-00890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consortium GNCG. The German National Cohort: aims, study design and organization. Eur J Epidemiol. 2014;29:371–382. doi: 10.1007/s10654-014-9890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bamberg F, Kauczor HU, Weckbach S, et al. Whole-body MR imaging in the German National Cohort: rationale, design, and technical background. Radiology. 2015;277:206–220. doi: 10.1148/radiol.2015142272. [DOI] [PubMed] [Google Scholar]
- 10.The Royal College of Radiologists. London: Royal College of Radiologists; 2011. management of incidental findings detected during research imaging. [Google Scholar]
- 11.Booth TC, Waldman AD, Wardlaw JM, Taylor SA, Jackson A. Management of incidental findings during imaging research in „healthy“ volunteers: current UK practice. Br J Radiol. 2012;85:11–21. doi: 10.1259/bjr/73283917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth TC, Jackson A, Wardlaw JM, Taylor SA, Waldman AD. Incidental findings found in „healthy“ volunteers during imaging performed for research: current legal and ethical implications. Br J Radiol. 2010;83:456–465. doi: 10.1259/bjr/15877332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegenscheid K, Seipel R, Schmidt CO, et al. Potentially relevant incidental findings on research whole-body MRI in the general adult population: frequencies and management. Eur Radiol. 2013;23:816–826. doi: 10.1007/s00330-012-2636-6. [DOI] [PubMed] [Google Scholar]
- 14.Hegedüs P, von Stackelberg O, Neumann C, et al. How to report incidental findings from population whole-body MRI: view of participants of the German National Cohort. Eur Radiol. 2019;29:5873–5878. doi: 10.1007/s00330-019-06077-z. [DOI] [PubMed] [Google Scholar]
- 15.Schuppert C, Krüchten RV, Hirsch JG, et al. Whole-body magnetic resonance imaging in the large population-based German National Cohort Study: predictive capability of automated image quality assessment for protocol repetitions. Invest Radiol. 2022;57:478–487. doi: 10.1097/RLI.0000000000000861. [DOI] [PubMed] [Google Scholar]
- 16.Zhou W, Bovik AC. A universal image quality index. IEEE Signal Processing Letters. 2002;9:81–84. [Google Scholar]
- 17.Wood ML, Henkelman RM. MR image artifacts from periodic motion. Med Phys. 1985;12:143–151. doi: 10.1118/1.595782. [DOI] [PubMed] [Google Scholar]
- 18.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 19.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 20.Gaser C, Dahnke R, Thompson P, Kurth F, Luders E. Alzheimer’s Disease Neuroimaging I: CAT—a computational anatomy toolbox for the analysis of structural MRI data. bioRxiv. 2022 doi: 10.1093/gigascience/giae049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Völzke H. [Study of Health in Pomerania (SHIP). Concept, design and selected results] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55:790–794. doi: 10.1007/s00103-012-1483-6. [DOI] [PubMed] [Google Scholar]
- 22.Caspers S, Moebus S, Lux S, et al. Studying variability in human brain aging in a population-based German cohort—rationale and design of 1000BRAINS. Front Aging Neurosci. 2014;6:149. doi: 10.3389/fnagi.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding EL, Powe NR, Manson JE, Sherber NS, Braunstein JB. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med. 2007;167:905–912. doi: 10.1001/archinte.167.9.905. [DOI] [PubMed] [Google Scholar]
- 24.Doyal L. Sex, gender, and health: the need for a new approach. BMJ. 2001;323:1061–1063. doi: 10.1136/bmj.323.7320.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen SE, Matthews PM, Bamberg F, et al. Imaging in population science: cardiovascular magnetic resonance in 100,000 participants of UK Biobank—rationale, challenges and approaches. J Cardiovasc Magn Reson. 2013;15:46. doi: 10.1186/1532-429X-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2000;343:475–480. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- 27.Dekkers IA, Jansen PR, Lamb HJ. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK biobank study. Radiology. 2019;291:763–771. doi: 10.1148/radiol.2019181012. [DOI] [PubMed] [Google Scholar]
- 28.Fischer B, Sedlmeier AM, Hartwig S, et al. Anthropometrische Messungen in der NAKO Gesundheitsstudie—mehr als nur Größe und Gewicht. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2020;63:290–300. doi: 10.1007/s00103-020-03096-w. [DOI] [PubMed] [Google Scholar]
- 29.Gatidis S, Kart T, Fischer M, et al. Better together: data harmonization and cross-study analysis of abdominal MRI data from UK biobank and the German National Cohort. Invest Radiol. 2023;58:346–354. doi: 10.1097/RLI.0000000000000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bundesausschuss G. Richtlinie des Gemeinsamen Bundesausschusses über Kriterien zur Qualitätsbeurteilung in der radiologischen Diagnostik nach § 135b Absatz 2 SGB V (Qualitätsbeurteilungs-Richtlinie Radiologie/QBR-RL) Bundesanzeiger (BAnz AT) 2020:1–28. [Google Scholar]
- 31.Narvacan K, Treit S, Camicioli R, Martin W, Beaulieu C. Evolution of deep gray matter volume across the human lifespan. Hum Brain Mapp. 2017;38:3771–3790. doi: 10.1002/hbm.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fjell AM, Westlye LT, Amlien I, et al. Minute effects of sex on the aging brain: a multisample magnetic resonance imaging study of healthy aging and Alzheimer‘s disease. J Neurosci. 2009;29:8774–8783. doi: 10.1523/JNEUROSCI.0115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchis-Segura C, Ibañez-Gual MV, Aguirre N, Cruz-Gómez ÁJ, Forn C. Effects of different intracranial volume correction methods on univariate sex differences in grey matter volume and multivariate sex prediction. Sci Rep. 2020;10:12953. doi: 10.1038/s41598-020-69361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruigrok AN, Salimi-Khorshidi G, Lai MC, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]