Abstract
Both type I and type II diabetes are powerful and independent risk factors for coronary artery disease (CAD), stroke, and peripheral arterial disease. Atherosclerosis accounts for virtually 80% of all deaths among diabetic patients. Prolonged exposure to hyperglycemia is now recognized a major factor in the pathogenesis of atherosclerosis in diabetes. Hyperglycemia induces a large number of alterations at the cellular level of vascular tissue that potentially accelerate the atherosclerotic process. Animal and human studies have elucidated three major mechanisms that encompass most of the pathological alterations observed in the diabetic vasculature: 1) Nonenzymatic glycosylation of proteins and lipids which can interfere with their normal function by disrupting molecular conformation, alter enzymatic activity, reduce degradative capacity, and interfere with receptor recognition. In addition, glycosylated proteins interact with a specific receptor present on all cells relevant to the atherosclerotic process, including monocyte-derived macrophages, endothelial cells, and smooth muscle cells. The interaction of glycosylated proteins with their receptor results in the induction of oxidative stress and proinflammatory responses 2) oxidative stress 3) protein kinase C (PKC) activation with subsequent alteration in growth factor expression. Importantly, these mechanisms may be interrelated. For example, hyperglycemia-induced oxidative stress promotes both the formation of advanced glycosylation end products and PKC activation.
Both type I and type II diabetes are powerful and independent risk factors for coronary artery disease (CAD), stroke, and peripheral arterial disease [1-3]. Atherosclerosis accounts for virtually 80% of all deaths among North American diabetic patients, compared with one third of all deaths in the general North American population [1]. More then 75% of all hospitalizations for diabetic complications are attributable to cardiovascular disease.
Prolonged exposure to hyperglycemia is now recognized as the primary casual factor in the pathogenesis of diabetic complications [4-6]. Hyperglycemia induces a large number of alterations in vascular tissue that potentially promote accelerated atherosclerosis. Currently, three major mechanisms have emerged that encompass most of the pathological alterations observed in the vasculature of diabetic animals and humans: 1) Nonenzymatic glycosylation of proteins and lipids 2) oxidative stress 3) protein kinase C (PKC) activation. Importantly, these mechanisms are not independent. For example, hyperglycemia-induced oxidative stress promotes the formation of advanced glycosylation end products and PKC activation [7].
Advanced glycosylation end products
The effects of hyperglycemia are often irreversible and lead to progressive cell dysfunction [8]. For example, in diabetic patients with functioning pancreatic transplants renal pathology continues to progress for at least 5 years after diabetes has been cured [8]. The mechanism for these observations is unclear, but suggests that cellular perturbations may persist despite the return of normoglycemia (the so-called memory effect). Thus, persistent rather than transient, acute metabolic changes are of pivotal importance in the pathogenesis of diabetic complications.
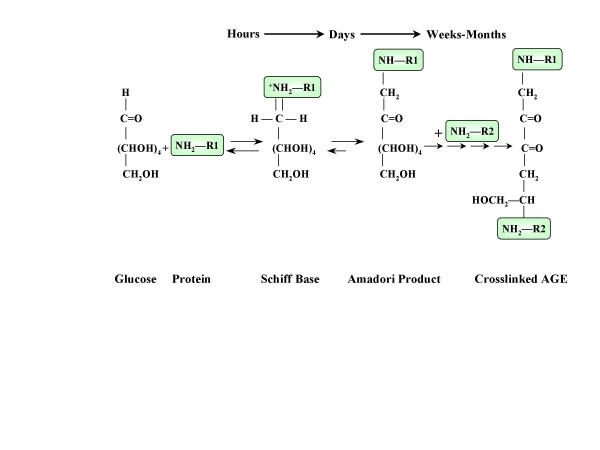
One of the important mechanisms responsible for the accelerated atherosclerosis in diabetes is the nonenzymatic reaction between glucose and proteins or lipoproteins in arterial walls, collectively known as Maillard, or browning reaction [9]. Glucose forms chemically reversible early glycosylation products with reactive amino groups of circulating or vessel wall proteins (Schiff bases), which subsequently rearrange to form the more stable Amadori-type early glycosylation products. Equilibrium levels of Schiff-base and Amadori products (the best known of which is hemoglobin A1C) are reached in hours and weeks, respectively [10] (Figure 1). Some of the early glycosylation products on long-lived proteins (e.g. vessel wall collagen) continue to undergo complex series of chemical rearrangement to form advanced glycosylation end products (AGEs) [10]. Once formed, AGE-protein adducts are stable and virtually irreversible. Although AGEs comprise a large number of chemical structures, carboxymethyl-lysine-protein adducts are the predominant AGEs present in vivo [11,12].
Figure 1.
The formation of advanced glycosylation end products.
AGEs accumulate continuously on long-lived vessel wall proteins with aging and at an accelerated rate in diabetes [10]. The degree of nonenzymatic glycation is determined mainly by the glucose concentration and time of exposure [10]. However, another critical factor to the formation of AGEs is the tissue microenvironment redox potential. Thus, situations in which the local redox potential has been shifted to favor oxidant stress, AGEs formation increases substantially [7,13-17].
AGEs can accelerate the atherosclerotic process by diverse mechanism, which can be classified as non-receptor dependent (Table 1) and receptor-mediated (Table 2).
Table 1.
Atherosclerosis promoting effects of AGEs: Non-Receptor Mediated Mechanisms
| Extracellular matrix |
| Collagen cross linking [74] |
| Enhanced synthesis of extracellular matrix components [10] |
| Trapping of LDL in the subendothelium [75] |
| Glycosylated subendothelial matrix quenches nitric oxide [76] |
| Functional alterations of regulatory proteins |
| bFGF glycosylation reduces its heparin binding capacity and its mitogenic activity on endothelial cells [13] |
| Inactivation of the complement regulatory protein CD59 [26] |
| Lipoprotein modifications |
| Glycosylated LDL [19,20] |
| Reduced LDL recognition by cellular LDL receptors [21] |
| Increased susceptibility of LDL to oxidative modification [19] |
Table 2.
Atherosclerosis promoting effects of AGEs: Receptor Mediated Mechanisms
| Promoting inflammation |
| Secretion of cytokines such as TNF-α, IL-1 [74]. |
| Chemotactic stimulus for monocyte-macrophages [37,38] |
| Induction of cellular proliferation |
| Stimulation of PDGF [37] and IGF-I [40] secretion from monocytes and possibly SMC. |
| Endothelial dysfunction |
| Increased permeability of EC monolayers [34,35] |
| Increased procoagulant activity [35] |
| Increased expression of adhesion molecules [33] |
| Increased Intracellular oxidative stress [31,34] |
IGF-I = Insulin-like growth factor I; IL-1 = Interleukin-1; PDGF = Platelet-derived growth factor; TNF-α = Tumor necrosis factor-α.
Non-receptor mediated mechanisms
Glycosylation of proteins and lipoproteins can interfere with their normal function by disrupting molecular conformation, alter enzymatic activity, reduce degradative capacity, and interfere with receptor recognition (Table 1). Thus, changes in the normal physiology of proteins that are relevant to atherogenesis, may promote atherosclerosis in diabetic individuals.
Perhaps the most studied example is interference of the normal physiology of the low-density lipoprotein (LDL) particle. The glycosylation process occurs both on the apoprotein B [18] and phospholipid [19] components of LDL, leading to both functional alternations in LDL clearance and increased susceptibility to oxidative modifications.
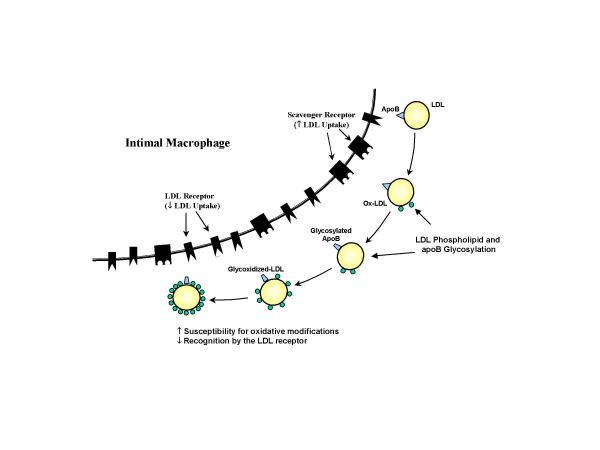
Clinical studies have shown an increased level of AGEs on LDL obtained from diabetics compared with normal individuals [19,20]. Glycosylation of LDL apo B (the surface protein of LDL) occurs mainly on a positively charged lysine residues within the putative LDL receptor binding domain which are essential for the specific recognition of LDL by the LDL receptor [20]. LDL glycosylation is increased in correlation with glucose levels, and AGE-ApoB levels are up to 4-fold higher in diabetic patients [19,20]. Glycosylation of ApoB results in a significant impairment of LDL-receptor-mediated uptake decreasing the in vivo clearance of LDL compared to native LDL [21] (Figure 2). Several studies have shown that degradation of glycated LDL is impaired in cultured human fibroblasts (which posses LDL receptor) compared with normal LDL, and that this impairment is proportional to the extent of glycation [21]. In contrast to fibroblasts, human monocyte-derived macrophages recognize glycated LDL to a greater extent than native LDL [22]. The uptake of glycated LDL by these cells, however, is not mediated by the LDL receptor pathway, but by a high-capacity, low-affinity receptor pathway [22]. Thus, glycated LDL are poorly recognized by the specific LDL receptor and are preferentially recognized by a nonspecific (scavenger) receptor present on human macrophages. Because LDL glycosylation enhances its uptake by human aortic intimal cells [23] and monocyte-derived macrophages [22] with stimulation of foam cells formation, the recognition of glycated LDL by the scavenger receptor pathway is thought to promote intracellular accumulation of cholesteryl esters and promote atherosclerosis (Figure 2).
Figure 2.
Potential mechanisms by which LDL glycosylation increases its atherogenicity. Advanced glycosylation of the phospholipid component of LDL is accompanied by the progressive oxidative modification of unsaturated fatty acid residues. Glycosylation of LDL apoB reduces its recognition by the LDL receptor and increases uptake through the scavenger receptor (see text for details).
Another atherogenic effect of glycation is to confer increased susceptibility of LDL to oxidative modification. Oxidation reactions occur normally during glycation can oxidize the amine-containing phospholipids component of LDL, independently of transition metals or exogenous free radical-generating systems [19]. Advanced glycosylation of an amine-containing phospholipids component of LDL is accompanied by progressive oxidative modification of unsaturated fatty acid residues [21]. LDL oxidation following AGE-LDL formation occurs in direct proportion to glucose concentration and can be inhibited by the AGE formation inhibitor aminoguanidine [19]. Thus, glycation confers increased susceptibility of LDL to oxidative modification [21,24], which is considered a critical step in its atherogenicity.
Another example is the alterations in normal function of the complement regulatory protein. Deposition of the membrane attack complex of complement (MAC) in blood vessels stimulates proliferation of fibroblasts and smooth muscle cells, in part by releasing growth factors such as fibroblast growth factor and platelet derived growth factor from MAC-targeted endothelium [25]. MAC deposition is normally restricted because cells express the regulatory membrane protein CD59, which limits complement activation and MAC formation. Glycation of the complement regulatory protein CD59 results in its inactivation [26] and may increased the sensitivity of the diabetic endothelium to MAC-induced release of growth factors and cytokines.
Glycosylation of matrix components such as collagen VI, laminin, and vitronectin decreased binding of anionic heparan sulfate, leading to greater turnover of heparan sulfate [10]. The absence of HS is thought to stimulate a compensatory overproduction of other matrix components through altered partitioning of growth-regulatory factors between matrix bound proteoglycans and cells [10]. AGEs on matrix also alter the normal interactions of transmembrane integrin receptors with three specific matrix liganeds. For example, modification of the cell binding domains of type IV collagen causes decreased endothelial cell adhesion [10].
Receptor-mediated mechanisms
The cellular interactions of AGEs are mediated through a specific receptor for AGE determinants on cell surfaces [16]. The presence of the AGE receptor (RAGE), a member of the immunoglobulin superfamily of receptors [27], has been demonstrated in all cells relevant to the atherosclerotic process including monocyte-derived macrophages, endothelial cells, and smooth muscle cells [16,28] (Table 2). The macrophage AGE receptor system is closely tied to AGE turnover, and is thought to represent a mechanism that responds to raising AGE levels with aging and degrade senescent proteins [29].
In mature animals, RAGE expression on these cells is low [28]. However, under certain pathological circumstances, sustained upregulation of RAGE occurs. In pathological lesions, abundance of RAGE expressing cells is usually associated with sites of accumulated RAGE ligands. In diabetic vasculature, cells expressing high levels of RAGE are often proximal to areas in which AGEs are abundant [17,30].
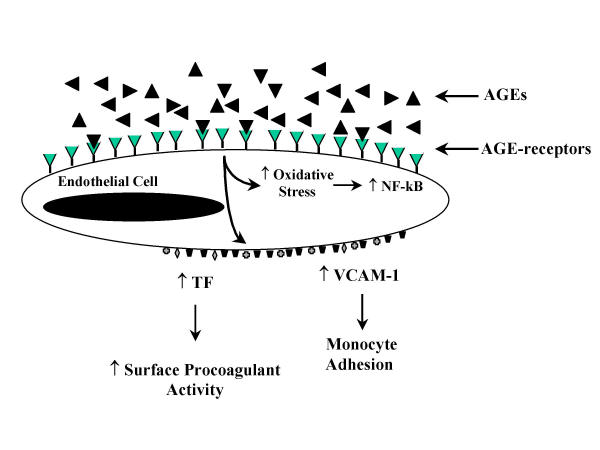
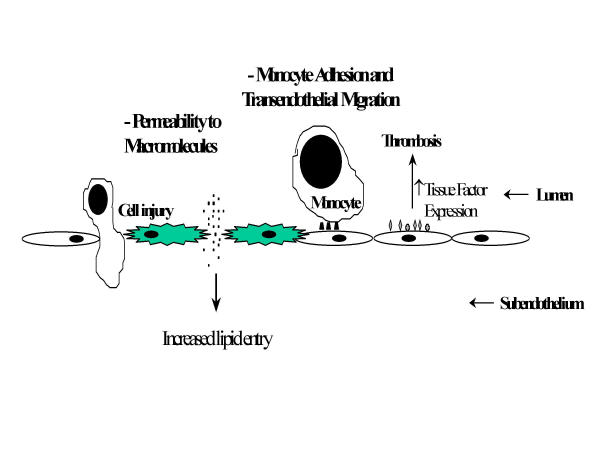
AGE interaction with RAGE on endothelial cells results in the induction of oxidative stress and consequently of the transcription factor NF-κB [31,32] and VCAM-1 [33] (Figure 3). In addition, engagement of AGEs with their specific receptors results in reduced endothelial barrier function [33,34]. With increased permeability of endothelial cell monolayers [34,35]. Thus, the interaction of AGEs with RAGE-bearing endothelial cells can mediate initiating events in atherogenesis. For example, increased endothelial permeability can lead to increased lipid entry into the subendothelium. Enhancement of adhesive interactions of monocytes with the endothelial surface can subsequently result in transendothelial migration (Figure 4).
Figure 3.
Consequence of AGE interaction with the AGE receptor on endothelial cells.
Figure 4.
Mechanisms by which AGEs induce endothelial dysfunction and promote atherosclerosis.
Binding of soluble AGEs to RAGE-bearing monocytes induces chemotaxis [36], followed by mononuclear infiltration through an intact endothelial monolayer [37,38]. Pathological studies of human atherosclerotic plaques showed infiltration of RAGE-expressing cells in the expanded intima [28]. Monocyte-macrophage interaction with AGEs results also in the production of mediators such as interleukin-1, tumor necrosis factor-α, platelet-derived growth factor, and insulin growth factor-I [37,39,40], which have a pivotal role in the pathogenesis of atherosclerosis [41].
In smooth muscle cells, binding of AGE-modified proteins to RAGE is associated with increased cellular proliferation [42]. Although the precise mechanism of this response is unknown, the growth-promoting effects mediated by the RAGE are likely to be cytokine or growth-factor mediated. Thus, under conditions of enhanced tissue AGE deposition, receptor-mediated interaction of AGE-proteins with vascular wall cells facilitate the migration of inflammatory cells into the lesion with the subsequent release of growth-promoting cytokines.
The potential role of RAGE in the atherogenic process in diabetes has been demonstrated by Park and associates [43]. In the model of atherosclerosis-prone mice due to homozygous deletion of apolipoprotein E (apoE) gene, the induction of diabetes using streptozotocin resulted in atherosclerosis of increased severity compared to euglycemic apoE controls. The development of vascular was more rapid with the formation of more complex lesions (fibrous caps, extensive monocyte infiltration, etc) and atherosclerosis extending distally in the aorta and major arteries. Increased expression of RAGE and the presence of AGEs in the vessel wall, especially at sites of vascular lesions were also evident. Blockade of AGE-RAGE interaction using a truncated soluble extracellular domain of RAGE resulted in a striking suppression of lesions in diabetic mice, with lesions largely arrested at the fatty streak stage and a large reduction in complex lesions. These effects were independent of glucose and lipid levels [43].
Protein kinase c
The metabolic consequences of hyperglycemia can be expressed in cells in which glucose transport is largely independent of insulin. The resulting intracellular hyperglycemia has been implicated in the pathogenesis of diabetic complications through the activation of the protein kinase C (PKC) system [44-46].
High ambient glucose concentrations activate PKC by increasing the formation of diacylglycerol (DAG), the major endogenous cellular co-factor for PKC activation, from glycolytic intermediates such as dihydroxy-acetone phosphate and glyceraldehyde-3-phosphate. The elevation of DAG and subsequent activation of PKC in the vasculature can be maintained chronically [47].
PKC is a family of at least 12 isoforms of serine and threonine kinases. Although several PKC isoforms are expressed in vascular tissue, in the rat model of diabetes there is a preferential activation of PKC β2 in the aorta, heart, and retina, and PKC β1 in the glomeruli [48,49].
The PKC system is ubiquitously distributed in cells and is involved in the transcription of several growth factors, and in signal transduction in response to growth factors [48,50,51]. In vascular smooth muscle cells, PKC activation has been shown to modulate growth rate, DNA synthesis, and growth factor receptor turnover [46,48]. For example, hyperglycemia-induced PKC activation also results in increased platelet derived growth factor-β receptor expression on smooth muscle cells and other vascular wall cells (e.g., endothelial cells, monocyte-macrophages) [52,53].
PKC activation increases the expression of transforming growth factor-β (TGF-β), which is one of the most important growth factor regulating extracellular matrix production by activating gene expression of proteoglycans and collagen and decreasing the synthesis of proteolytic enzymes that degrade matrix proteins [54]. Increased expression of TGF-β is thought to lead to thickening of capillary basement membrane – one of the early structural abnormalities observed in almost all tissues in diabetes. PKC β selective inhibitor (LY333531) attenuates glomerular expression of TGF-β and ECM proteins such as fibronectin and type IV collagen [49,50].
Oxidative stress
Oxidative stress is widely invoked as a pathogenic mechanism for atherosclerosis. Among the sequelae of hyperglycemia, oxidative stress has been suggested as a potential mechanism for accelerated atherosclerosis [7,55,56]. Hyperglycemia can increase oxidative stress through several pathways. A major mechanism appears to be the hyperglycemia-induced intracellular reactive oxygen species (ROS), produced by the proton electromechanical gradient generated by the mitochondrial electron transport chain and resulting in increased production of superoxide [7].
Two other mechanisms have been proposed that may explain how hyperglycemia causes increased ROS formation. One mechanism involves the transition metal-catalyzed autoxidation of free glucose, as described in cell-free systems. Through this mechanism, glucose itself initiates autoxidative reaction and free radical production yielding superoxide anion (O2-) and hydrogen peroxide (H2O2) [57]. The other mechanism involves the transition metalcatalyzed autoxidation of protein-bound Amadori products, which yields superoxide and hydroxyl radicals and highly reactive dicarbonyl compounds [55] (see Glycoxidation).
There is also evidence that hyperglycemia may compromise natural antioxidant defenses. Under normal circumstances, free radicals are rapidly eliminated by antioxidants such as reduced glutathione, vitamin C, and vitamin E. Reduced glutathione content [58,59], as well as reduced vitamin E [60,61] have been reported in diabetic patients. Plasma and tissue levels of vitamin C are 40–50% lower in diabetic patients compared with nondiabetic subjects [62,63].
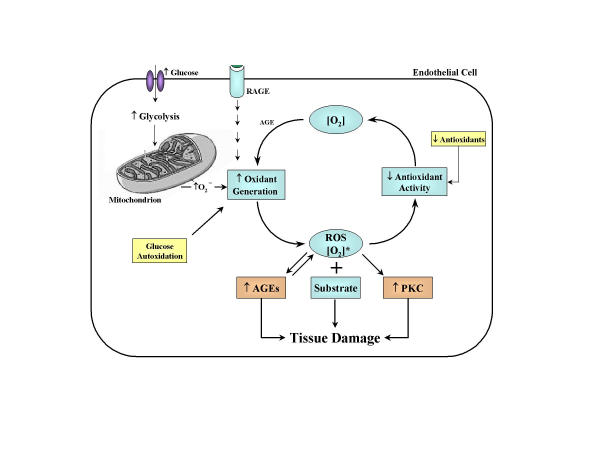
Importantly, there appears to be a tight pathogenic link between hyperglycemia-induced oxidant stress and the two hyperglycemia-dependent mechanisms of vascular damage described above, namely AGEs formation and PKC activation (Figure 5).
Figure 5.
Relationship between rates of oxidant generation, antioxidant activity, oxidative stress, and oxidative damage in diabetes. [O2]* represents various forms of reactive oxygen species [ROS]. The overall rate of formation of oxidative products leading to oxidative tissue damage is dependent on ambient levels of both [O2]* and substrate. Increased generation of [O2]* depends on several sources including glucose autoxidation, increased mitochondrial superoxide production, and as a result of the receptor for advanced glycosylation end products activation. [O2]* deactivation is reduced because antioxidant defenses are compromised in diabetes. Note that oxidative stress also promotes other hyperglycemia-induced mechanisms of tissue damage. Oxidative stress activates protein kinase C (PKC) and accelerates the formation of advanced glycosylation endproducts (AGEs).
Glycoxidation
Some of the individual advanced glycosylation products such as Nε-carboxymethyl)lysine (CML) and pentosidine are formed in reactions of protein with glucose only under oxidative conditions [15,64-66]. Thus, some AGEs are produced by combined processes of glycation and oxidation and have been termed glycoxidation products [56]. Each AGE structure has its own formation mechanism and thus its own dependence on oxidative stress. However, since glycoxidation products on proteins are irreversible, it has been suggested that they may be an integrative biomarker for the accumulated oxidative stress the respective tissue has been exposed to [14,55].
As previously discussed, the interaction between AGE epitopes and the cell surface AGE receptor upregulate oxidative stress response genes [31] and release oxygen radicals [67]. Thus, hyperglycemia simultaneously enhances both AGEs formation and oxidative stress, and the mutual facilitatory interactions between glycation and oxidation chemistry can contribute sinergistically to the formation of AGEs, oxidative stress, and diabetic complications (Figure 5). Indeed, there are strong correlations between levels of glycoxidation products in skin collagen and the severity of diabetic retinal, renal, and vascular disease [68,69].
Oxidative stress and PKC activation
Oxidative stress may also be involved in the activation of DAG-PKC in vascular tissue [7]. Oxidants produced in the setting of hyperglycemia can activate PKC [70]. Furthermore, several studies have shown that antioxidants such as vitamin E can inhibit PKC activation probably by decreasing DAG levels [7,71-73].
Contributor Information
Doron Aronson, Email: daronson@netvision.net.il.
Elliot J Rayfield, Email: ejrayfield@aol.com.
References
- American Diabetes association. Consensus Statement Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. Diabetes Care. 1993;16:72–78. doi: 10.2337/diacare.12.8.573. [DOI] [PubMed] [Google Scholar]
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Cayatte AJ, Rozek MM. Pathogenesis of the atherosclerotic lesion. Implications for diabetes mellitus. Diabetes Care. 1992;15:1156–1167. doi: 10.2337/diacare.15.9.1156. [DOI] [PubMed] [Google Scholar]
- Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48:937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association [see comments]. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation [see comments]. N Engl J Med. 1998;339:69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- Maillard L. Action des acides amines sur les sucres: formation des melanoidines par voie methodique. C R Hebd Seances Acad Sci. 1912;154:66–68. [Google Scholar]
- Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Higashi T, Sano H, Jinnouchi Y, Yoshida M, Araki T, Ueda S, Horiuchi S. N (epsilon)-(carboxymethyl) lysine protein adduct is a major immunological epitope in proteins modified with advanced gly cation end products of the Maillard reaction. Biochemistry. 1996;35:8075–8083. doi: 10.1021/bi9530550. [DOI] [PubMed] [Google Scholar]
- Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:N10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- Giardino I, Edelstein D, Brownlee M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J Clin Invest. 1996;97:1422–1428. doi: 10.1172/JCI118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MX, Knecht KJ, Thorpe SR, Baynes JW. Role of oxygen in cross-linking and chemical modification of collagen by glucose. Diabetes. 1992;41:42–48. doi: 10.2337/diab.41.2.s42. [DOI] [PubMed] [Google Scholar]
- Dunn JA, Ahmed MU, Murtiashaw MH, Richardson JM, Walla MD, Thorpe SR, Baynes JW. Reaction of ascorbate with lysine and protein under autoxidizing conditions: formation of N epsilon-(carboxymethyl)lysine by reaction between lysine and products of autoxidation of ascorbate. Biochemistry. 1990;29:10964–10970. doi: 10.1021/bi00501a014. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Hori O, Brett J, Yan SD, Wautier JL, Stern D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14:1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84:489–497. doi: 10.1161/01.res.84.5.489. [DOI] [PubMed] [Google Scholar]
- Bucala R, Mitchell R, Arnold K, Innerarity T, Vlassara H, Cerami A. Identification of the major site of apolipoprotein B modification by advanced glycosylation end products blocking uptake by the low density lipoprotein receptor. J Biol Chem. 1995;270:10828–10832. doi: 10.1074/jbc.270.18.10828. [DOI] [PubMed] [Google Scholar]
- Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci USA. 1993;90:6434–6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci USA. 1994;91:9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher UP, Witztum JL. Glucosylation of low-density lipoproteins to an extent comparable to that seen in diabetes slows their catabolism. Diabetes. 1984;33:130–134. doi: 10.2337/diab.33.2.130. [DOI] [PubMed] [Google Scholar]
- Klein RL, Laimins M, Lopes-Virella MF. Isolation, characterization, and metabolism of the glycated and nonglycated subfractions of low-density lipoproteins isolated from type I diabetic patients and nondiabetic subjects. Diabetes. 1995;44:1093–1098. doi: 10.2337/diab.44.9.1093. [DOI] [PubMed] [Google Scholar]
- Sobenin IA, Tertov VV, Koschinsky T, Bunting CE, Slavina ES, Dedov II, Orekhov AN. Modified low density lipoprotein from diabetic patients causes cholesterol accumulation in human intimal aortic cells. Atherosclerosis. 1993;100:41–54. doi: 10.1016/0021-9150(93)90066-4. [DOI] [PubMed] [Google Scholar]
- Bowie A, Owens D, Collins P, Johnson A, Tomkin GH. Glycosylated low density lipoprotein is more sensitive to oxidation: implications for the diabetic patient? Atherosclerosis. 1993;102:63–67. doi: 10.1016/0021-9150(93)90084-8. [DOI] [PubMed] [Google Scholar]
- Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet-derived growth factor from endothelial cells. J Exp Med. 1994;179:985–992. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta J, Hettinga J, Fluckiger R, Krumrei N, Goldfine A, Angarita L, Halperin J. Molecular basis for a link between complement and the vascular complications of diabetes. Proc Natl Acad Sci USA. 2000;97:5450–5455. doi: 10.1073/pnas.97.10.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- Brett J, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–1712. [PMC free article] [PubMed] [Google Scholar]
- Vlassara H. Receptor-mediated interactions of advanced glycosylation end products with cellular components within diabetic tissues. Diabetes. 1992;41:52–56. doi: 10.2337/diab.41.2.s52. [DOI] [PubMed] [Google Scholar]
- Ritthaler U, Deng Y, Zhang Y, Greten J, Abel M, Sido B, Allenberg J, Otto G, Roth H, Bierhaus A, et al. Expression of receptors for advanced glycation end products in peripheral occlusive vascular disease. Am J Pathol. 1995;146:688–694. [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- Wautier JL, Wautier MP, Schmidt AM, Anderson GM, Hori O, Zoukourian C, Capron L, Chappey O, Yan SD, Brett J, et al. Advanced gly cation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: a link between surface-associated AGEs and diabetic complications. Proc Natl Acad Sci USA. 1994;91:7742–7746. doi: 10.1073/pnas.91.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97:238–243. doi: 10.1172/JCI118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito C, Gerlach H, Brett J, Stern D, Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989;170:1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Brett J, Mora R, Nowygrod R, Stern D. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J Clin Invest. 1993;91:2155–2168. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein M, Brett J, Radoff S, Ogawa S, Stern D, Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci USA. 1990;87:9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc Natl Acad Sci USA. 1992;89:12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H, Brownlee M, Manogue KR, Dinarello CA, Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988;240:1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- Kirstein M, Aston C, Hintz R, Vlassara H. Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product-modified proteins. J Clin Invest. 1992;90:439–446. doi: 10.1172/JCI115879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease [see comments]. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994;70:138–151. [PubMed] [Google Scholar]
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- Feener EP, King GL. Vascular dysfunction in diabetes mellitus. Lancet. 1997;350:SI9–13. doi: 10.1016/s0140-6736(97)90022-2. [DOI] [PubMed] [Google Scholar]
- Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor [see comments]. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes. 1994;43:1122–1129. doi: 10.2337/diab.43.9.1122. [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest. 1997;100:115–126. doi: 10.1172/JCI119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. Faseb J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- Park JY, Takahara N, Gabriele A, Chou E, Naruse K, Suzuma K, Yamauchi T, Ha SW, Meier M, Rhodes CJ, et al. Induction of endothelin-1 expression by glucose: an effect of protein kinase C activation. Diabetes. 2000;49:1239–1248. doi: 10.2337/diabetes.49.7.1239. [DOI] [PubMed] [Google Scholar]
- Kawano M, Koshikawa T, Kanzaki T, Morisaki N, Saito Y, Yoshida S. Diabetes mellitus induces accelerated growth of aortic smooth muscle cells: association with overexpression of PDGF beta-receptors. Eur J Clin Invest. 1993;23:84–90. doi: 10.1111/j.1365-2362.1993.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Inaba T, Ishibashi S, Gotoda T, Kawamura M, Morino N, Nojima Y, Kawakami M, Yazaki Y, Yamada N. Enhanced expression of platelet-derived growth factor-beta receptor by high glucose. Involvement of platelet-derived growth factor in diabetic angiopathy. Diabetes. 1996;45:507–512. doi: 10.2337/diab.45.4.507. [DOI] [PubMed] [Google Scholar]
- Nabel E, Shum L, Pompili V, Yang Z-Y, San H, Shu H, Liptay S, Gold L, Gordon D, Derynck R, et al. Direct transfer of transforming growth factor b1 gene into arteries stimulates fibrocellular hyperplasia. Proc Natl Acad Sci USA. 1993;90:10759–10763. doi: 10.1073/pnas.90.22.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. 1993;49:642–652. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Hirokawa J, Tagami S, Kawakami Y, Urata Y, Kondo T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia. 1995;38:201–210. doi: 10.1007/s001250050271. [DOI] [PubMed] [Google Scholar]
- Dominguez C, Ruiz E, Gussinye M, Carrascosa A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents [see comments]. Diabetes Care. 1998;21:1736–1742. doi: 10.2337/diacare.21.10.1736. [DOI] [PubMed] [Google Scholar]
- Sano T, Umeda F, Hashimoto T, Nawata H, Utsumi H. Oxidative stress measurement by in vivo electron spin resonance spectroscopy in rats with streptozotocin-induced diabetes. Diabetologia. 1998;41:1355–1360. doi: 10.1007/s001250051076. [DOI] [PubMed] [Google Scholar]
- Karpen CW, Cataland S, O'Dorisio TM, Panganamala RV. Production of 12-hydroxyeicosatetraenoic acid and vitamin E status in platelets from type I human diabetic subjects. Diabetes. 1985;34:526–531. doi: 10.2337/diab.34.6.526. [DOI] [PubMed] [Google Scholar]
- Chen MS, Hutchinson ML, Pecoraro RE, Lee WY, Labbe RF. Hyperglycemia-induced intracellular depletion of ascorbic acid in human mononuclear leukocytes. Diabetes. 1983;32:1078–1081. doi: 10.2337/diab.32.11.1078. [DOI] [PubMed] [Google Scholar]
- Yue DK, McLennan S, Fisher E, Heffernan S, Capogreco C, Ross GR, Turtle JR. Ascorbic acid metabolism and polyol pathway in diabetes. Diabetes. 1989;38:257–261. doi: 10.2337/diab.38.2.257. [DOI] [PubMed] [Google Scholar]
- Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991;266:11654–11660. [PubMed] [Google Scholar]
- Wells-Knecht MC, Thorpe SR, Baynes JW. Pathways of formation of glycoxidation products during glycation of collagen. Biochemistry. 1995;34:15134–15141. doi: 10.1021/bi00046a020. [DOI] [PubMed] [Google Scholar]
- Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995;34:3702–3709. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, Scott CW, Caputo C, Frappier T, Smith MA, et al. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci USA. 1994;91:7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisswenger PJ, Moore LL, Brinck-Johnsen T, Curphey TJ. Increased collagen-linked pentosidine levels and advanced glycosylation end products in early diabetic nephropathy. J clin Invest. 1993;92:212–217. doi: 10.1172/JCI116552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance DR, Dyer DG, Dunn JA, Bailie KE, Thorpe SR, Baynes JW, Lyons TJ. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993;91:2470–2478. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki M, Fumio U, Nawata H, King GL. Vitamin E normalizes diacylglycerol-protein kinase C activation induced by hyperglycemia in rat vascular tissues. Diabetes. 1996;45:S117–119. doi: 10.2337/diab.45.3.s117. [DOI] [PubMed] [Google Scholar]
- Kunisaki M, Bursell SE, Umeda F, Nawata H, King GL. Normalization of diacylglycerol-protein kinase C activation by vitamin E in aorta of diabetic rats and cultured rat smooth muscle cells exposed to elevated glucose levels. Diabetes. 1994;43:1372–1377. doi: 10.2337/diab.43.11.1372. [DOI] [PubMed] [Google Scholar]
- Kunisaki M, Bursell SE, Clermont AC, Ishii H, Ballas LM, Jirousek MR, Umeda F, Nawata H, King GL. Vitamin E prevents diabetes-induced abnormal retinal blood flow via the diacylglycerol-protein kinase C pathway. Am J Physiol. 1995;269:E239–246. doi: 10.1152/ajpendo.1995.269.2.E239. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation products on collagen covalently trap low-density lipoprotein. Diabetes. 1985;34:938–941. doi: 10.2337/diab.34.9.938. [DOI] [PubMed] [Google Scholar]
- Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]