Abstract
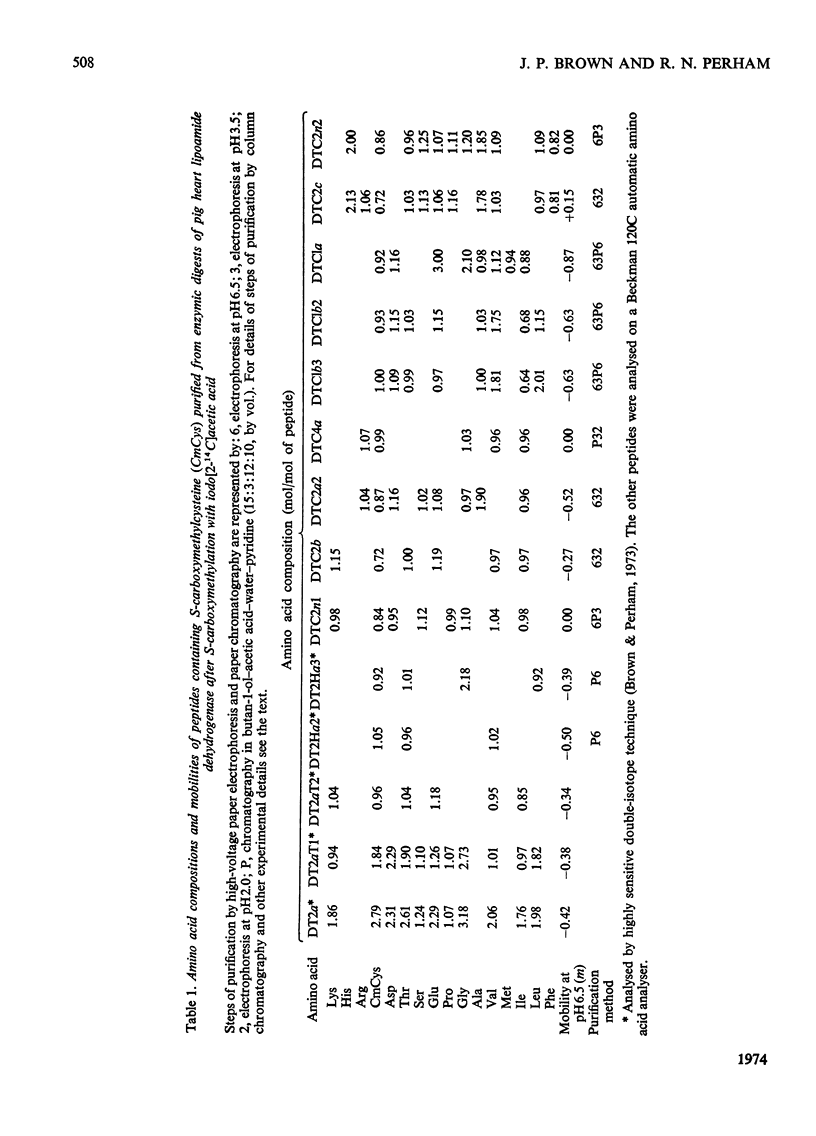
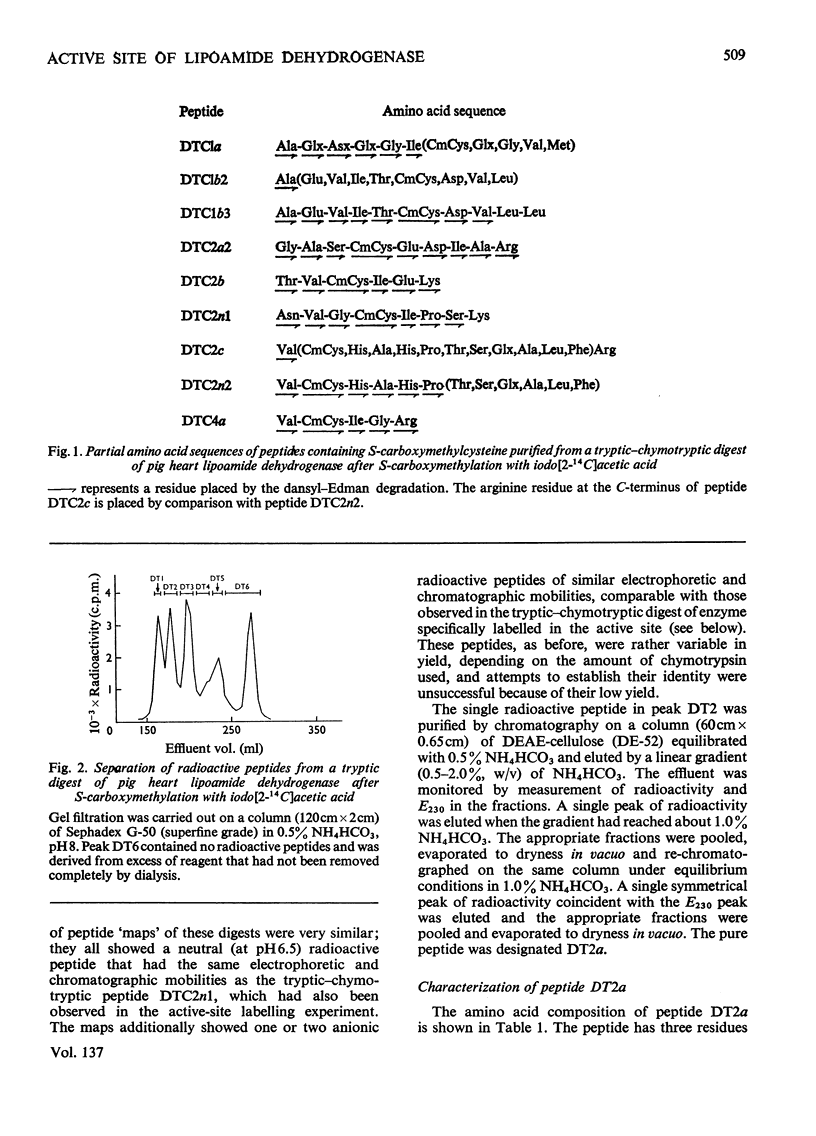
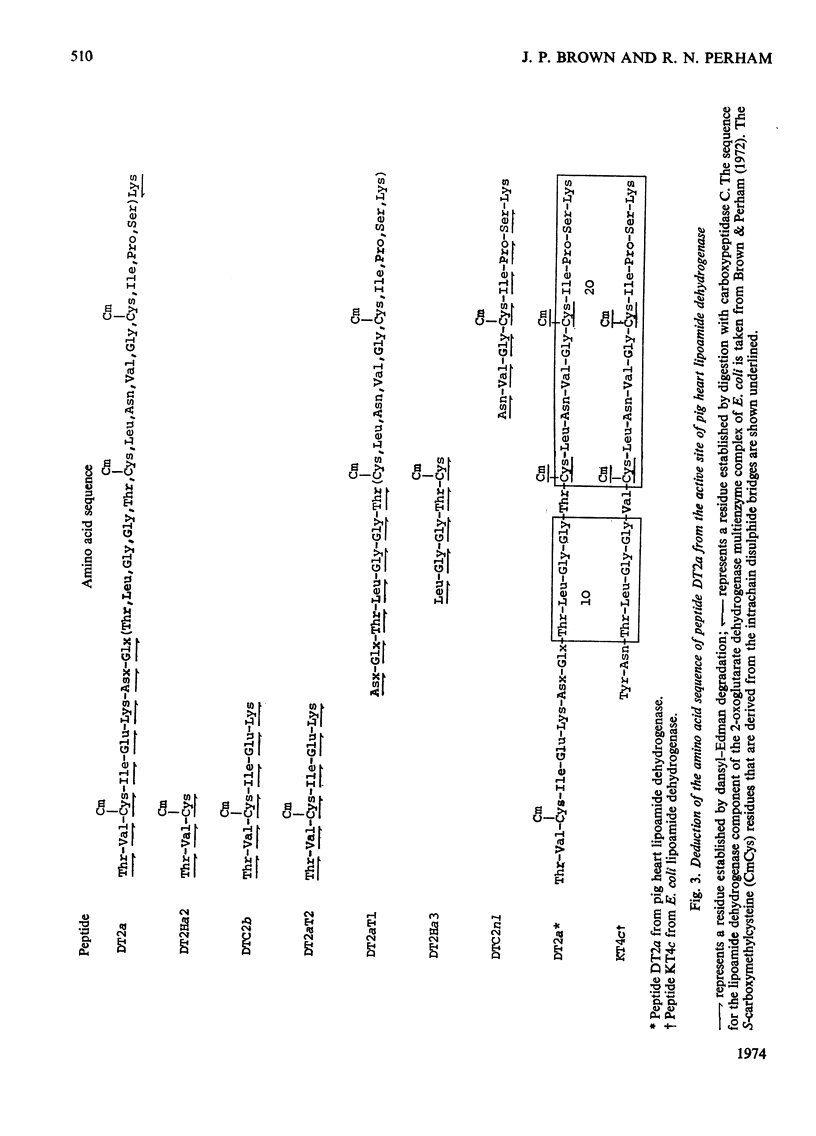
1. The two cysteine residues forming the disulphide bridge that comprises part of the active site of lipoamide dehydrogenase from pig heart were specifically labelled with iodo[2-14C]acetic acid. 2. A tryptic peptide containing these carboxymethylcysteine residues was isolated from digests of reduced and S-carboxymethylated lipoamide dehydrogenase and its amino acid sequence of 23 residues was determined. 3. The sequence is highly homologous with a similar sequence containing the active-site disulphide bridge of lipoamide dehydrogenase derived from the 2-oxoglutarate dehydrogenase complex of Escherichia coli (Crookes strain) and it is probable that, as in the bacterial enzyme, the disulphide bridge forms an intrachain loop containing six residues. The results indicate that the bacterial and mammalian proteins have a common genetic origin. 4. Amino acid sequences containing six other unique carboxymethylcysteine residues were also partly determined. 5. The analysis of the primary structure thus far is consistent with the view that the enzyme (mol.wt. approx. 110000) is composed of two identical polypeptide chains.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. P., Perham R. N. A highly sensitive method for amino-acid analysis by a double-isotope-labelling technique using dansyl chloride. Eur J Biochem. 1973 Nov 1;39(1):69–73. doi: 10.1111/j.1432-1033.1973.tb03104.x. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Perham R. N. An amino acid sequence in the active site of lipoamide dehydrogenase from the 2-oxoglutarate dehydrogenase complex of E. coli (Crookes strain). FEBS Lett. 1972 Oct 1;26(1):221–224. doi: 10.1016/0014-5793(72)80577-5. [DOI] [PubMed] [Google Scholar]
- Burleigh B. D., Jr, Williams C. H., Jr The isolation and primary structure of a paptide containing the oxidation-reduction active cystine of Escherichia coli lipoamide dehydrogenase. J Biol Chem. 1972 Apr 10;247(7):2077–2082. [PubMed] [Google Scholar]
- Gibbons I., Anderson P. J., Perham R. N. Amino acid sequence homology in the active site of rabbit and sturgeon muscle aldolases. FEBS Lett. 1970 Sep 18;10(1):49–53. doi: 10.1016/0014-5793(70)80413-6. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R., Creaghan I. T. Gene-protein relationships of the alpha-keto acid dehydrogenase complexes of Escherichia coli K12: isolation and characterization of lipoamide dehydrogenase mutants. J Gen Microbiol. 1973 Mar;75(1):197–210. doi: 10.1099/00221287-75-1-197. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. 6. The amino acid sequence of the protein from escherichia coli B. Eur J Biochem. 1968 Dec 5;6(4):475–484. doi: 10.1111/j.1432-1033.1968.tb00470.x. [DOI] [PubMed] [Google Scholar]
- KOIKE M., SHAH P. C., REED L. J. alpha-Keto acid dehydrogenation complexes. III. Purification and properties of dihydrolipoic dehydrogenase of Escherichia coli. J Biol Chem. 1960 Jul;235:1939–1943. [PubMed] [Google Scholar]
- MASSEY V., GIBSON Q. H., VEEGER C. Intermediates in the catalytic action of lipoyl dehydrogenase (diaphorase). Biochem J. 1960 Nov;77:341–351. doi: 10.1042/bj0770341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSEY V., HOFMANN T., PALMER G. The relation of function and structure in lipoyl dehydrogenase. J Biol Chem. 1962 Dec;237:3820–3828. [PubMed] [Google Scholar]
- MASSEY V., VEEGER C. Studies on the reaction mechanism of lipoyl dehydrogenase. Biochim Biophys Acta. 1961 Mar 18;48:33–47. doi: 10.1016/0006-3002(61)90512-1. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Perham R. N. A diagonal paper-electrophoretic technique for studying amino acid sequences around the cysteine and cystine residues of proteins. Biochem J. 1967 Dec;105(3):1203–1207. doi: 10.1042/bj1051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L. J., Oliver R. M. The multienzyme alpha-keto acid dehydrogenase complexes. Brookhaven Symp Biol. 1968 Jun;21(2):397–412. [PubMed] [Google Scholar]
- Ronchi S., Williams C. H., Jr The isolation and primary structure of a peptide containing the oxidation-reduction active cystine of Escherichia coli thioredoxin reductase. J Biol Chem. 1972 Apr 10;247(7):2083–2086. [PubMed] [Google Scholar]
- Sakurai Y., Fekuyoshi Y., Hamada M., Hayakawa T., Koike M. Mammalian alpha-keto acid dehydrogenase complexes. VI. Nature of the multiple forms of pig heart lipoamide dehydrogenase. J Biol Chem. 1970 Sep 10;245(17):4453–4462. [PubMed] [Google Scholar]
- Shapiro A. L., Maizel J. V., Jr Molecular weight estimation of polypeptides by SDS-polyacrylamide gel electrophoresis: further data concerning resolving power and general considerations. Anal Biochem. 1969 Jun;29(3):505–514. doi: 10.1016/0003-2697(69)90335-2. [DOI] [PubMed] [Google Scholar]
- Thelander L. The amino acid sequence of a peptide containing the active center disulfide of thioredoxin reductase from Escherichia coli. J Biol Chem. 1970 Nov 25;245(22):6026–6029. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams C. H., Jr, Arscott L. D. Sequence around the active center cystine of lipoamide dehydrogenase from pig heart, comparison with the E. coli enzyme. Z Naturforsch B. 1972 Sep;27(9):1078–1080. doi: 10.1515/znb-1972-0925. [DOI] [PubMed] [Google Scholar]
- Williams C. H., Jr, Zanetti G., Arscott L. D., McAllister J. K. Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and thioredoxin. J Biol Chem. 1967 Nov 25;242(22):5226–5231. [PubMed] [Google Scholar]


