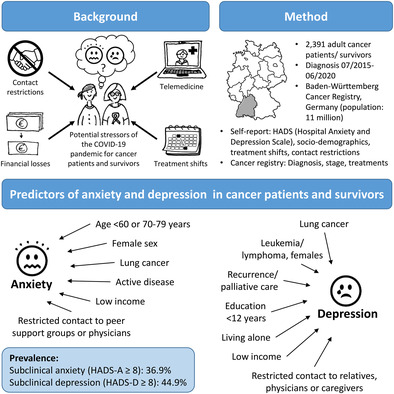
Abstract
Treatment modifications and contact restrictions were common during the COVID‐19 pandemic and can be stressors for mental health. There is a lack of studies assessing pandemic‐related risk factors for anxiety and depression of cancer patients and survivors systematically in multifactorial models. A total of 2391 participants, mean age 65.5 years, ≤5 years post‐diagnosis of either lung, prostate, breast, colorectal cancer, or leukemia/lymphoma, were recruited in 2021 via the Baden‐Württemberg Cancer Registry, Germany. Sociodemographic information, pandemic‐related treatment modifications, contact restrictions, and anxiety/depression (Hospital Anxiety and Depression Scale, HADS) were assessed via self‐administered questionnaire. Clinical information (diagnosis, stage, and treatment information) was obtained from the cancer registry. Overall, 22% of participants reported oncological care modifications due to COVID‐19, mostly in follow‐up care and rehabilitation. Modifications of active cancer treatment were reported by 5.8%. Among those, 50.5% had subclinical anxiety and 55.4% subclinical depression (vs. 37.4% and 45.4%, respectively, for unchanged active treatment). Age <60 years, female sex, lung cancer, low income, and contact restrictions to peer support groups or physicians were identified as independent risk factors for anxiety. Risk factors for depression were lung cancer (both sexes), leukemia/lymphoma (females), recurrence or palliative treatment, living alone, low income, and contact restrictions to relatives, physicians, or caregivers. The study demonstrates that changes in active cancer treatment and contact restrictions are associated with impaired mental well‐being. The psychological consequences of treatment changes and the importance for cancer patients to maintain regular contact with their physicians should be considered in future responses to threats to public health.
Keywords: cancer registry, mental health, SARS‐CoV‐2, social contact, treatment modifications, well‐being
What's new?
Many cancer patients are at increased risk of mental health issues. This risk likely was heightened during the COVID‐19 pandemic, though understanding of the pandemic's impact on mental health in cancer remains uncertain. Here, clinical and sociodemographic risk factors for anxiety and depression were analyzed for cancer patients and survivors during the COVID‐19 pandemic. Female sex, lung cancer, active disease, and low income or restricted contact with relatives or physicians were key risk factors for anxiety and depression. In particular, the findings highlight the importance of social contact as a modifiable risk factor for the mental health of cancer patients.
1. INTRODUCTION
The COVID‐19 pandemic has affected health systems worldwide. Medical resources have been reallocated to contain the spread of the virus and to prepare for the (potential) care of COVID‐19 patients. 1 , 2 These developments have impacted oncological care in many countries, 3 including Germany. 4 Cancer screening programs have been reduced or suspended by the state, and patients were more reluctant to seek healthcare services. 5 For cancer patients, treatment pathways were altered to prevent treatment in intensive care units, 5 and telehealth became more important. 6 A global study among cancer centers in 54 countries found that 88% of the centers had reduced their level of care due to precautionary measures, capacity restrictions, staff shortage, and lack of access to medications. 7 A study among Comprehensive Cancer Centers in Germany showed that capacities were decreased throughout the pandemic. The highest and most enduring restrictions were reported for follow‐up care and psycho‐oncology, but surgical treatment was also notably affected. 4
1.1. Mental health of cancer patients/survivors in the context of the COVID‐19 pandemic
Common risk factors for depression in cancer patients and survivors include female sex, comorbidities, advanced cancer stage, metastases, low physical functioning, low education and income, lack of partnership, low social support, hopelessness, and dysfunctional coping styles. 8 Low cognitive function, fatigue and insomnia could also play a role. 9 Predictors of anxiety in cancer survivors are researched less well, and they include poor social support and pain. 10 A systematic review on distress, anxiety, and depression in adolescent and young adult cancer survivors reported being female and being out of school/work as risk factors for anxiety. 11 A German study in adult cancer survivors further found associations of anxiety with younger age, living alone, low education, financial difficulties, low cognitive function, fatigue, and insomnia. 9 In times of COVID‐19, further risk factors have emerged. Social life of all society was affected due to stay‐at‐home mandates and physical contact restrictions. The prevalence of loneliness rose, and thereby also the prevalence of depression and anxiety. 12 A large cohort study in the general population in Germany reported that perceived stress increased in all age groups during the pandemic, while increases in depressive symptoms and anxiety symptoms were limited to those under 60 years of age. 13 This might be due to work‐related strains, such as job loss, financial problems, and insecurity regarding the future. 14 Patients with pre‐existing chronic conditions reported significantly more anxiety and depression during the pandemic than the general population and healthcare workers. 15 An online survey in Germany, comparing anxiety and depression in cancer patients in March 2020 and before the outbreak of the pandemic (retrospectively assessed), reported a significant increase in the rates of depression (from 9.3% to 16.7%) and of anxiety (from 8.0% to 20.7%). 16 An international systematic review of anxiety and depression in cancer patients during the COVID‐19 pandemic found even higher rates, with an overall prevalence of depression of 37% and an overall prevalence of anxiety of 38%. 17 Risk factors for mental health problems in cancer patients during the COVID‐19 pandemic included younger age, female gender, lower education, economic instability, living in urban areas, having negative coping styles, and lack of social support. 18 Cancer patients and survivors worried about limited access to healthcare, the fear of infection or of contracting the virus, insecurity, loneliness and isolation, 18 , 19 , 20 as well as practical issues about employment, finances, and transportation. 21 Studies from Turkey 22 and Germany 23 found that treatment delays or disruptions were associated with psychological problems. A large Chinese study also reported that pandemic‐related treatment shifts were a risk factor for anxiety and depression in cancer patients, while social support, being employed, and longer time since diagnosis were protective factors. 24
1.2. Study objective
Previous research on the consequences of COVID‐19 on cancer patients and survivors is often based on small samples, qualitative data or nonvalidated instruments, or is limited to patients undergoing treatment, and most included studies were conducted in the same country (China) and potentially not comparable to Europe. Therefore, the aims of the current study are to assess (1) the prevalence of anxiety and depression 1 year after the beginning of the COVID‐19 pandemic in cancer patients and survivors in Germany, (2) differences in anxiety and depression between cancer patients and survivors with changes in active oncological treatment, compared to those without changes, and (3) risk and protective factors for anxiety and depression in cancer patients and survivors, including treatment changes and contact restrictions, but also sociodemographic and clinical characteristics.
2. METHODS
2.1. Sample
The study is based on the population‐based cross‐sectional study “Consequences of Corona Restrictions for Cancer Therapy and Survivorship” (CroKuS [Folgen der Corona‐Beschränkungen für Krebstherapie und Survivorship]). A total of 2439 participants (1563 of those with cancer diagnosis between 07/2019 and 06/2020, defined as “patients,” and 876 with cancer diagnosis between 07/2015 and 06/2019, defined as “survivors”) were recruited between May and December 2021 via the Baden‐Württemberg Cancer Registry (BWCR), Germany.
Inclusion criteria for recruitment were: Histologically confirmed diagnosis of lung (ICD‐10 C33‐34), prostate (ICD‐10 C61), breast (ICD‐10 C50), colorectal (ICD‐10 C33‐34), or hematological (ICD‐10 C81‐C96) cancer, 18–85 years of age, and capable of providing consent. Exclusion criteria were death prior to receipt of the questionnaire, refusal to have identity data recorded in the BWCR, or general refusal to participate in studies. We excluded participants with second malignant tumors (ICD‐10 C00‐C97, excluding C44) and participants who reported comorbid carcinoma in situ being likely to have been treated during the study period.
Potentially eligible cancer patients and survivors (n = 14,184) were randomly selected from the BWCR, according to a stratified sampling scheme by tumor and time since diagnosis. Invitations were sent in two recruitment waves (April and August 2021) by the registry's trust center via postal mail. Those willing to participate (n = 2978) gave written informed consent and subsequently received a paper questionnaire by post from the German Cancer Research Center (DKFZ). Overall, 2509 persons returned the questionnaire (response rate: ~85% among those who received the questionnaire, 21% overall). Of these, 5 were excluded because the questionnaire had too many missing responses in relevant questions, 49 because of a malign or in situ second cancer (previously not known to the registry), and 16 because of an invalid cancer diagnosis, due to a downgrading of a previously malign to an in situ primary diagnosis in the registry, or cancer type unknown to the participant and no consent to link the registry data (Flowchart: Figure S1). For the current analysis, we further excluded N = 48 participants who did not consent to link their cancer registry information and who did not indicate whether they were in active cancer treatment during the pandemic, resulting in a final sample of N = 2391.
2.2. Measures
Participants' clinical data (diagnosis, stage, and treatment information) were linked from the cancer registry after written consent was obtained from the participants. Sociodemographic information and the frequency and burden of individual restrictions in social life and oncological care were assessed by self‐report in the questionnaire. For those participants without self‐reported changes in oncological care, we determined—based on the dates of registered treatments—whether surgery, systemic therapy or radiotherapy was scheduled between January 2020 (beginning of the COVID‐19 pandemic) and the time of the survey. Age at survey was defined as year of participation minus year of birth. Anxiety and depression were assessed by the Hospital Anxiety and Depression Scale (HADS). 25 For both the anxiety (HADS‐A) and the depression (HADS‐D) subscale, scores of 8 and above have been proposed to identify subclinical cases and scores of 11 and higher to identify clinically significant cases. 26 Over the past decades, different cutoffs have been used and recommended, 27 , 28 also specifically for cancer patients. 25 , 29 Nevertheless, in the current study, the most widely used cutoff scores of ≥8 and ≥11 were used to enable comparison of the results with other studies. 17 In the regression models, body mass index (BMI) was calculated as weight (kg)/height (m)2 and classified according to the WHO guidelines as underweight (below 18.5), normal weight (18.5 to <25), pre‐obesity (25 to <30), and obesity (30 and higher). For persons living alone, low household income was classified as 1200 € and less (2000 € and less for shared households), average as 1201–2000 € (2001–4000 € for shared households), and higher average to high as more than 2000 € (more than 4000 € for shared households). 30
2.3. Statistical analyses
To compare patients and survivors with respect to sociodemographic and clinical characteristics and COVID‐19 restrictions, we employed chi‐square tests for categorical variables and t tests for continuous variables. Logistic regression was used to model the prevalence of anxiety and depression in participants with and without changes in their active cancer treatment due to the COVID‐19 pandemic, adjusted for age, sex, education, tumor site, and stage. In a second step, further factors that are potentially associated with anxiety and depression (partner, employment, time since diagnosis, treatment phase, previous COVID‐19 infection, BMI, and contact restrictions) were included in the logistic regression models to calculate odds ratios (ORs) for anxiety and depression based on these factors. Statistical analyses were performed using SAS Enterprise Guide 7.15; p <0.05 was considered statistically significant. Multiple imputation (MI) with 25 imputations was used to handle missing values for all relevant scales except the questions on treatment changes, in order not to overestimate the actual number of pandemic‐related treatment changes in Germany. MI estimates missing values based on the distribution of variables in the data set and on their associations. In contrast to single imputation, MI takes into account the uncertainty of the estimation by drawing multiple values from the likely range of values and thus creating multiple imputed data sets to be used as a basis for subsequent analyses. The final estimates are the combined (averaged) results from all data sets. 31 Graphics were created using Microsoft Excel 2016.
3. RESULTS
3.1. Comparison of nonrespondents and respondents
Based on the cancer registry data, we compared respondents and nonrespondents to check the representativeness of the sample (Table S1). While there was no difference regarding sex, respondents were 2.5 years younger at survey and at diagnosis. Response was slightly higher for those with more recent diagnosis (1–2 years before the survey). Patients and survivors with breast or prostate cancers as well as hemato‐oncological diseases were more likely to participate, compared to those with colorectal and especially lung cancer. Stages I and II were slightly overrepresented, while the prevalence of Stage IV was lower in respondents. Overall, although most differences were statistically significant, most differences were small, and respondents showed sufficient variability regarding their sociodemographic and clinical characteristics.
3.2. Sample characteristics
The mean age of participants at the time of the survey was 65.5 years (Table 1). There was a balanced representation across all levels of education. The majority lived with their spouse/partner. Half of the sample was retired, and three out of four participants had an average to high household income. There were no substantial differences in socio‐demographics between patients and survivors (Table 1). At the time of the survey in the second half of 2021 (after a 6‐month lockdown in Germany from December 2020 to May 2021), 85% of the participants still reported restrictions in public life, about 75% stated restricted contact with relatives and 29% reported restricted contact with peer support groups (Table 1). Patients were more likely than survivors to report contact restrictions to relatives and peer support groups. Patients also indicated a higher burden resulting from these restrictions (Table 1).
TABLE 1.
Sample characteristics (sociodemographic).
| Overall | Patients a | Survivors b | p diff | ||||
|---|---|---|---|---|---|---|---|
| Mean c | SD d | Mean | SD | Mean | SD | p (t) | |
| Mean age at survey | 65.5 | 11.8 | 65.4 | 11.7 | 65.5 | 11.9 | .78 |
| N | % | n | % | n | % | p (χ 2) | |
|---|---|---|---|---|---|---|---|
| Total | 2391 | 100 | 1535 | 100 | 856 | 100 | |
| Age at survey | .69 | ||||||
| 18–49 years | 186 | 7.8 | 121 | 7.9 | 65 | 7.6 | |
| 50–59 years | 453 | 18.9 | 295 | 19.2 | 158 | 18.5 | |
| 60–69 years | 774 | 32.4 | 504 | 32.8 | 270 | 31.5 | |
| 70–79 years | 726 | 30.4 | 450 | 29.3 | 276 | 32.2 | |
| 80–86 years | 252 | 10.5 | 165 | 10.7 | 87 | 10.2 | |
| Sex | .16 | ||||||
| Female | 1231 | 51.5 | 774 | 50.4 | 457 | 53.4 | |
| Education | .72 | ||||||
| ≤9 years | 823 | 34.4 | 521 | 33.9 | 303 | 35.4 | |
| 10–11 years | 719 | 30.1 | 462 | 30.1 | 257 | 30.0 | |
| ≥12 years | 849 | 35.5 | 553 | 36.0 | 296 | 34.6 | |
| Living situation/partnership | .2 | ||||||
| Living with spouse/partner | 1814 | 75.9 | 1187 | 77.3 | 628 | 73.4 | |
| Having partner, living alone | 101 | 4.2 | 59 | 3.8 | 42 | 4.9 | |
| No partner, living alone | 383 | 16.0 | 232 | 15.1 | 151 | 17.6 | |
| Living with others | 61 | 2.6 | 38 | 2.5 | 23 | 2.7 | |
| Senior or nursing home | 7 | 0.3 | 6 | 0.4 | 1 | 0.1 | |
| Other | 24 | 1.0 | 13 | 0.8 | 11 | 1.3 | |
| Employment situation | .23 | ||||||
| Employed | 574 | 24.0 | 389 | 25.3 | 186 | 21.7 | |
| Freelancer | 103 | 4.3 | 72 | 4.7 | 31 | 3.6 | |
| Civil servant | 97 | 4.0 | 64 | 4.2 | 32 | 3.8 | |
| Retired | 1308 | 54.7 | 808 | 52.7 | 500 | 58.4 | |
| Unemployed | 207 | 8.7 | 137 | 8.9 | 71 | 8.3 | |
| Other | 58 | 2.4 | 38 | 2.5 | 20 | 2.4 | |
| Multiple jobs | 44 | 1.8 | 28 | 1.8 | 16 | 1.9 | |
| Household income e | .62 | ||||||
| Low | 537 | 22.5 | 342 | 22.3 | 196 | 22.9 | |
| Average | 1161 | 48.6 | 757 | 49.3 | 404 | 47.2 | |
| High | 693 | 29.0 | 437 | 28.4 | 256 | 29.9 | |
| Contact restrictions | |||||||
| … with relatives | 1811 | 75.8 | 1192 | 77.7 | 619 | 72.3 | .004 |
| … with peer support groups | 694 | 29.0 | 477 | 31.0 | 217 | 25.4 | .003 |
| … with physicians | 380 | 15.9 | 247 | 16.1 | 133 | 15.6 | .75 |
| … with caregivers | 401 | 16.8 | 269 | 17.5 | 133 | 15.5 | .20 |
| … with public | 2039 | 85.3 | 1324 | 86.2 | 715 | 83.5 | .07 |
| Burdened by contact restrictions f | |||||||
| … with relatives | 828 | 34.6 | 553 | 36.0 | 275 | 32.1 | .0077 |
| … with peer support groups | 147 | 6.1 | 106 | 6.9 | 41 | 4.8 | .0131 |
| … with physicians | 104 | 4.3 | 70 | 4.6 | 34 | 4.0 | .79 |
| … with caregivers | 104 | 4.3 | 71 | 4.6 | 33 | 3.9 | .43 |
| … with public | 1041 | 43.5 | 686 | 44.7 | 355 | 41.5 | .05 |
Note: Bold p values mark statistically significant differences (p < .05) between cancer patients and survivors in global comparison.
Patients: cancer patients diagnosed between 07/2019 and 06/2020.
Survivors: cancer survivors diagnosed between 01/2015 and 06/2019.
All results are based on 25 imputations of missing values (except items on burden as these are conditional). Missings were generally not higher than 5%–10% per variable. Percentages might not add up to 100% due to rounding of multiple imputation results.
SD: standard deviation.
Low: single households ≤1200 €, shared households ≤2000 €; Average: single households 1201–2000 €, shared households 2001–4000 €; and high: single households >2000 €, shared households >4000 €.
Feeling “burdened” by contact restrictions includes the answers “very much” and “quite a bit” (compared to “partially,” “rather not,” and “not at all”).
The sample was also balanced in terms of cancer types and stages, with no significant differences between patients and survivors (Table 2). More than half of the sample were in follow‐up care at the time of the survey, with patients significantly more likely to be in primary treatment and survivors significantly more likely to be in palliative care. Of the whole sample, 46% indicated that their treatment had ended, and 61% still perceived themselves as a cancer patient (regardless of treatment phase). About one third of the sample still reported a substantial physical and/or mental burden from cancer, with patients reporting a slightly higher burden than survivors (Table 2). Overall, 21.9% of participants (patients 25.1% and survivors 16.1%) reported a modification of their oncological care due to the COVID‐19 pandemic (Table 2). Patients with more recent diagnoses reported significantly more changes (25%) compared to survivors (16%). Most of these changes referred to rehabilitation and follow‐up care (Table S2), while 5.8% of the overall sample reported a change in their active cancer treatment (Table 2). Considering that only 60% of the sample had active treatment during the relevant time period (Table 2), this means that 9.6% of planned active treatments had been changed (11.8% of planned surgeries, 6.2% of planned systemic therapies, and 3.2% of planned radiotherapies, Table S2).
TABLE 2.
Sample characteristics (clinical).
| Overall | Patients a | Survivors b | p diff | ||||
|---|---|---|---|---|---|---|---|
| N c | % | n | % | n | % | p (χ2) | |
| Tumor | .2 | ||||||
| Breast cancer | 318 | 13.3 | 207 | 13.5 | 111 | 13.0 | |
| Colorectal cancer | 628 | 26.3 | 399 | 26.0 | 229 | 26.8 | |
| Lung cancer | 523 | 21.9 | 318 | 20.7 | 205 | 23.9 | |
| Prostate cancer | 303 | 12.7 | 209 | 13.6 | 94 | 11.0 | |
| Leukemia or lymphoma | 619 | 25.9 | 402 | 26.2 | 217 | 25.4 | |
| Stage (UICC) | .94 | ||||||
| I | 472 | 19.8 | 303 | 19.7 | 170 | 19.8 | |
| II | 483 | 20.2 | 303 | 19.7 | 181 | 21.1 | |
| III | 461 | 19.3 | 297 | 19.3 | 165 | 19.2 | |
| IV | 355 | 14.8 | 231 | 15.0 | 124 | 14.5 | |
| n.a. (leukemia/lymphoma) | 619 | 25.9 | 402 | 26.2 | 217 | 25.4 | |
| Treatment phase (at survey) | .0087 | ||||||
| Diagnosis | 89 | 3.7 | 58 | 3.8 | 31 | 3.7 | .87 |
| Primary treatment | 269 | 11.2 | 190 | 12.4 | 79 | 9.2 | .0185 |
| Remission | 502 | 21.0 | 336 | 21.9 | 165 | 19.3 | .14 |
| Follow‐up | 1293 | 54.1 | 819 | 53.3 | 474 | 55.3 | .35 |
| Recurrence | 127 | 5.3 | 71 | 4.6 | 56 | 6.6 | .0378 |
| Palliative care | 111 | 4.7 | 61 | 4.0 | 50 | 5.9 | .0345 |
| Has your cancer treatment ended? | .16 | ||||||
| Yes | 1089 | 45.6 | 683 | 44.5 | 406 | 47.5 | |
| Do you still perceive yourself as being a cancer patient? | .44 | ||||||
| Yes | 1451 | 60.7 | 941 | 61.3 | 511 | 59.6 | |
| To which extent do you feel currently burdened by the cancer? | .0036 | ||||||
| Not at all | 531 | 22.2 | 317 | 20.7 | 214 | 25.0 | .0144 |
| | | 1079 | 45.1 | 701 | 45.7 | 378 | 44.1 | .47 |
| | | 537 | 22.5 | 339 | 22.1 | 198 | 23.2 | .53 |
| Very much | 244 | 10.2 | 178 | 11.6 | 66 | 7.7 | .0023 |
| Any change in cancer care d | |||||||
| Yes | 533 | 22.3 | 392 | 25.5 | 141 | 16.5 | <.0001 |
| Active cancer treatment planned between 01/2020 and survey e | |||||||
| Yes | 1436 | 60.1 | 1132 | 73.7 | 304 | 35.5 | <.0001 |
| Change in cancer treatment e | |||||||
| Any | 138 | 5.8 | 114 | 7.4 | 24 | 2.8 | <.0001 |
| Surgery | 72 | 3.0 | 63 | 4.1 | 9 | 1.1 | <.0001 |
| Systemic therapy | 65 | 2.7 | 50 | 3.3 | 15 | 1.8 | <.0001 |
| Radiotherapy | 16 | 0.7 | 14 | 0.9 | 2 | 0.2 | <.0001 |
Note: Bold p values mark statistically significant differences (p < .05) between cancer patients and survivors in global comparison. For statistically significant global differences, also the results of level‐wise comparison are shown.
Patients: cancer patients diagnosed between 07/2019 and 06/2020.
Survivors: cancer survivors diagnosed between 01/2015 and 06/2019.
All results are based on 25 imputations of missing values (except items on changes in cancer care and treatment). Missings were generally not higher than 5%–10% per variable. Percentages might not add up to 100% due to rounding of multiple imputation results.
Cancer care includes surgery, systemic therapy, radiotherapy, follow‐up care, rehabilitation, psycho‐oncology, and/or nursing care.
Active cancer treatment includes surgery, systemic therapy, and/or radiotherapy.
3.3. Prevalence of anxiety and depression
The prevalence of anxiety in the overall study sample was 16.3% (subclinical: 36.7%, Table S3). The prevalence for participants with a change in active cancer treatment (surgery, systemic, or radiotherapy) was 25.2% (subclinical: 50.5%) compared to 15.6% (subclinical: 37.4%) in the group with “unchanged” active cancer treatment, and 14.4 (subclinical: 37.1%) in the group with no active cancer treatment during the relevant time period (adjusted for age, sex, education, cancer site, and stage, Figure 1). The overall prevalence of depression was 19.4% (subclinical: 44.8%, Table S3) and was higher in participants who reported a change in active cancer treatment (30%; subclinical: 55.4%) than among those without active treatment change (19.0%; subclinical: 45.4%) and among those without active cancer treatment (15.2%; subclinical: 42.4%); adjusted for age, sex, education, cancer site, and stage (Figure 1, all differences in the anxiety and depression prevalence between the subgroup with changed treatment and those without altered treatment were statistically significant).
FIGURE 1.
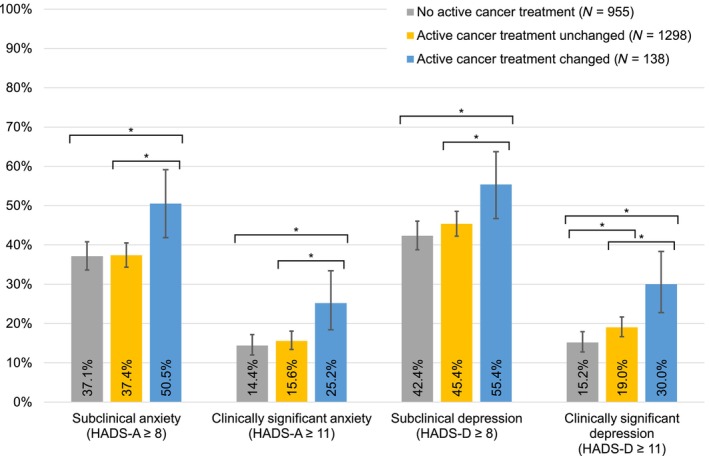
Prevalence of anxiety and depression, stratified by change of active treatment due to the COVID‐19 pandemic. Active treatment refers to surgery, systemic, or radiotherapy in the time between January 2020 and the survey in 2021, according to self‐report or cancer registry. Change refers to self‐report of cancellation, postponement or other change of surgery, systemic or radiotherapy due to the COVID‐19 pandemic. Participants who had only changes in further domains like rehabilitation are not included in the group with changes due to a lack of information on the number of planned treatments for these domains. All percentages were adjusted for age at survey, sex, education, tumor site, and stage. The spans of the lines with asterisks (*) indicate which subgroups differ statistically significant (p < .05) in pairwise comparison, e.g. participants with a change in active cancer treatment reported more subclinical anxiety than the two other groups, whereas there was no significant difference in anxiety between those with unchanged treatment and those without treatment.
3.4. Risk factors for anxiety and depression
To analyze risk and protective factors for anxiety and depression, we included additional factors in the models and calculated ORs (Tables S4 and S5). When contact restrictions to physicians were included, the change of active treatment was no longer relevant in explaining anxiety and depression. Independent risk factors for anxiety were age <60 years, female sex (for colorectal cancer, leukemia/lymphoma, and lung cancer), lung cancer, low income, contact restrictions to peer support groups and to physicians (Figure 2). The prevalence of anxiety was lower in individuals aged 70–79 years and being in remission. Independent risk factors for depression were lung cancer, leukemia/lymphoma (only females), recurrence or palliative treatment, low income, living alone, and contact restrictions to relatives, physicians, and caregivers. Higher education was associated with lower risk of depression (Figure 3). The risk factors for the cutoff scores of ≥11 for clinically significant anxiety and depression showed a similar pattern. The main differences were that Stage IV emerged as additional predictor for anxiety, and age 50–59 years was additionally associated with depression, while the associations of cancer site/sex and education with depression were not statistically significant (Figures S2 and S3).
FIGURE 2.
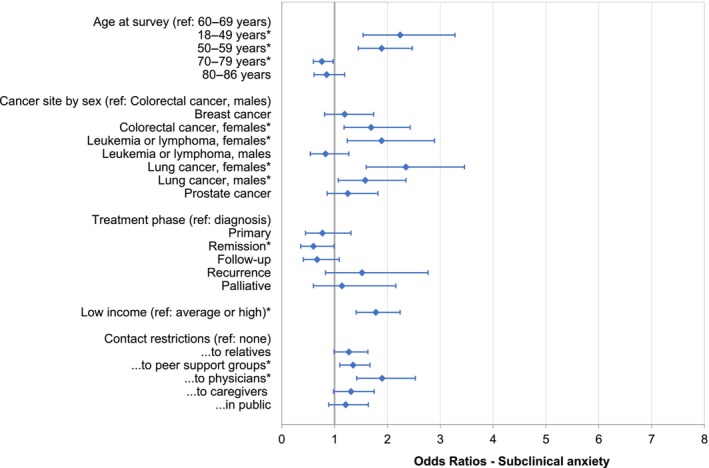
Odds ratios for subclinical anxiety (HADS‐A cutoff ≥8). The asterisks (*) reflect statistically significant factors in the overall model. Further nonsignificant factors in the model were: Treatment change, employment, education, living situation, stage, patient versus survivor, previous COVID‐19 infection, and BMI (data not shown). All results are based on 25 imputations of missing values in the nonconditional variables. BMI, body mass index; HADS‐A, anxiety subscale of the Hospital Anxiety and Depression Scale.
FIGURE 3.
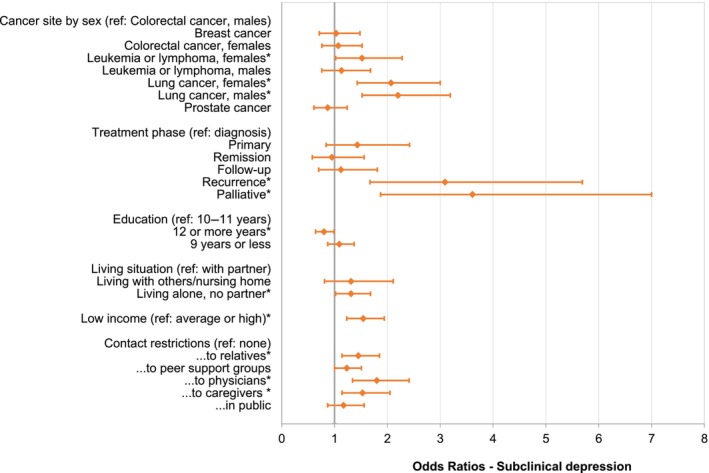
Odds ratios for subclinical depression (HADS‐D cutoff ≥8). The asterisks (*) reflect statistically significant factors in the overall model. Further nonsignificant factors in the model were: Treatment change, age at survey, employment, stage, patient versus survivor, previous COVID‐19 infection, and BMI (data not shown). All results are based on 25 imputations of missing values in the nonconditional variables. BMI, body mass index; HADS‐D, depression subscale of the Hospital Anxiety and Depression Scale.
We further explored whether the reasons behind changes in active cancer treatment, and one's own involvement in the decision‐making process contribute to feelings of anxiety and depression. The questions on these aspects had too much missing data and the results did not show a clear pattern (Table S6). For surgeries and radiotherapies, an alteration due to high infection risk seemed to be associated with higher anxiety and depression but none of the comparisons was statistically significant. Patients' inclusion in the decision‐making process, but also not knowing who made the decision about treatment change (compared to the decision by the physician alone) was associated with higher anxiety (Table S6).
4. DISCUSSION
The media reported on treatment shifts in oncological care very early in the pandemic, and it is important to quantify the proportion of patients and survivors affected by this problem and potential long‐term consequences. Although the impact of COVID‐19 itself on the care systems worldwide has diminished in the meantime, treatment shifts can also happen due to other reasons like lack of personnel in the health sector or delivery problems of medication, and it is important to be aware of the physical and psychological consequences. Pre‐pandemic studies have shown that even short treatment delays of 4 weeks can be associated with a 6%–8% increased mortality in cancer patients. 32
As decreases in the absolute numbers of cancer therapies might also result from decreases in diagnoses, 33 we analyzed the proportions of diagnosed cancer survivors reporting changes. In our sample, 22% of participants stated modifications of their oncological care, which is comparable to a large Dutch study, where 19%–29% of patients reported modifications, including changes to telehealth. 34 In our sample, 12% of the planned surgeries were affected by changes. This is in line with the nonoperation rates during full lockdowns found in an international study 35 and also with German cancer registry data 36 and capacity restrictions reported by German Comprehensive Cancer Centers. 4 Systemic and radiotherapy were less likely to be changed than surgery, which is consistent with a study among clinicians worldwide. 37 Further studies in the coming years should investigate whether and which treatment modifications will be associated with lower quality of life or a higher rate of recurrence in the long term.
Not only medical care, but also contact with clinicians, peer support groups or family and friends are important for the recovery of cancer patients. Repeated lockdowns and social isolation regulations in various intensities affected everyone. The second lockdown in Germany ended shortly before the study, but a broad majority of the study population still reported contact restrictions in public and in their own lives at the time of the survey. Hospital visits were restricted for a long time in various intensities (no visits at all, only one person, only vaccinated or tested persons), which might be reflected in the higher rate of restricted contact with relatives in cancer patients compared to survivors and the higher burden by these restrictions. Besides this, fear of infection during the active treatment could have led to a prolonged period of self‐initiated isolation for cancer patients, beyond the “official” regulations. Another reason for the higher burden might be that relatives often act as additional caregivers during treatment.
The COVID‐19 pandemic has increased feelings of insecurity, fear, and worry in the whole population. 38 , 39 For cancer patients, modifications in care or contact restrictions to caregivers, physicians, or peer support groups could have further increased the risk of psychological distress. In our sample, 36.7% of the cancer patients/survivors showed subclinical anxiety, which is comparable to a meta‐analysis in cancer patients during COVID‐19 that found a prevalence of 38%, while the prevalence of 44.8% for subclinical depression in our sample was higher than in the meta‐analysis (37% overall, only 27% in studies using HADS‐D). 17 The frequency of anxiety and depression found in our study are also higher than in studies of the general population from the beginning of the pandemic, where prevalence of 31.9% for anxiety and 33.7% for depression were reported. 40 However, differences to other studies should not be over‐interpreted due to different instruments and age ranges of the samples and time of assessment. The current study was conducted more than a year after the beginning of the pandemic, whereas most published studies report data from the beginning of the pandemic. Fear and hopelessness may have increased as exposure to the unpleasant situation prolonged, but on the other hand, participants might also have developed more coping strategies.
A change of active treatment of the tumor during the COVID‐19 pandemic was associated with higher levels of anxiety and depression, which is in line with previous studies. 41 , 42 As our study was cross‐sectional and we did not have information on participants' level of anxiety and depression prior to the pandemic, this finding cannot be interpreted causally. We controlled for active treatment and found that the differences in anxiety and depression between those with “unchanged” active treatment and those who only received follow‐up care or no treatment were small. However, there might be further factors that explain the association. Patients who were more anxious or depressive might also have been more likely to postpone or cancel their treatment, either on their own initiative due to fears because of feelings of guilt and worthlessness, or because they did not feel stable enough to “fight” for the planned treatment. Further, anxious and depressive cancer survivors might also be more focused on negative experiences, remembering or reporting minor modifications of treatment that mentally stable patients did not consider relevant.
The results showed that once the contact restrictions to the physician were taken into account, treatment changes no longer explained the variance in anxiety and depression. This is in line with a German study in cancer patients finding that satisfaction with information provision was a predictor of anxiety. 43 In our study, those who reported that their clinician alone decided on the treatment change were less anxious, compared to those involved in the decision‐making process. At first sight, this might be counter‐intuitive, because patient involvement is supposed to be beneficial, but it could be a sign that the doctor's explanation gave them a sense of control and connection, while deciding alone is also a sign of a lack of contact. Besides, only nonurgent surgeries were supposed to be rescheduled, suggesting that patients did not have mental problems due to suffering from the direct consequences of not receiving treatment but rather from the feeling of being left alone. The high frequency of mental health problems in the group with changed treatment can be understood as an alarm signal, especially as the survey took place after the end of the “actual” lockdown. Participants under the age of 70 years, females, those with lung cancer, and those with a recurrence or in palliative care were at higher risk of mental health problems.
Anxiety and depression are not only a personal burden for patients, but are also associated with higher cancer‐specific and all‐cause mortality, 44 so it is important to monitor the mental well‐being of vulnerable cancer patients and survivors at risk and to help them strengthen their resilience. A German study found that the perception of nature, silence, and wondering, as well as the meaning of life and religious trust were important resources for cancer patients during the pandemic, and the authors suggest offering guided forest walks or virtual walks, meditation, and mindfulness interventions. 45 In the general population, it has been shown that regular and stable moderate to vigorous physical activity during the COVID‐19 pandemic was associated with less depressive and anxiety symptoms. 46 Information satisfaction has also previously been shown to be associated with anxiety levels in cancer patients. 47 Telehealth can be a compromise to allow patients to be informed and to stay in contact with their physicians in times of social distancing. The COVID‐19 pandemic has helped to improve such services, and for rare cancers or patients from rural areas, online services may even be an advantage to consult specialists they otherwise would not reach. 48
4.1. Strengths and limitations
Several limitations need to be considered when interpreting the results of this study. Unfortunately, the overall response rate was lower than expected. Cancer patients/survivors without restrictions might have been less motivated to participate and to share their experiences, while at the same time there is the possibility of healthy survivor bias. Cancer patients/survivors with lower incomes might be underrepresented in the sample. Nevertheless, the differences between respondents and nonrespondents were small regarding sex, age, and clinical characteristics, and a substantial proportion of participants with lower levels of education, with lung cancer and Stage IV cancers were included, who potentially may also have a higher burden. The relatively high rates of anxiety and depression also show that not only healthy survivors have participated.
The cross‐sectional design of the study does not allow for causal interpretations to explain anxiety and depression. We sought to include cancer survivors diagnosed more than half a year before the start of the pandemic as a control group with fewer modifications in treatment, but due to the heterogeneity of diagnoses and stages in the overall sample, many “survivors” were still in active treatment and thus reported changes in treatment, while some “patients” had already completed treatment before the pandemic. Considering that only about half of the sample was still in active treatment during the pandemic, the absolute rate of care modifications in cancer patients and survivors might in fact have been even higher.
Delays in case notification to and processing at the cancer registry (both not related to the COVID‐19 pandemic) caused the survey could take up only more than a year after the pandemic had started and we asked participants retrospectively about care modifications. This may have led to recall bias; however, to our knowledge this is the first study to ask a large cohort of cancer patients/survivors about their experiences beyond the first wave of the pandemic.
5. CONCLUSION
It is reassuring that the majority of study participants did not encounter major difficulties regarding oncological care during the COVID‐19 pandemic. However, for those who did experience changes in oncological care, potential long‐term consequences are still pending. In addition, changes in active treatment and contact restrictions were associated with mental well‐being, and the number of participants suggestive of anxiety or depression was generally high. Health management in times of crisis should balance the physical and psychological consequences against the benefits of reducing the numbers of infections. It is important to monitor the mental well‐being of cancer patients and survivors at risk and to support their resilience.
AUTHOR CONTRIBUTIONS
Daniela Doege: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; visualization; writing – original draft. Julien Frick: Methodology; writing – review and editing. Rachel D. Eckford: Conceptualization; validation; writing – review and editing. Lena Koch‐Gallenkamp: Resources; writing – review and editing. Michael Schlander: Conceptualization; funding acquisition; writing – review and editing. Susanne Bergbold: Data curation; resources; writing – review and editing. Silke Hermann: Resources; writing – review and editing. Dagmar Schuldt: Resources; writing – review and editing. Volker Arndt: Conceptualization; funding acquisition; methodology; project administration; supervision; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Medical Faculty of the University of Heidelberg (January 13, 2021, S‐974/2020). Written informed consent was obtained from all individual participants included in the study. The study is registered in the German Clinical Trials Register (DRKS00025155).
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGMENTS
We thank the data managers Folke Thormann and Anja Wolf (Division of Clinical Epidemiology and Aging Research, DKFZ, Heidelberg) for their assistance in the study, including study monitoring, questionnaire mailing, scanning of questionnaires, and data processing and verification. This work was supported by a grant (25.000 €) from the Trial Pool of the National Center for Tumor Diseases (NCT) Heidelberg. Open Access funding enabled and organized by Projekt DEAL.
Doege D, Frick J, Eckford RD, et al. Anxiety and depression in cancer patients and survivors in the context of restrictions in contact and oncological care during the COVID‐19 pandemic. Int J Cancer. 2025;156(4):711‐722. doi: 10.1002/ijc.35204
Members of Baden‐Württemberg Cancer Registry: Susanne Bergbold, Epidemiological Cancer Registry of Baden‐Württemberg, German Cancer Research Center (DKFZ), Heidelberg, Germany. Silke Hermann, Epidemiological Cancer Registry of Baden‐Württemberg, German Cancer Research Center (DKFZ), Heidelberg, Germany. Dagmar Schuldt, Trust Center of Baden‐Württemberg Cancer Registry, German Federal Pension Insurance, Karlsruhe, Germany.
Contributor Information
Daniela Doege, Email: d.doege@dkfz.de.
Baden‐Württemberg Cancer Registry:
DATA AVAILABILITY STATEMENT
All source code is publicly available on Github (https://github.com/DDoege/CroKuS-study-German-Cancer-Research-Center.git). The other data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Burki TK. Cancer care in the time of COVID‐19. Lancet Oncol. 2020;21:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bundestag . Gesetz zum Ausgleich COVID‐19 bedingter finanzieller Belastungen der Krankenhäuser und weiterer Gesundheitseinrichtungen (COVID‐19‐Krankenhausentlastungsgesetz). [Act to compensate hospitals and other healthcare facilities for financial burdens caused by COVID‐19] Bundesgesetzblatt Teil I, Nr. 14 vom 27.03.2020: Bundesanzeiger, 2020.
- 3. Alom S, Chiu CM, Jha A, Lai SHD, Yau THL, Harky A. The effects of COVID‐19 on cancer care provision: a systematic review. Cancer Control. 2021;28:1‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arndt V, Doege D, Fröhling S, et al. Cancer care in German centers of excellence during the first 2 years of the COVID‐19 pandemic. J Cancer Res Clin Oncol. 2023;149:913‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID‐19 pandemic on cancer care. Nat Cancer. 2020;1:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garfan S, Alamoodi AH, Zaidan BB, et al. Telehealth utilization during the Covid‐19 pandemic: a systematic review. Comput Biol Med. 2021;138:104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jazieh AR, Akbulut H, Curigliano G, et al. The impact of COVID‐19 pandemic on cancer care: a global collaborative study. JCO Glob Oncol. 2020;6:1428‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riedl D, Schüßler G. Factors associated with and risk factors for depression in cancer patients—a systematic literature review. Transl Oncol. 2022;16:101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Götze H, Friedrich M, Taubenheim S, Dietz A, Lordick F, Mehnert A. Depression and anxiety in long‐term survivors 5 and 10 years after cancer diagnosis. Support Care Cancer. 2020;28:211‐220. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long‐term cancer survivors compared with spouses and healthy controls: a systematic review and meta‐analysis. Lancet Oncol. 2013;14:721‐732. [DOI] [PubMed] [Google Scholar]
- 11. Osmani V, Hörner L, Klug SJ, Tanaka LF. Prevalence and risk of psychological distress, anxiety and depression in adolescent and young adult (AYA) cancer survivors: a systematic review and meta‐analysis. Cancer Med. 2023;12:18354‐18367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berger K, Riedel‐Heller S, Pabst A, Rietschel M, Richter D. Loneliness during the first wave of the SARS‐CoV‐2 pandemic‐results of the German National Cohort (NAKO). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2021;64:1157‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters A, Rospleszcz S, Greiser KH, Dallavalle M, Berger K. The impact of the COVID‐19 pandemic on self‐reported health. Dtsch Arztebl Int. 2020;117:861‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dragano N, Reuter M, Peters A, et al. Increase in mental disorders during the COVID‐19 pandemic—the role of occupational and financial strains. Dtsch Arztebl Int. 2022;119:179‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo M, Guo L, Yu M, Jiang W, Wang H. The psychological and mental impact of coronavirus disease 2019 (COVID‐19) on medical staff and general public—a systematic review and meta‐analysis. Psychiatry Res. 2020;291:113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bäuerle A, Musche V, Schmidt K, et al. Mental health burden of German cancer patients before and after the outbreak of COVID‐19: predictors of mental health impairment. Int J Environ Res Public Health. 2021;18:2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ayubi E, Bashirian S, Khazaei S. Depression and anxiety among patients with cancer during COVID‐19 pandemic: a systematic review and meta‐analysis. J Gastrointest Cancer. 2021;52:499‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verma R, Kilgour HM, Haase KR. The psychosocial impact of COVID‐19 on older adults with cancer: a rapid review. Curr Oncol. 2022;29:589‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bartels M, Gal R, van der Velden JM, Verhoeff JJC, Verlaan JJ, Verkooijen HM. Impact of the COVID‐19 pandemic on quality of life and emotional wellbeing in patients with bone metastases treated with radiotherapy: a prospective cohort study. Clin Exp Metastasis. 2021;38:209‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pichler T, Frank T, Maier S, et al. The views of cancer out‐patients on the impact of the COVID‐19 pandemic. Dtsch Med Wochenschr. 2022;147:41‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edge R, Mazariego C, Li Z, et al. Psychosocial impact of COVID‐19 on cancer patients, survivors, and carers in Australia: a real‐time assessment of cancer support services. Support Care Cancer. 2021;29:5463‐5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yildirim OA, Poyraz K, Erdur E. Depression and anxiety in cancer patients before and during the SARS‐CoV‐2 pandemic: association with treatment delays. Qual Life Res. 2021;30:1903‐1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eckford RD, Gaisser A, Arndt V, et al. The COVID‐19 pandemic and cancer patients in Germany: impact on treatment, follow‐up care and psychological burden. Front Public Health. 2022;9:788598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Duan Z, Ma Z, et al. Epidemiology of mental health problems among patients with cancer during COVID‐19 pandemic. Transl Psychiatry. 2020;10:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Annunziata MA, Muzzatti B, Bidoli E, et al. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support Care Cancer. 2020;28:3921‐3926. [DOI] [PubMed] [Google Scholar]
- 26. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361‐370. [DOI] [PubMed] [Google Scholar]
- 27. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69‐77. [DOI] [PubMed] [Google Scholar]
- 28. Wu Y, Levis B, Sun Y, et al. Accuracy of the Hospital Anxiety and Depression Scale Depression subscale (HADS‐D) to screen for major depression: systematic review and individual participant data meta‐analysis. BMJ. 2021;373:n972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singer S, Kuhnt S, Götze H, et al. Hospital anxiety and depression scale cutoff scores for cancer patients in acute care. Br J Cancer. 2009;100:908‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Statistisches Bundesamt (Destatis) . Net Income of Households by Household Type, 2021, 2023.
- 31. Li P, Stuart EA, Allison DB. Multiple imputation: a flexible tool for handling missing data. JAMA. 2015;314:1966‐1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta‐analysis. BMJ. 2020;371:m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nogueira LM, Schafer EJ, Fan Q, et al. Assessment of changes in cancer treatment during the first year of the COVID‐19 pandemic in the US. JAMA Oncol. 2024;10:109‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van de Poll‐Franse LV, de Rooij BH, Horevoorts NJE, et al. Perceived care and well‐being of patients with cancer and matched norm participants in the COVID‐19 crisis: results of a survey of participants in the Dutch PROFILES Registry. JAMA Oncol. 2021;7:279‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. COVIDSurg Collaborative . Effect of COVID‐19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. 2021;22:1507‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Justenhoven C, Rieger B. The impact of the Corona pandemic on reported data relating to cancer diagnoses, therapy, and follow‐up. Dtsch Arztebl Int. 2022;119:724‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Onesti CE, Tagliamento M, Curigliano G, et al. Expected medium‐ and long‐term impact of the COVID‐19 outbreak in oncology. JCO Glob Oncol. 2021;7:162‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hossain MM, Tasnim S, Sultana A, et al. Epidemiology of mental health problems in COVID‐19: a review. F1000Research. 2020;9:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Santomauro DF, Mantilla Herrera AM, Shadid J, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID‐19 pandemic. Lancet. 2021;398:1700‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salari N, Hosseinian‐Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID‐19 pandemic: a systematic review and meta‐analysis. Glob Health. 2020;16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Swainston J, Chapman B, Grunfeld EA, Derakshan N. COVID‐19 lockdown and its adverse impact on psychological health in breast cancer. Front Psychol. 2020;11:2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gaisser A, Eckford RD, Arndt V, et al. Nearly two years of the coronavirus pandemic from the perspective of people affected by cancer. Der Onkologe. 2022;28:248‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frank T, Pichler T, Maier S, et al. Stressors related to the COVID‐19 pandemic and their association with distress, depressive, and anxiety symptoms in cancer out‐patients. Front Psychol. 2023;14:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Y‐H, Li J‐Q, Shi J‐F, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta‐analysis of cohort studies. Mol Psychiatry. 2020;25:1487‐1499. [DOI] [PubMed] [Google Scholar]
- 45. Büssing A, Hübner J, Walter S, Gießler W, Büntzel J. Tumor patients' perceived changes of specific attitudes, perceptions, and behaviors due to the COVID‐19 pandemic and its relation to reduced wellbeing. Front Psych. 2020;11:574314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wolf S, Seiffer B, Zeibig JM, et al. Is physical activity associated with less depression and anxiety during the COVID‐19 pandemic? A rapid systematic review. Sports Med. 2021;51:1771‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goerling U, Faller H, Hornemann B, et al. Information needs in cancer patients across the disease trajectory. A prospective study. Patient Educ Couns. 2020;103:120‐126. [DOI] [PubMed] [Google Scholar]
- 48. Nekhlyudov L, Duijts S, Hudson SV, et al. Addressing the needs of cancer survivors during the COVID‐19 pandemic. J Cancer Surviv. 2020;14:601‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
All source code is publicly available on Github (https://github.com/DDoege/CroKuS-study-German-Cancer-Research-Center.git). The other data that support the findings of this study are available from the corresponding author upon reasonable request.


