Abstract
Dilute povidone-iodine (polyvinylpyrrolidone iodine [PVP-I]) irrigation in spine surgery and total joint arthroplasty has seen a rapid and substantial increase in its use during the past decade. Yet, most surgeons do not know the chemistry and biochemistry that explain its efficacy in preventing infections. PVP-I forms a complex with molecular iodine (I2), facilitating the delivery of I2 to the membrane of the infectious organism. Here, PVP-I establishes an equilibrium between complexed and noncomplexed (free) I2 in the aqueous solution. The I2 acts at numerous cellular targets of infecting organisms augmenting its role as a biocidal molecule. The paradoxical increase in the concentration of I2 that occurs with dilution of PVP-I is a result of equilibrium kinetics and is associated with an enhanced antimicrobial activity. Cytotoxicity studies have yielded conflicting results, but most endorse diluted concentrations as being less damaging to tissues. Clinical studies have verified notable reductions in surgical site infections with a 3-minute soak of 0.35% dilute povidone-iodine irrigation. Guidelines from the World Health Organization, Centers for Disease Control and Prevention, and International Consensus Meeting on Musculoskeletal Infection support the use of prophylactic incisional wound irrigation with aqueous PVP-I to reduce and prevent surgical site infections.
Iodine has been applied as a disinfectant since the mid-nineteenth century. It is currently used for preoperative/preprocedural skin preparation of the patient, for disinfection of the hands of the surgical team, and as a prophylactic intraoperative incisional wound irrigant (pIOWI). In orthopaedic surgery, antiseptic pIOWI has increased substantially in the subspecialties of total joint arthroplasty (TJA) and spine surgery (Figure 1).1 The protocols used for an antiseptic pIOWI with povidone-iodine (referred to colloquially as a Betadine bath, Texas tea, home brew) are varied, but most surgeons use an on-site dilute povidone-iodine preparation because of its anticipated increased bactericidal activity and lower toxicity. This increased bacterial killing is caused by a paradoxical increase in molecular iodine (I2), the active biocide. Concerns have been expressed regarding on-site dilutions because they ignore the substantiveness of chemical equilibrium kinetics related to the biocidal efficacy of povidone-iodine compounds. This can cause large variations in the concentration of I2 and may explain inconsistencies in some of the clinical and preclinical results. In addition, sterility issues have been identified when the on-site povidone-iodine is acquired from a nonsterile container, and therefore, this technique is not recommended. However, the use of sterile aqueous povidone-iodine solution as a pIOWI to prevent surgical site infections (SSIs) is endorsed by the World Health Organization and Centers for Disease Control and Prevention.
Figure 1.
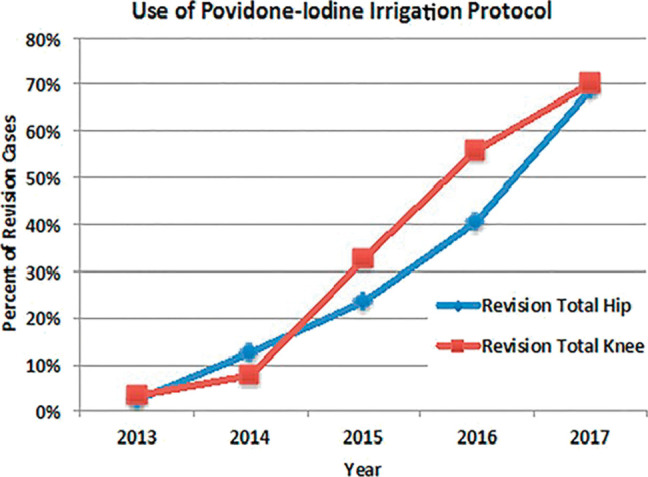
Graph demonstrating the percentage of revision total hip and revision total knee arthroplasties in which the dilute povidone-iodine irrigation protocol was used at Mayo Clinic from 2013 to 2017 (Reproduced/adapted with permission from Hart A, Hernandez NM, Abdel MP, Mabry TM, Hanssen AD, Perry KI: Povidone-iodine wound lavage to prevent infection after revision total hip and knee arthroplasty: an analysis of 2884 cases. J Bone Joint Surg Am 2019;101(13):1151-1159). Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Iodine Chemistry and Biochemistry
In mammals, iodine is present in vivo in several distinct forms including iodide, organically bound iodine, and molecular iodine (synonymous with “free” or unbound iodine, noncomplexed iodine, elemental iodine, diatomic iodine, or I2).2 In chemistry, iodide is a hydrophilic monoatomic ion and iodine (I2) is an elemental diatomic molecule that is hydrophobic. Iodide is a nutritional requirement (Recommended Dietary Allowance for adults between 100 and 200 μg) that is absorbed from the blood and concentrated in the thyroid gland where it is oxidized to form I2 by thyroid peroxidase. Both I2 and hypoiodous acid, created by the reaction of I2 with water, are responsible for iodination of thyroglobulin-bound tyrosines. This iodination then leads to formation of the 2 thyroid hormones (thyroxine and thyroglobulin) that are important in regulating metabolism.3,4
Lugol Solution
Soon after the discovery of a new substance in seaweed ash by Bernard Courtois in 1811 and its subsequent identification as a new element in 1813, iodine's potential medical uses as a tincture and topical antiseptic were applied. Lugol solution was developed in 1829 by a French physician Jean Guillaume August Lugol who used iodine extensively for topical application and oral administration in medical settings.5 It is a mixture of I2 and potassium iodide in distilled water. It contains approximately 170 ppm of I2 but also contains other iodide species including tri-iodide (I3-). This tincture of iodine was used for traumatic wound disinfection up until and including the First World War. The idea that I2 in Lugol solution is responsible for skin staining and irritation has been widely held for over a century; however, there are no controlled data to support this assumption. In fact, a recent investigation demonstrated that the tri-iodide ion and not I2 was responsible for the cytotoxicity.6,7
Iodophors
Iodophors are formulations containing iodine complexed with a solubilizing agent (carrier) such as water-soluble polymers. They are highly acidic compositions that provide a small concentration of the active biocide, that is, unbound or free I2.8 Complexation of I2 is used to stabilize it in an aqueous environment.9 The small amount of unbound or free I2 is in equilibrium with the large concentrations of iodide/tri-iodide ions and with the polymers that complex it.10 Polyvinylpyrrolidone-iodine (PVP-I, synonymous with povidone-iodine [PVI]), first introduced in 1956, is an iodophor antiseptic widely used in health care. Structurally, povidone-iodine consists of protonated PVP units that are linked together by hydrogen bonds and incorporate iodide/tri-iodide anions (Figure 2). A 10% povidone-iodine solution, an example being the brand name Betadine, generally contains 90% water, 8.5% PVP (carrier), and 1% available iodine, although substantial variability in formulations exist between different manufacturers. The US Pharmacopeia analytical standards accept a 35% variation in available iodine, so a 10% povidone-iodine product can have between 85% and 120% of the labeled iodine concentration. It is also important to note that the labeled concentration of “iodine” in iodophors is unrelated to the active biocide I2 concentration. These 1% available iodine molecules in a 10% povidone-iodine solution (approximately 10,000 ppm available iodine) take the form of 9 different chemical equilibria producing at least 10 separate iodine species, including only 3-5 parts per million (ppm) or 0.0003% to 0.0005% of the active biocide I2.11 To gain a perspective, based on an Recommended Dietary Allowance of 125 μg/d for iodide, the follicular lumen of the thyroid gland converts 6.25 μg iodide/hour into I2 in a volume of approximately 7.5 mL, which equates to generating approximately 900 ppm I2/hr, which is about 2 orders of magnitude (×100) higher than the I2 concentration found in the commonly used antiseptic PVP-I.7
Figure 2.

Illustration demonstrating the PVP-I (povidone-iodine) complex serving as a carrier and as a reservoir for molecular iodine (I2). When noncomplexed I2 is removed from the solution as it penetrates and disrupts the pathogen membrane, it is replaced by I2 released from the tri-iodide that diffuses off the PVP-I complex because of equilibrium kinetics (Reproduced with permission from I2Pure).
Unique Biocidal and Antimicrobial Properties of I2
Berkelman et al12 were the first to describe a paradoxical increase in bactericidal activity of dilute preparations of povidone-iodine. They hypothesized that the concentration of “free” or unbound I2 in solution was the main source of the biocidal activity in povidone-iodine, and within a range of dilutions, the I2 levels increased, reaching approximately 25 ppm at 0.1% (Figure 3). This was thought to be related to the equilibrium between bound and unbound iodine with the carrier PVP. Indeed, PVP is one of many carriers that yield a complex equilibrium of chemicals that provides a relatively low concentration of the active biocide of I2. The bactericidal mechanism of action has subsequently been shown to be the delivery of the iodine species I2 by the PVP-I carrier to the bacterium cell, where the I2 diffuses off the carrier and oxidizes fatty/amino acids, nucleotides, and cytosolic enzymes in the respiratory chain, causing them to become denatured and deactivated13,14 (Figure 4). The result is a simultaneous action against multiple molecular targets, which accounts for the lack of resistance to iodine.15 Hence, iodophors containing I2 have a broad antimicrobial spectrum with activity against gram-positive and gram-negative bacteria, including antibiotic-resistant and antiseptic-resistant strains, fungi, and protozoa. They are also active against a wide range of enveloped and nonenveloped viruses and some bacterial spores with increased exposure time.16
Figure 3.
Graph demonstrating paradoxical increase in I2 (free iodine or molecular iodine) in dilute povidone-iodine aqueous solutions (Reproduced/adapted with permission from Zamora JL: Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am J Surg 1986;151:400-406). Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Figure 4.
Image demonstrating the active moiety I2, oxidizing pathogen nucleotides and fatty/amino acids and thus disrupting the pathogen membrane and deactivating proteins and DNA/RNA (Adapted by I2Pure, licensed under the Creative Commons Attribution-Share-Alike 4.0 international licenses/by/4.0/). Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Gottardi et al also verified that the biocidal efficacy of germicidal antiseptics was proportional to the concentration of I2, showing that iodophors with lower total iodine concentrations (available iodine) but high levels of I2 killed Staphylococcus aureus and spores of Bacillus subtilis more rapidly than nondilute Betadine, which contains low levels of I2.6 This confirmed the frequently observed positive correlation between the concentration of I2 and the rate of microbial kill (Figure 5).9
Figure 5.

Plot demonstrating correlation of S aureus survival data with the concentration of I2 and length of exposure (Reproduced/adapted with permission from Berkelman RL, Holland BW, Anderson RL: Increased bactericidal activity of dilute preparations of povidone-iodone solutions. J Clin Microbiol 1982;15(4):635-639).
In additional research, Gottardi demonstrated that I2, but not the other species of iodine found in iodine-based disinfectants, diffuses into skin and provides a prolonged biocidal effect because of a dynamic back-diffusion of I2 from treated areas.17 I2 partitions into the fatty tissue of the hypodermis when applied to skin and then slowly back-diffuses out, delivering a biocidal iodine atmosphere in, on, and at the surface (Supplemental Digital Content 1, Video 1: molecular iodine and its interaction with skin [epidermis, dermis, hypodermis]).
Residual I2 activity from the back-diffusion associated with topical I2 was recently reinforced by Freeman et al.7 They observed an outgassing from pig skin for 3.3 hours after being treated with 66,000 ppm of I2 compared with 4 hours for porcine hypodermis tissue treated directly with 15,200 ppm of I2. They hypothesized that the lipophilic I2 is absorbed into regions of unsaturated lipid in the hypodermis, from which it back-diffuses out for a period related to concentration and exposure time.
Biofilm
Biofilms can be defined as microbial aggregates irrespective of attachment to a biotic or abiotic (implant) surface.18 These bacteria colonies exist in free-floating and surface-associated forms and are known for an increased antibiotic tolerance and the presence of an extracellular polymeric substance. The extracellular polymeric substance is a hydrogel-like matrix that encases the cells in a biofilm and includes proteins, lipids, nucleic acids (extracellular DNA), and polysaccharides.
The use of povidone-iodine in treating biofilms, as either a topical application or part of an iodophor dressing, has been well documented in the literature. Human studies conducted in various settings have established the effectiveness of povidone-iodine in reducing the bacterial loads in both acute and chronic wounds.19-22
Investigations into the use of dilute povidone-iodine and its paradoxical increase in I2 concentrations have been conducted in both in vitro settings and animal models of implant-related infections and biofilms. Oduwole et al23 studied the antibiofilm activity of subinhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. Using a minimum inhibitory concentration of 1.4% povidone-iodine, they tested the effect of serial twofold dilutions (0.17%, 0.35%, 0.7%) on the development of staphylococcal biofilms. In addition to inhibiting growth of S epidermidis and S aureus reference strains and isolates from prosthetic joint infections, subinhibitory concentrations of povidone-iodine also suppressed S epidermidis and S aureus biofilm development at least in part by repressing transcription of the icaADBC operon (responsible for polysaccharide intercellular adhesion). In a separate study, Gilotra et al24 investigated the effectiveness of a dilute Betadine lavage protocol (3.5%) compared with normal saline lavage in a rabbit knee model of acute prosthetic joint infection. The knees irrigated with dilute Betadine exhibited a notable reduction in bacterial counts on metal implants (20-fold decrease) and 10-fold decrease in polyethylene implant-related counts, supporting the antibiofilm efficacy of the dilute Betadine lavage protocol.
Povidone-Iodine (Betadine) Dilution
van Meurs et al25 hypothesized that the ideal irrigation solution should maximize antimicrobial effects while minimizing cytotoxicity (achieving a high therapeutic index) during the brief exposure time (2 minutes) typically used in surgical procedures. Their in vitro research sought to identify an antiseptic dilution that minimized cytotoxicity at the minimal bactericidal concentration (MBC). They observed a steep dose-response curve for bacterial killing with povidone-iodine, starting at 1 g/L and reaching the MBC for all strains of bacteria tested at approximately 1.32 g/L, a 75-fold dilution of the standard concentration of 100 g/L. They concluded that povidone-iodine at a dilution of 1.3 g/L (MBC of S aureus, S epidermidis tested: 1.32 g/L) was the preferred irrigation solution. This finding advocated for a more dilute solution than in previous clinical studies that used povidone-iodine diluted to a 0.35% concentration (=35 g/L).
Nuckolls examined the complex chemistry behind povidone-iodine dilution into water or saline and cautioned against the routine performance of on-site dilution of commercial 5-10% povidone-iodine solutions.26 He determined that the on-site dilution of povidone-iodine, such as that done intraoperatively when povidone-iodine is mixed with saline or water, compared with a manufactured dilution, can negatively affect the biocidal potency and stability of I2. This is due to perturbations in the chemical equilibria of the numerous iodine fractions (including I2) in aqueous solution that are caused by pH changes with water and saline dilution.
Different commercial preparations of povidone-iodine can also affect changes in the concentration of I2 because of the iodine-complexing properties of the various additives and surfactants. A comparison of 10 commercially available povidone-iodine antiseptics marketed as containing 10% povidone-iodine and 1% available iodine exhibited a range of 0.2 to 10 ppm of I2.27 Because of a possible range of 2 orders of magnitude in the concentration of I2 before dilution, variability in bactericidal activity is almost certain to occur with dilution.
Despite the uncertainties related to the amount of I2 that is generated by an on-site dilution, any increase in I2 levels resulting from a diluted PVP-I concentration should provide an enhanced bactericidal activity and less tissue toxicity.
Potential Cytotoxicity
Research examining the potential cytotoxicity of povidone-iodine–based formulations in musculoskeletal tissues has yielded conflicting results. Some studies report negative effects, including toxic injury to cells, while others describe beneficial effects and anabolic cellular activities. Most of these investigations have been in vitro cell culture studies, which inherently struggle to replicate the complex biologic processes that occur in living tissues. For example, von Keudell et al explored the effects of dilute povidone-iodine on articular cartilage and found a strong correlation between chondrocyte viability in the superficial layer and both the duration of exposure and concentration of povidone-iodine. Notably, the most diluted concentration studied, 0.35% povidone-iodine, which has a higher I2 concentration, showed the least cytotoxic effect.28
Conversely, Newton Ede et al29 examined the in vitro effects of dilute povidone-iodine (0.35%) on osteoblasts and concluded that “PVI has a rapid and detrimental effect on human osteoblast cellular proliferation, metabolic function, and bone nodule mineralization.”
Liu et al used human primary osteoblasts, fibroblasts, and myoblasts in cell culture and subjected them to various concentrations of povidone-iodine (0%, 0.001%, 0.01%, 0.1%, 0.35%, 1%) for 3 minutes. They concluded that concentrations of PVI used in irrigation protocols in spine surgery (0.35%) exerted a cytotoxic effect and inhibited migration of these cell lines, but that povidone-iodine in concentrations of less than 0.1% did not have a negative effect on cell survival or migration.30
Jiang et al used cells isolated from human joint tissue, including cartilage-derived progenitor cells, subchondral bone–derived osteoblasts, and bone marrow–derived mesenchymal stem cells. They concluded that “the biocompatibility and pro-osteogenic effects of low-concentration PVP-I on cells from joint tissue in vitro and the enhanced subchondral bone formation in low-concentration PVP-I–treated scaffolds in an in vivo rabbit model provided important new insights into the use of PVP-I for osteochondral defect repair.”31
In a different analysis evaluating the effects of PVP-I on tendon-bone healing, also in an in vivo rabbit model, Zhang et al32 found that dilute PVP-I at 100uM was osteoinductive and that it promoted bone-tendon healing by osteogenesis.
Although in vitro studies with povidone-iodine have shown disparate results depending on the experimental conditions, a common finding is that diluted povidone-iodine is less toxic than nondiluted, thereby providing indirect evidence that I2 is not the cytotoxic iodine species.
Clinical Science/Studies
The practice of including povidone-iodine as an antiseptic irrigation solution in surgery first emerged in the literature in the 1970s, and primarily in patients undergoing abdominal, genitourinary, and cardiothoracic procedures. After the recognition of a paradoxical increased bactericidal activity of dilute povidone-iodine preparations by Berkelman et al in 1982, articles describing the use of dilute povidone-iodine irrigation in surgery began to appear. Throughout the 1980s and 1990s, the number of journal articles with dilute povidone-iodine formulations as an intervention increased considerably, expanding into the disciplines of ophthalmology; neurosurgery; and ear, nose, and throat.
A 2007 meta-analysis in the Canadian Journal of Surgery identified 15 studies from 1977 to 2005 that evaluated the efficacy of povidone-iodine irrigation to prevent SSIs.33 A total of 15 studies were included with varying povidone-iodine dilutions (10% PVP-I, 1% PVP-I, 0.5% PVP-I, 0.35% PVP-I) in the intervention/treatment groups. Among these, 11 randomized control trials (RCTs) provided high-quality evidence, with 3 studies offering level 1 evidence and 8 providing level 2 evidence. Of all the studies, 10 demonstrated that povidone-iodine irrigation was markedly more effective at preventing SSIs compared with using saline, water, or no irrigation at all.
In the field of orthopaedic surgery, a clinical investigation using intraoperative, dilute Betadine irrigation was first reported in the spine literature in 2005. Cheng et al34 conducted a RCT of 414 consecutive patients undergoing spinal surgery. Surgical procedures included decompression, fusion, and fixation for degenerative scoliosis or stenosis, fixation for traumatic spinal fracture, diskectomy for disk prolapse, and excision with fixation for spinal metastatic lesions. In the group with a 0.35% dilute Betadine soak for 3 minutes, there were 0 superficial or deep infections (of 208), compared with 1 superficial infection and 6 deep infections (of 206) in the control group with normal saline irrigation. This resulted in a significantly worse deep infection rate and total infection rate (P = 0.0146 for deep infection rate and P = 0.0072 for total infection rate) in the control group.
After this, Chang et al35 completed a similar RCT in 2006 that included 244 consecutive patients and 435 patients with primary instrumented lumbosacral posterolateral fusion levels for degenerative spinal disorder. Group 1 (0.35% dilute povidone-iodine irrigation) consisted of 120 patients (212 fusion levels), and group 2 (normal saline irrigation) consisted of 124 patients (213 fusion levels). 0 infections in group 1 patients and 6 infections in group 2 were observed, for a statistically significant difference (P = 0.029). No difference in spine fusion was achieved between the groups suggesting the minimal or absence of a negative cytotoxic effect from the 0.35% dilute povidone-iodine irrigation.
In TJA, Della Valle et al in 2012 conducted a retrospective analysis that included 1,862 cases (630 total hip arthroplasties [THAs] and 1,232 total knee arthroplasties [TKAs]) done using a normal saline lavage protocol and 688 cases (274 THAs and 414 TKAs) after instituting a 0.35% dilute Betadine lavage protocol that was based on the protocol described by Cheng et al.34,36 A prosthetic joint infection (PJI) developed in 18 of 1,862 TJAs before the use of 0.35% dilute Betadine lavage versus 1 of 688 TJAs after adopting the protocol, for a significant difference (P = 0.018). A noteworthy difference from the spine studies was the use of a 10% Betadine paint with a sponge stick before skin closure in the 0.35% dilute Betadine lavage group.
In a 2020 follow-up publication, Della Valle et al conducted a RCT of aseptic revision TJAs.37 A total of 457 patients were included, with 234 patients (153 knees and 83 hips) in the normal saline lavage group and 223 (144 knees and 79 hips) in the 0.35% dilute povidone-iodine lavage group. They recorded 8 PJIs in the saline group and 1 PJI in the 0.35% dilute povidone-iodine group (3.4% versus 0.4%, P = 0.038). Similar to their previous study, in the 0.35% dilute povidone-iodine lavage cohort, the wound edges were painted with 10% povidone-iodine with a sponge stick before closure.
2 large studies published in 2019 from the Mayo Clinic Joint Replacement Database, one analyzing 11,738 primary hip and knee arthroplasties and the other analyzing 2,884 revision hip and knee arthroplasties, did not find a benefit to dilute povidone-iodine irrigation (0.25%) versus no dilute povidone-iodine irrigation. Both investigations were retrospective with level 3 evidence.1,38 A separate retrospective study, also with level 3 evidence, from the Rothman Institute in 2022 analyzed 31,331 cases from their institutional registry. They reported 8,659 surgeries irrigated with dilute povidone-iodine (0.30%) and 22,672 irrigated with sterile saline and water before closure. They described an absolute risk reduction of 0.73% for prosthetic joint infection and 2.34 times lower rate of PJI (0.6% versus 1.3%).39 The disparate outcomes across these 3 registry investigations potentially stem from different parameters used in propensity score modeling and potential surgeon bias.
The World Health Organization and US Centers for Disease Control and Prevention have issued clinical practice guidelines recommending the use of prophylactic incisional wound irrigation with a sterile aqueous povidone-iodine solution to prevent SSIs.40-42 Furthermore, during the second International Consensus Meeting on Musculoskeletal Infection, experts overwhelmingly agreed, with a “super majority, strong consensus,” on the benefits of using dilute povidone-iodine for wound irrigation in surgical procedures.43
Conclusion
The evidence for including iodine as part of the antiseptic principles in surgery continues to strengthen. A recent systemic review and network meta-analysis concluded with a high certainty of evidence that pIOWI with an aqueous antiseptic solution was associated with the reduction of SSIs.44 Of 32 studies that compared antiseptic irrigation with either antibiotic irrigation or saline, 22 included povidone-iodine and 18 of these were RCTs. The author described serious concerns regarding antimicrobial resistance with antibiotic irrigation and confirmed that no signs of resistance to iodine have been shown.
The use of dilute povidone-iodine as an antiseptic in a pIOWI protocol has increased dramatically in orthopaedic surgery during the past decade. Its efficacy is attributed to the chemistry of I2 and its role as an active biocide and antimicrobial agent. The paradoxical increase in I2 that occurs with dilution of povidone-iodine is well established. The germicidal properties of I2 formulated in a topical iodine-based composition, augmented by the phenomenon of back-diffusion or outgassing from the hypodermis and dermis after application, was validated by the PREPARE (A Pragmatic Randomized Trial Evaluation Preoperative Alcohol Skin Solutions in Fractured Extremities) study in orthopaedic trauma.45
Because the amount of I2 is not specified in a commercially prepared povidone-iodine preparation, an exact recommendation regarding the ideal dilution that provides the maximum therapeutic index is not possible. Current knowledge supports a range of 0.01% to 1.0% povidone-iodine dilution as correlating with increased I2 levels, with a maximum of 25 ppm of I2 at 0.1% povidone-iodine concentration. All the clinical studies mentioned herein described a 3-minute soak for povidone-iodine irrigation, but data are lacking regarding an optimal period.
Future research endeavors include developing iodine-based compounds with a high functional concentration of I2 that have the potential to provide a more robust and effective antimicrobial activity in orthopaedic surgery.
Footnotes
Neither Dr. Meehan nor any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal's Web site (www.jaaos.org).
References
- 1.Hart A, Hernandez NM, Abdel MP, Mabry TM, Hanssen AD, Perry KI: Povidone-iodine wound lavage to prevent infection after revision total hip and knee arthroplasty: An analysis of 2,884 cases. J Bone Joint Surg Am 2019;101:1151-1159. [DOI] [PubMed] [Google Scholar]
- 2.Ahad F, Ganie SA: Iodine, iodine metabolism and iodine deficiency disorders revisited. Indian J Endocrinol Metab 2010;14:13-17. [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler J, Obinger C, Eales G: Factors influencing the study of peroxidase-generated iodine species and implications for thyroglobulin synthesis. Thyroid 2008;18:769-774. [DOI] [PubMed] [Google Scholar]
- 4.Cooper RA: Iodine revisited. Int Wound J 2007;4:124-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan J: Lugol, his work and his solution. Ann Med Hist 1928;10:202-208. [PMC free article] [PubMed] [Google Scholar]
- 6.Hickey J, Panicucci R, Duan Y, et al. : Control of the amount of free molecular iodine in iodine germicides. J Pharm Pharmacol 1997;49:1195-1199. [DOI] [PubMed] [Google Scholar]
- 7.Freeman C, Duan E, Kessler J: Molecular iodine is not responsible for cytotoxicity in iodophors. J Hosp Infect 2022;122:194-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada H, Nojima Y, Ogawa S, et al. : Relationship between virucidal efficacy and free iodine concentration of povidone-iodine in buffer solution. Biocontrol Sci 2016;21:21-27. [DOI] [PubMed] [Google Scholar]
- 9.Gottardi W: [Aqueous iodine solutions as disinfectants: Composition, stability, comparison with chlorine and bromine solution (author's transl) [in German]. Zentralbl Bakteriol B 1978;167:206-215. [PubMed] [Google Scholar]
- 10.Gottardi W: Iodine and disinfection: Theoretical study on mode of action, efficiency, stability, and analytical aspects in the aqueous system. Arch Pharm (Weinheim) 1999;332:151-157. [DOI] [PubMed] [Google Scholar]
- 11.Makhayeva DN, Irmukhametova GS, Khutoryanskiy VV: Polymeric iodophors: Preparation, properties, and biomedical applications. Rev J Chem 2020;10:40-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkelman RL, Holland BW, Anderson RL: Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol 1982;15:635-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA: Povidone iodine in wound healing: A review of current concepts and practices. Int J Surg 2017;44:260-268. [DOI] [PubMed] [Google Scholar]
- 14.Schreier H, Erdos G, Reimer K, König B, König W, Fleischer W: Molecular effects of povidone-iodine on relevant microorganisms: An electron-microscopic and biochemical study. Dermatology 1997;195(suppl 2):111-116. [DOI] [PubMed] [Google Scholar]
- 15.Lepelletier D, Maillard JY, Pozzetto B, Simon A: Povidone iodine: Properties, mechanisms of action, and role in infection control and Staphylococcus aureus decolonization. Antimicrob Agents Chemother 2020;64:e00682-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wada H, Nojima Y, Ogawa S, et al. : Relationship between virucidal efficacy and free iodine concentration of povidone-iodine in buffer solution. Biocontrol Sci 2016;21:21-27. [DOI] [PubMed] [Google Scholar]
- 17.Gottardi WJ: The uptake and release of molecular iodine by the skin: Chemical and bactericidal evidence of residual effects caused by povidone-iodine preparations. J Hosp Infect 1995;29:9-18. [DOI] [PubMed] [Google Scholar]
- 18.Sauer K, Stoodley P, Goeres DM, et al. : The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat Rev Microbiol 2022;20:608-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daróczy J: Antiseptic efficacy of local disinfecting povidone-iodine (Betadine) therapy in chronic wounds of lymphedematous patients. Dermatology 2002;204(suppl 1):75-78. [DOI] [PubMed] [Google Scholar]
- 20.Homann HH, Rosbach O, Moll W, et al. : A liposome hydrogel with polyvinyl-pyrrolidone iodine in the local treatment of partial-thickness burn wounds. Ann Plast Surg 2007;59:423-427. [DOI] [PubMed] [Google Scholar]
- 21.Vogt PM, Hauser J, Rossbach O, et al. : Polyvinyl pyrrolidone-iodine liposome hydrogel improves epithelialization by combining moisture and antisepis. A new concept in wound therapy. Wound Repair Regen 2001;9:116-122. [DOI] [PubMed] [Google Scholar]
- 22.Capriotti K, Pelletier J, Barone S, Capriotti J: Efficacy of dilute povidone-iodine against multi-drug resistant bacterial biofilms, fungal biofilms and fungal spores. J Clin Res Dermatol 2018;5:1-5. [Google Scholar]
- 23.Oduwole KO, Glynn AA, Molony DC, et al. : Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J Orthopaedic Res 2010;28:1252-1256. [DOI] [PubMed] [Google Scholar]
- 24.Gilotra M, Nguyen T, Jaffe D, Sterling R: Dilute betadine lavage reduces implant-related bacterial burden in a rabbit knee prosthetic infection model. Am J Orthop (Belle Mead NJ) 2015;44:E38-E41. [PubMed] [Google Scholar]
- 25.van Meurs SJ, Gawlitta D, Heemstra KA, Poolman RW, Vogely HC, Kruyt MC: Selection of an optimal antiseptic solution for intraoperative irrigation: An in vitro study. J Bone Joint Surg Am 2014;96:285-291. [DOI] [PubMed] [Google Scholar]
- 26.Nuckolls C: Chemical considerations related to the dilution of commercial 10% povidone-iodine for use in the COVID-19 pandemic, 2020. Available at: 10.31219/osf.io/8kepw. Accessed February 14, 2024. [DOI]
- 27.Gottardi W, Koller W: The concentration of free iodine in aqueous povidone-iodine containing systems and its variation with temperature. Monatsch Chem 1986;117:1011-1020. [Google Scholar]
- 28.von Keudell A, Canseco JA, Gomoll AH: Deleterious effects of diluted povidone-iodine on articular cartilage. J Arthroplast 2013;28:918-921. [DOI] [PubMed] [Google Scholar]
- 29.Newton Ede MP, Philp AM, Philp A, Richardson SM, Mohammad S, Jones SW: Povidone-iodine has a profound effect on in vitro osteoblast proliferation and metabolic function and inhibits their ability to mineralize and form bone. Spine (Phila Pa 1976) 2016;41:729-734. [DOI] [PubMed] [Google Scholar]
- 30.Liu JX, Werner JA, Buza JA, III, Kirsch T, Zuckerman JD, Virk MS: Povidone-iodine solutions inhibit cell migration and survival of osteoblasts, fibroblasts, and myoblasts. Spine (Phila Pa 1976) 2017;42:1757-1762. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y, Chen L, Zhang S, et al. : Incorporation of bioactive polyvinylpyrrolidone-iodine within bilayered collagen scaffolds enhances the differentiation and subchondral osteogenesis of mesenchymal stem cells. Acta Biomater 2013;9:8089-8098. [DOI] [PubMed] [Google Scholar]
- 32.Zhang P, Zhi Y, Fang H, et al. : Effects of polyvinylpyrrolidone-iodine on tendon-bone healing in a rabbit extra-articular model. Exp Ther Med 2017;13:2751-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chundamala J, Wright JG: The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: An evidence-based review. Can J Surg 2007;50:473-481. [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH: Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976) 2005;30:1689-1693. [DOI] [PubMed] [Google Scholar]
- 35.Chang FY, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH: Can povidone-iodine solution be used safely in a spinal surgery? Eur Spine J 2006;15:1005-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ: Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty 2012;27:27-30. [DOI] [PubMed] [Google Scholar]
- 37.Calkins TE, Culvern C, Nam D, et al. : Dilute betadine lavage reduces the risk of acute postoperative periprosthetic joint infection in aseptic revision total knee and hip arthroplasty: A randomized controlled trial. J Arthroplasty 2020;35:538-543.e1. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez NM, Hart A, Taunton MJ, et al. : Use of povidone-iodine irrigation prior to wound closure in primary total hip and knee arthroplasty: An analysis of 11,738 cases. J Bone Joint Surg Am 2019;101:1144-1150. [DOI] [PubMed] [Google Scholar]
- 39.Shohat N, Goh GS, Harrer SL, Brown S: Dilute povidone-iodine irrigation reduces the rate of periprosthetic joint infection following hip and knee arthroplasty: An analysis of 31,331 cases. J Arthroplasty 2022;37:226-231.e1. [DOI] [PubMed] [Google Scholar]
- 40.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. , Healthcare Infection Control Practices Advisory Committee: Centers for Disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017;152:784-791. [DOI] [PubMed] [Google Scholar]
- 41.Allegranzi B, Zayed B, Bischoff P, et al. , WHO Guidelines Development Group: New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect Dis 2016;16:e288-e303. [DOI] [PubMed] [Google Scholar]
- 42.Global Guidelines for the Prevention of Surgical Site Infection. Geneva, Switzerland: World Health Organization; 2016. [PubMed] [Google Scholar]
- 43.Blom A, Cho J, Fleischman A, et al. : General assembly, prevention, antiseptic irrigation solution: Proceedings of international consensus on orthopedic infections. J Arthroplasty 2019;34:S131-S138. [DOI] [PubMed] [Google Scholar]
- 44.Groenen H, Bontekoning N, Jalalzadeh H, et al. : Incisional wound irrigation for the prevention of surgical site infection: A systematic review and network meta-analysis. JAMA Surg 2024;159:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.PREP-IT Investigators, Sprague S, Slobogean G, Wells JL, et al. The PREP-IT Investigators: Skin antisepsis before surgical fixation of extremity fractures. N Engl J Med 2024;390:409-420. [DOI] [PubMed] [Google Scholar]




