Abstract
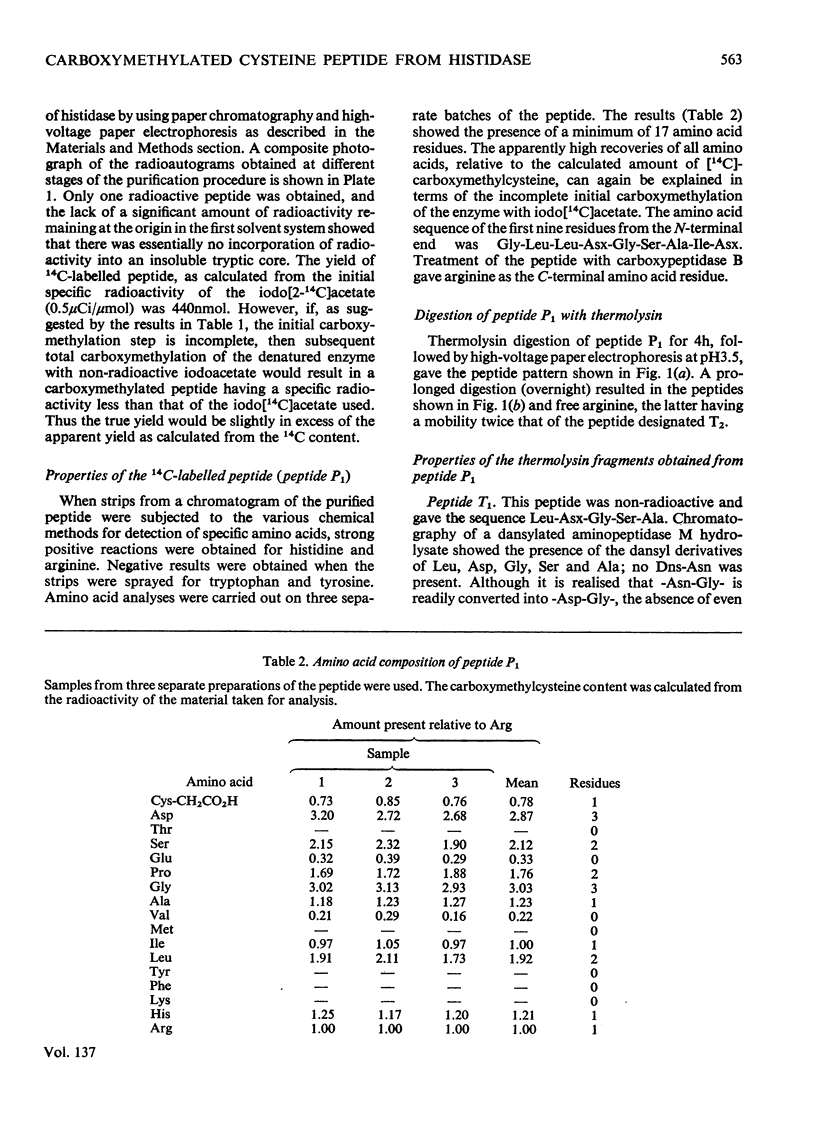
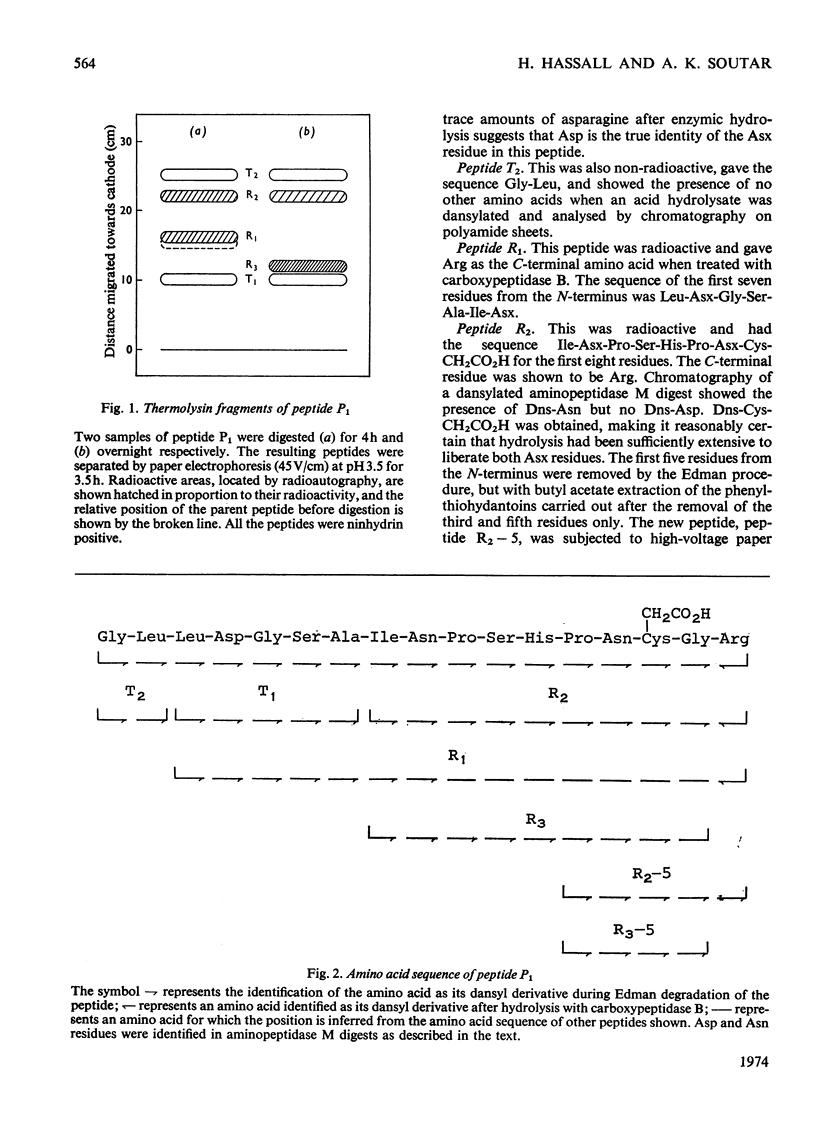
1. Oxidized (polymerized) histidine ammonia-lyase from Pseudomonas testosteroni was activated with dithiothreitol and the reduced disulphide-linked cysteine residues of the native enzyme were carboxymethylated with iodo[14C]acetate. 2. The activity of the carboxymethylated enzyme was similar to that of the polymerized form and approx. 15% of that of the fully reduced form. 3. A tryptic digest of the [14C]carboxymethylated enzyme contained only one radioactive peptide. 4. The amino acid sequence of this peptide was shown to be Gly-Leu-Leu-Asp-Gly-Ser-Ala-Ile-Asn-Pro-Ser-His-Pro-Asn-Cys- (CH2CO2H)-Gly-Arg. 5. These findings show that, during polymerization, the disulphide bonds are formed between identical regions of the enzyme, and that the cysteine residue involved is also the one required in the reduced state for full activity of the enzyme.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., CROCKER C. Réactions colorées spécifiques de l'arginine et de la tyrosine réalisées après chromatographie sur papier. Biochim Biophys Acta. 1952 Dec;9(6):704–705. doi: 10.1016/0006-3002(52)90236-9. [DOI] [PubMed] [Google Scholar]
- Asquith R. S., Carthew P. The preparation and subsequent identification of a dehydroalanyl peptide from alkali-treated oxidised glutathione. Biochim Biophys Acta. 1972 Dec 28;285(2):346–351. doi: 10.1016/0005-2795(72)90319-4. [DOI] [PubMed] [Google Scholar]
- CECIL R., McPHEE J. R. The sulfur chemistry of proteins. Adv Protein Chem. 1959;14:255–389. doi: 10.1016/s0065-3233(08)60613-0. [DOI] [PubMed] [Google Scholar]
- Cass J. S. Use of rare and threatened species of nonhuman primates as subjects in biomedical research. Fed Proc. 1970 Sep-Oct;29(5):1590–1590. [PubMed] [Google Scholar]
- Coote J. G., Hassall H. The control of the enzymes degrading histidine and related imidazolyl derivates in Pseudomonas testosteroni. Biochem J. 1973 Mar;132(3):423–433. doi: 10.1042/bj1320423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- George S. G., Kenny J. Studies on the enzymology of purified preparations of brush border from rabbit kidney. Biochem J. 1973 May;134(1):43–57. doi: 10.1042/bj1340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givot I. L., Smith T. A., Abeles R. H. Studies on the mechanism of action and the structure of the electrophilic center of histidine ammonia lyase. J Biol Chem. 1969 Dec 10;244(23):6341–6353. [PubMed] [Google Scholar]
- HALL D. A. Histidine alpha-deaminase and the production of urocanic acid in the mammal. Biochem J. 1952 Jul;51(4):499–504. doi: 10.1042/bj0510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassall H., Lunn P. J., Ryall-Wilson J. Detection of histidase and urocanase after disc electrophoresis on polyacrylamide gels. Anal Biochem. 1970 Jun;35(2):326–334. doi: 10.1016/0003-2697(70)90192-2. [DOI] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- Klee C. B., Gladner J. A. Isolation of a cysteine-peptide at the active site of histidine ammonia-lyase. J Biol Chem. 1972 Dec 25;247(24):8051–8057. [PubMed] [Google Scholar]
- Klee C. B. Metal activation of histidine ammonia-lyase. Metal ion-sulfhydryl group relationship. J Biol Chem. 1972 Mar 10;247(5):1398–1406. [PubMed] [Google Scholar]
- Klee C. B. Reversible polymerization of histidine ammonia lyase. The role of sulfhydryl groups in the activity and polymeric state of the enzyme. J Biol Chem. 1970 Jun;245(12):3143–3152. [PubMed] [Google Scholar]
- PETERKOFSKY A. The mechanism of action of histidase: amino-enzyme formation and partial reactions. J Biol Chem. 1962 Mar;237:787–795. [PubMed] [Google Scholar]
- SMITH I. Colour reactions on paper chromatograms by a dipping technique. Nature. 1953 Jan 3;171(4340):43–44. doi: 10.1038/171043a0. [DOI] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Dehydroalanine in histidine ammonia lyase. J Biol Chem. 1969 Dec 10;244(23):6550–6552. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]