Abstract
Background:
Primary lymphedema, a condition characterized by impaired lymphatic function, has long remained underexplored. Current diagnostic approaches rely on clinical history and genetic testing, yet the genetic underpinnings remain elusive in many cases. Traditional thinking suggests that primary lymphedema is confined to specific anatomical regions, but our experience challenges this notion. We hypothesize that primary lymphedema is systemic lymphatic dysfunction.
Methods:
All patients with clinical diagnosis of primary lymphedema from January 2020 to April 2022 were included in our study. Demographic data, medical and surgical history, and indocyanine green (ICG) lymphographic findings were collected.
Results:
A total of 152 patients met our inclusion criteria. We observed a predominance of female patients (75%) and a mean age of 43.9 years. The onset of swelling varied, with most patients (82.3%) experiencing it in their lower extremities. Notably, ICG lymphography revealed abnormal lymphatic findings in all symptomatic limbs, affecting multiple extremities in 97.4% of patients. Importantly, even among patients initially presenting with limited symptoms, asymptomatic extremities exhibited lymphatic defects. In addition, the extent of lymphatic disease, assessed through ICG lymphography, surpassed clinical symptoms in 80% of cases, underscoring the systemic nature of primary lymphedema.
Conclusions:
Our study suggests that primary lymphedema is a systemic lymphatic insufficiency, affecting the entire lymphatic system. This underscores the importance of comprehensive assessments, even with limited symptoms, to facilitate earlier diagnosis and more effective treatment approaches.
Takeaways
Question: Is primary lymphedema anatomically isolated or a systemic lymphatic dysfunction?
Findings: In a retrospective review of all patients diagnosed with primary lymphedema, we have found that 80% of patients had indocyanine green lymphographic disease that was more extensive than their clinical presentation, indicating lymphedema in asymptomatic extremities. This demonstrates that primary lymphedema represents a global lymphatic insufficiency.
Meaning: Our study suggests that primary lymphedema is systemic lymphatic insufficiency, affecting the entire lymphatic system.
INTRODUCTION
Lymphology research has made significant progress in secondary lymphedema, yet primary lymphedema remains relatively unexplored. Clinicians currently rely on a history devoid of lymphatic injury to diagnose both sporadic and hereditary primary lymphedema,1 with genetic testing confirming hereditary cases.2,3 However, due to our incomplete understanding of the involved genes, a negative genetic test does not conclusively rule out genetic primary lymphedema. Despite established fundamental concepts from the last century, little progress has been made.
Clinicians often categorize primary lymphedema by age of onset, although its clinical significance remains unclear.4–6 A traditional assumption is that primary lymphedema respects anatomic borders, affecting a single region in isolation.7–9 In practice, however, many patients report disease progression over time, involving a larger portion of their body.
Based on these observations, we hypothesized that primary lymphedema is a systemic condition involving the entire lymphatic system, rather than a specific region.10 Our study aims to test this hypothesis by using indocyanine green (ICG) lymphography to assess all 4 limbs of patients with suspected primary lymphedema. Our hypothesis posits that if primary lymphedema is a global lymphatic insufficiency, lymphographic abnormalities should manifest not only in symptomatic limbs but also in asymptomatic ones. By examining lymphatic function throughout the body, we seek to gain deeper insights into the extent and nature of primary lymphedema and its systemic ramifications.
METHODS
Study Approval and Participants
This study received approval from the Cleveland Clinic institutional review board and adhered to the Declaration of Helsinki. All patients with primary lymphedema presenting to our multidisciplinary lymphedema service from January 1, 2020, to April 30, 2022, were enrolled. Diagnosis of primary lymphedema was established through a comprehensive assessment, which included a detailed patient history confirming the absence of acquired lymphatic trauma and the exclusion of confounding conditions such as venous edema, heart failure, obesity, and lipedema.
ICG Lymphography Protocol
We conducted ICG lymphography following a standardized protocol, as previously described.11,12 Specifically, we intradermally injected 0.1 mL of 0.25% ICG (Akorn, Lake Forest, IL) at 3 injection sites: 2 interdigital web spaces and the wrist for the arms, and the medial malleolus for the legs. Lymphatic imaging was performed using Quest Spectrum HD (Olympus, Hamburg, Germany). Each patient underwent two 3-minute scans: an immediate postinjection scan conducted immediately after the fluorophore injection, and a 6-hour delayed scan. All ICG scans were performed, analyzed, and interpreted by a single provider, and results were confirmed by the senior author. The immediate scan allowed assessment of lymphatic pump velocity, anatomical features, and collateralization. The delayed scan facilitated visualization of plateaued normal and pathologic dermal backflow patterns, indicative of disease severity. Normal ICG lymphography results showed linear lymphatic vessels coursing toward axillae and groins, following expected anatomical patterns, and lacking pathologic dermal backflow patterns. Abnormal ICG lymphography scans displayed delayed pump velocity, abnormal/nonanatomic linear patterns, collateralized lymphatic vessels, and/or dermal backflow patterns.
Data Collection and Statistical Analysis
We collected demographic data, including sex, age, body mass index, and medical history. Additional information gathered included ICG lymphangiography results, age of lymphedema symptom onset, the affected extremity, family history of lymphedema, and any prior diagnostic studies or lymphedema treatments.
RESULTS
Patient Demographics and Characteristics
A total of 152 patients met our inclusion criteria. Among these, 114 (75.0%) were women, and 38 (25.0%) were men. The average age of the patients was 43.9 ± 19.2 years (ranging from 2 to 88 years), with an average body mass index of 27.0 ± 5.7 kg/m2. The mean age of swelling onset was 30.7 ± 18.8 years, with onset occurring at birth in some cases. Notably, 10 (6.6%) patients reported a positive family history of lymphedema (Table 1).
Table 1.
Demographics
| N | % | |
|---|---|---|
| Patients | 152 | |
| Age, y | 43.9 ± 19.2 | |
| BMI, kg/m2 | 27.0 ± 5.7 | |
| Sex | ||
| Female | 114 | 75.0 |
| Male | 38 | 25.0 |
| Age of swelling onset | 31.0 ± 18.8 | |
| Family history of lymphedema | 10 | 6.6 |
Disease Presentation
The presentation of lymphedema symptoms varied among the patients. a total of 81 (53.3%) patients presented with lymphedema symptoms affecting multiple extremities, whereas 68 (44.7%) patients experienced symptoms isolated to a single extremity. In contrast, 3 (2.0%) patients had symptoms limited to nonextremity regions (face/trunk). Among isolated extremity involvement cases, the left lower extremity was the most frequently affected, reported by 39 (25.7%) patients. Bilateral lower extremities were affected in 55 (36.2%) patients, and all 4 extremities showed involvement in 14 (9.2%) patients (Table 2).
Table 2.
First Extremity Swelling
| N | % | |
|---|---|---|
| Upper extremity | 11 | 7.2 |
| Isolated right upper extremity | 7 | |
| Isolated left upper extremity | 1 | |
| Bilateral upper extremity | 3 | |
| Lower extremity | 125 | 82.3 |
| Isolated right lower extremity | 42 | |
| Isolated left lower extremity | 68 | |
| Bilateral lower extremity | 15 | |
| Bilateral upper and lower extremities | 0 | 0 |
| Others | 16 | 10.5 |
*Others are defined as mixed upper and lower extremity.
Onset and Affected Sites
A majority of patients,125 (82.3%), reported that swelling initially began in their lower extremities, whereas 11 (7.2%) experienced onset in their upper extremities. In addition, 13 (8.6%) patients reported swelling starting in both upper and lower extremities, and 3 (1.9%) had swelling limited to their face or trunk. The left lower extremity was the most commonly affected site for initial swelling, with 68 (44.7%) patients reporting it, followed by the right lower extremity in 42 (27.6%) (Table 3).
Table 3.
Lymphedema Symptoms
| N | % | |
|---|---|---|
| Upper extremity | 11 | 7.2 |
| Isolated right upper extremity | 5 | |
| Isolated left upper extremity | 2 | |
| Bilateral upper extremity | 4 | |
| Lower extremity | 116 | 76.4 |
| Isolated right lower extremity | 22 | |
| Isolated left lower extremity | 39 | |
| Bilateral lower extremity | 55 | |
| Bilateral upper and lower extremities | 14 | 9.2 |
| Others* | 11 | 7.2 |
Others are defined as mixed upper and lower extremity.
Lymphatic Dysfunction on ICG Lymphography
On ICG lymphography, all symptomatic extremities exhibited abnormal lymphographic findings. Of the 152 patients, 148 (97.4%) displayed lymphatic dysfunction in multiple extremities (Figs. 1, 2). The most prevalent distribution pattern involved lymphatic dysfunction in all 4 extremities, observed in 66 (43.4%) patients (Table 4). Moreover, 50 (32.9%) patients exhibited ICG lymphatic dysfunction in 2 limbs, 34 (22.4%) patients in 3 limbs, and only 4 (2.6%) patients in a single limb.
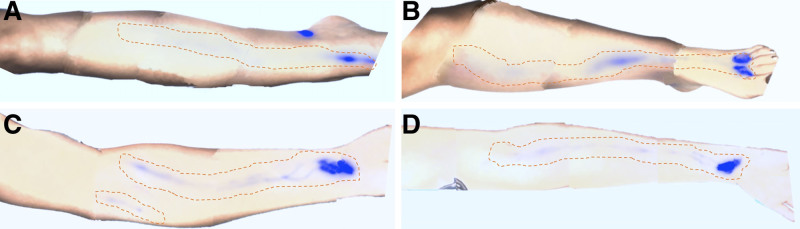
Fig. 1.
Four-limb ICG lymphography of a 38-year-old woman with primary lymphedema, symptomatic only in the right leg. Despite being only symptomatic in the right leg, the patient demonstrated significant functional deficits in all 4 limbs. The ICG signals became arrested at the mid-third of the leg, at the knee, and in the antecubital fossa in (A) right leg, (B) left leg, and (C, D) bilateral arms, respectively. These patterns deviate from healthy lymphatic drainage patterns, where ICG would progress to groins and axillae. The study demonstrated a systemic functional impairment, indicative of the extensive nature of lymphatic dysfunction in primary lymphedema.
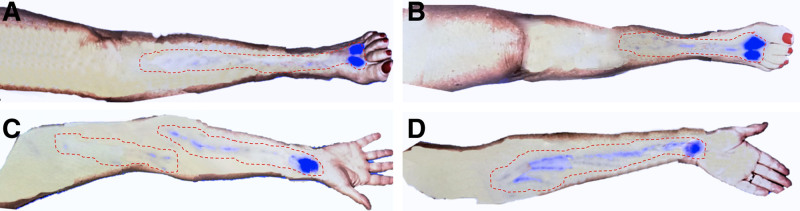
Fig. 2.
Four-limb ICG lymphography scan of a 72-year-old woman with primary lymphedema, initially reporting swelling only in left leg. A, ICG signal stagnation in the proximal left lower leg, failing to reach the groin. B, ICG signal arrested in the mid-right lower leg. C, Right arm showing ICG signal halting in the mid-upper arm. D, Left arm showing ICG signal prematurely terminated at the elbow and at the mid-upper arm. After the patient was made aware of her systemic, 4-limb functional impairment, she reported mild but recurrent swelling in both hands, exacerbated by repetitive movements. This case underscores the importance of thorough ICG evaluation, as initial patient reports may overlook less pronounced symptoms of lymphedema.
Table 4.
ICG Lymphography
| N | % | |
|---|---|---|
| Upper extremity | 6 | 4.0 |
| Isolated right upper extremity | 0 | |
| Isolated left upper extremity | 0 | |
| Bilateral upper extremity | 6 | |
| Lower extremity | 43 | 28.3 |
| Isolated right lower extremity | 2 | |
| Isolated left lower extremity | 2 | |
| Bilateral lower extremity | 39 | |
| Bilateral upper and lower extremities | 66 | 43.4 |
| Others* | 37 | 24.3 |
Others are defined as mixed upper and lower extremity.
Asymptomatic Extremities in Patients With Symptomatic Lymphedema
Among patients with 1 symptomatic extremity, 63 of 68 (92.6%) displayed lymphatic defects in 1 or more asymptomatic extremities. In patients with 2 symptomatic extremities, 43 of 64 (67.2%) had lymphatic defects in asymptomatic extremities. In patients with 3 symptomatic extremities, 1 of 3 (33.3%) showed lymphatic defects in asymptomatic extremities. Interestingly, all 3 (100%) patients with nonextremity symptoms (face/trunk) exhibited lymphatic defects in all 4 extremities.
Extent of Lymphatic Disease
Finally, we investigated the extent of lymphatic disease. Fourteen patients complained of swelling in all 4 extremities, which was consistent with ICG lymphography findings. Excluding these 14 patients, 110 of 138 (80%) patients had ICG lymphographic disease that was more extensive than their clinical symptoms, with ICG lymphography findings supporting the presence of lymphedema in asymptomatic extremities (Table 5).
Table 5.
Comparing ICG Lymphography Findings to Symptoms
| N | % | |
|---|---|---|
| More | 110 | 80 |
| Less | 0 | 0 |
| Same* | 28 | 20.3 |
n = 14 Patients had symptoms involving all 4 extremities, consistent with ICG lymphographic findings, excluded from analysis.
DISCUSSION
Primary lymphedema remains a significant challenge in the field of medicine, demanding increased attention and research focus. Although acquired lymphedema has received more extensive investigation, primary lymphedema remains relatively underexplored. Clinicians often rely on certain indicators, such as the absence of an inciting event, early age of disease onset, and a family history of lymphedema, to improve diagnostic accuracy. However, it is essential to recognize that these clinical features are not always present in patients with primary lymphedema, indicating the need for further investigation and refinement of diagnostic criteria.
Traditionally, the diagnosis of primary lymphedema has centered on the absence of an identifiable cause of lymphatic disruption. Patients frequently present with a lack of an identifiable trigger associated with the development of lymphedema. The age of disease onset is another diagnostic cue heavily relied upon. However, the clinical significance of classifying primary lymphedema into congenital lymphedema, lymphedema praecox, and lymphedema tarda in terms of predicting disease severity and guiding management remains unclear.6 Family history of lymphedema and genetic testing are also emphasized in diagnosing primary lymphedema; however, in our cohort, only 10 (6.6%) patients had a family history of lymphedema. Because the majority of our primary lymphedema cases were sporadic rather than hereditary, it suggests that although family history and genetic testing can provide valuable insights, they should not be relied upon exclusively for diagnosing primary lymphedema.9,10,13 Furthermore, the conventional belief has long held that primary lymphedema is anatomically restricted to a specific body region, akin to secondary lymphedema. However, findings from our cohort study challenge this entrenched notion.
In the past 2 decades, ICG lymphography has emerged as a pivotal imaging modality in lymphology. Researchers and surgeons have progressively utilized this technology not only for diagnostic purposes but also for severity staging; treatment planning—determining the most appropriate surgical procedures; and outcome tracking.14 Although finer interpretations of specific pathologic patterns, such as “stardust” versus “diffuse,” may vary among investigators, the determination of healthy/normal versus pathologic/abnormal is relatively clear-cut. ICG lymphography has shown high sensitivity in detecting both clinical and subclinical lymphatic dysfunction.15 A sensitivity of 1.0 in the upper limbs and 0.89 in the lower limbs has been reported by other authors for detecting symptomatic lymphedema.14 This sensitivity was found to be comparable to that of magnetic resonance lymphangiography (1.0) and higher than that of lymphoscintigraphy (0.62) and computed tomography scans (0.33). The specificity was found to be 1.0 with all the 4 imaging modalities. In our previously published study, we found that ICG lymphography had a sensitivity of 1.0 for symptomatic disease in both upper and lower limbs.16 In addition, ICG lymphography could identify lymphatic dysfunction in 54% of asymptomatic limbs where lymphoscintigraphy was normal.
This high sensitivity of ICG lymphography was utilized in the current study, which confirmed abnormal lymphatic patterns in all symptomatic and multiple asymptomatic extremities. A remarkable 97.4% of patients with primary lymphedema exhibited ICG-validated lymphatic dysfunction in multiple extremities. These compelling findings strongly attest to the notion that primary lymphedema transcends the confines of localized lymphatic dysfunction, manifesting as a systemic condition that impacts the entirety of the lymphatic system.17–19 This further corroborates our initial report, which highlighted the discovery of lymphatic dysfunction in contralateral limbs among patients diagnosed with primary lymphedema.10 The finding that 110 (80%) patients exhibited lymphatic dysfunction extending beyond their clinical symptoms underscores the need to evaluate the entire body, extending beyond the presenting symptomatic region in patients with primary lymphedema.
Our finding states that primary lymphedema is systemic and is supported by the embryologic framework of the lymphatic system, where early genetic defects are likely to affect the entire lymphatic network, even when clinical symptoms seem localized. The development of the lymphatic system begins in the fifth week of gestation, with endothelial cells differentiating from the paraxial mesoderm to form a systemic network of lymphatic vessels.20–22 This process involves the formation of 5 primary lymph sacs and the expansion of lymphatic vessels throughout the body, regulated by key genetic and molecular pathways, including PROX1 and VEGF-C/VEGFR-3 signaling pathways.3 Given the interconnected nature of these vessels and the uniformity of the developmental signals, any disruption or mutation during this stage would inherently lead to a systemic compromise of the entire lymphatic system.4 This underscores the global impact of early developmental anomalies, affirming the systemic nature of primary lymphedema. Mutations in the genes regulating these pathways have more commonly been found to be sporadic than hereditary, correlating with our observation of negative family history in most patients with primary lymphedema.3,23
Although we observed that primary lymphedema manifests as systemic lymphatic dysfunction, we noted that the majority of our patients initially presented with symptoms in a single limb, which later progressed to involve other extremities. In our cohort, the lower extremities were most commonly the first to become symptomatic, with the left lower extremity being the most frequently affected initially. These patients subsequently developed symptomatic lymphedema in other extremities. This suggests that at the initial presentation with symptoms in a single extremity, the asymptomatic limbs may already be experiencing underlying lymphatic dysfunction. This progression of primary lymphedema has not been previously reported; however, it is critical for the diagnosis and management of these patients. It is also important to note that ICG lymphography may yield false-negative results in early lymphedema.24 This implies that asymptomatic limbs with early lymphatic dysfunction may have a false-negative ICG study.
Recent advancements in surgical management, including supermicrosurgical lymphaticovenular anastomosis, lymph node–to-vein anastomosis, vascularized lymph vessel transplantation, and vascularized lymph node transplantation, which have notably improved the outlook for individuals with secondary lymphedema, have also been extended to the treatment of primary lymphedema without careful consideration of the nuanced differences between these 2 distinct disease entities.25,26 Our discovery of widespread lymphatic dysfunction in patients with primary lymphedema carries profound implications for the selection of appropriate management strategies. Notably, tissue transplant procedures such as vascularized lymph node transplantation and vascularized lymph vessel transplantation, when performed in patients with primary lymphedema, may potentially pose a higher risk of inducing donor site lymphedema by further compromising lymphatic function at a site with preexisting functional insufficiency.
Extending the diagnosis of lymphatic insufficiency beyond the symptomatic regions in patients with primary lymphedema not only enables timely intervention but also holds the potential to halt the progression toward symptomatic disease. Considering the routine practice of lymph node dissections and removal during cancer surgery, it is prudent to contemplate screening patients for preexisting asymptomatic primary lymphatic insufficiency—a predisposition to developing lymphedema—as a valuable measure in disease prevention or delaying its onset. The preoperative identification of asymptomatic lymphatic insufficiency would provide oncologic surgeons with critical information regarding a heightened risk of lymphedema development compared with their healthy counterparts, potentially guiding the use of more lymph-sparing techniques.27,28 Moreover, the consideration of immediate lymphatic reconstruction, or the lymphatic microsurgical preventive healing approach, and delayed distal lymphaticovenular anastomosis to mitigate the risk of developing symptomatic disease may be even more compelling for oncologic patients who commence their cancer treatment with a compromised lymphatic system.29–33
CONCLUSIONS
Our study provides compelling evidence that primary lymphedema is not confined to specific anatomic regions but represents a global lymphatic insufficiency affecting the entire lymphatic system. Our findings underscore the importance of evaluating the entire body, even when patients initially present with limited symptoms, leading to earlier diagnosis and more effective management strategies for primary lymphedema.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online 20 December 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Mellor RH, Hubert CE, Stanton AWB, et al. Lymphatic dysfunction, not aplasia, underlies Milroy disease. Microcirculation (New York, N.Y). 2010;17:281–296. [DOI] [PubMed] [Google Scholar]
- 2.Brouillard P, Boon L, Vikkula M. Genetics of lymphatic anomalies. J Clin Invest. 2014;124:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudduth CL, Greene AK. Primary lymphedema: update on genetic basis and management. Adv Wound Care (New Rochelle). 2022;11:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connell F, Brice G, Jeffery S, et al. A new classification system for primary lymphatic dysplasias based on phenotype. Clin Genet. 2010;77:438–452. [DOI] [PubMed] [Google Scholar]
- 5.Connell F, Gordon K, Brice G, et al. The classification and diagnostic algorithm for primary lymphatic dysplasia: an update from 2010 to include molecular findings. Clin Genet. 2013;84:303–314. [DOI] [PubMed] [Google Scholar]
- 6.Smeltzer DM, Stickler GB, Schirger A. Primary lymphedema in children and adolescents: a follow-up study and review. Pediatrics. 1985;76:206–218. [PubMed] [Google Scholar]
- 7.Senger JLB, Kadle RL, Skoracki RJ. Current concepts in the management of primary lymphedema. Medicina (Lithuania). 2023;59:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goss JA, Maclellan RA, Greene AK. Adult-onset primary lymphedema: a clinical-lymphoscintigraphic study of 26 patients. Lymphat Res Biol. 2019;17:620–623. [DOI] [PubMed] [Google Scholar]
- 9.Goss JA, MacLellan RA, Greene AK. Primary lymphedema of the upper extremities: clinical and lymphoscintigraphic features in 23 patients. Lymphat Res Biol. 2019;17:40–44. [DOI] [PubMed] [Google Scholar]
- 10.John T, Heineman WFC. Indocyaine green lymphography to diagnose primary lymphedema and the incidental discovery of primary asymptomatic lymphatic insufficiency. Plast Reconstr Surg Glob Open. 2018;6(Suppl 9):55. [Google Scholar]
- 11.Chen WF, Qin ES, Bowen MJ, et al. Exercise-enhanced ICG lymphography: a fast approach to diagnosis and staging of lymphedema. Int Microsurg J. 2021;5:3–6. [Google Scholar]
- 12.Chen WF, Zhao H, Yamamoto T, et al. Indocyanine green lymphographic evidence of surgical efficacy following microsurgical and supermicrosurgical lymphedema reconstructions. J Reconstr Microsurg. 2016;32:688–698. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Yoshimatsu H, Narushima M, et al. Indocyanine green lymphography findings in primary leg lymphedema. Eur J Vasc Endovasc Surg. 2015;49:95–102. [DOI] [PubMed] [Google Scholar]
- 14.Mihara M, Hara H, Araki J, et al. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS One. 2012;7:e38182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto T, Matsuda N, Doi K, et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow stage and concept of subclinical lymphedema. Plast Reconstr Surg. 2011;128:314e–321e. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa BA, Lammers JD, Al-Malak M, et al. Lymphoscintigraphy versus indocyanine green lymphography—which should be the gold standard for lymphedema imaging? Lymphatics. 2023;1:25–33. [Google Scholar]
- 17.Burnand KM, Glass DM, Mortimer PS, et al. Lymphatic dysfunction in the apparently clinically normal contralateral limbs of patients with unilateral lower limb swelling. Clin Nucl Med. 2012;37:9–13. [DOI] [PubMed] [Google Scholar]
- 18.Aldrich MB, Guilliod R, Fife CE, et al. Lymphatic abnormalities in the normal contralateral arms of subjects with breast cancer-related lymphedema as assessed by near-infrared fluorescent imaging. Biomed Opt Express. 2012;3:1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf S, von Atzigen J, Kaiser B, et al. Is lymphedema a systemic disease? A paired molecular and histological analysis of the affected and unaffected tissue in lymphedema patients. Biomolecules. 2022;12:1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Z, Zhao X, Wu Z, et al. Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets. Sig Transduct Target Ther. 2024;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. [DOI] [PubMed] [Google Scholar]
- 22.Oliver G, Srinivasan RS. Lymphatic vasculature development: current concepts. Ann N Y Acad Sci. 2010;1131:75–81. [DOI] [PubMed] [Google Scholar]
- 23.Mendola A, Schlögel MJ, Ghalamkarpour A, et al. ; The Lymphedema Research Group. Mutations in the VEGFR3 signaling pathway explain 36% of familial lymphedema. Mol Syndromol. 2013;4:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen WF, Lensing JN, Bowen M. Indocyanine green lymphography can be falsely negative in early extremity lymphedema. J Plast Reconstr Aesthet Surg. 2020;73:391–407. [DOI] [PubMed] [Google Scholar]
- 25.Raman S, Sanka SA, Ji J, et al. Vascularized lymph node transfer for the treatment of lymphedema: a systematic review and meta-analysis of clinical and patient-reported outcomes. Plast Aesthet Res. 2023;10:6. [Google Scholar]
- 26.Scaglioni MF, Arvanitakis M, Chen YC, et al. Comprehensive review of vascularized lymph node transfers for lymphedema: outcomes and complications. Microsurgery. 2018;38:222–229. [DOI] [PubMed] [Google Scholar]
- 27.Lessiani G, Iodice P, Nicolucci E, et al. Lymphatic edema of the lower limbs after orthopedic surgery: results of a randomized, open-label clinical trial with a new extended-release preparation. J Biol Regul Homeost Agents. 2015;29:805–812. [PubMed] [Google Scholar]
- 28.Yoshida S, Koshima I, Imai H, et al. Localized lymphedema after treatment for soft tissue sarcoma in the lower limbs: comparison of improvement according to duration before lymphaticovenular anastomosis. Clin Case Rep. 2019;7:1534–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boccardo F, Valenzano M, Costantini S, et al. LYMPHA technique to prevent secondary lower limb lymphedema. Ann Surg Oncol. 2016;23:3558–3563. [DOI] [PubMed] [Google Scholar]
- 30.Boccardo F, Casabona F, DeCian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery. 2014;34:421–424. [DOI] [PubMed] [Google Scholar]
- 31.Campisi CC, Ryan M, Boccardo F, et al. LYMPHA and the prevention of lymphatic injuries: a rationale for early microsurgical intervention. J Reconstr Microsurg. 2014;30:71–72. [DOI] [PubMed] [Google Scholar]
- 32.Levy AS, Murphy AI, Ishtihar S, et al. Lymphatic microsurgical preventive healing approach for the primary prevention of lymphedema: a 4-year follow-up. Plast Reconstr Surg. 2023;151:413–420. [DOI] [PubMed] [Google Scholar]
- 33.Chen WF, Orfahli LM, Huang TCT. Surgical prevention of breast cancer-related lymphedema: delayed distal lymphaticovenicular anastomosis: an alternative to the classic LYMPHA technique. Arch Breast Cancer. 2021;8:277–283. [Google Scholar]