Abstract
Background:
The use of minimally invasive (laparoscopic and robotic) pancreatoduodenectomy (PD) is being increasingly adopted despite the lack of hard evidence to support its utilisation. With recent randomised controlled trials (RCTs) comparing open pancreatoduodenectomy (OPD) with robotic or laparoscopic pancreatoduodenectomy (RPD or LPD), we undertook a network meta-analysis (NMA) comparing all 3 approaches to evaluate comparative outcomes.
Methods:
A systematic search of MEDLINE, EMBASE, and Cochrane CENTRAL was conducted up to May 2024 and relevant RCTs were identified. A random-effects meta-analysis and trial sequential analysis (TSA) were conducted for primary outcomes, followed by a Bayesian NMA of length of stay (LOS), duration of surgery, intraoperative blood loss, and pancreas resection-related outcomes
Results:
Seven RCTs involving 1336 patients were included, 5 investigating LPD compared with OPD and 2 RPD to OPD. Pairwise meta-analysis indicated that LPD was associated with shorter hospital stay (mean difference [MD], −1.39; 95% confidence interval [CI], −2.33 to −0.45) and lower intraoperative blood loss compared with OPD (MD, −131; 95% CI, −146 to −117). However, LPD was associated with significantly longer operative duration (MD, 39.5; 95% CI, 34–45). TSA confirmed the robustness of the positive and negative findings on pairwise meta-analysis. In comparison, there were no significant differences between RPD and OPD in pairwise meta-analysis, which could not be confirmed by TSA. Network meta-analysis tended to favour LPD in most outcome parameters including LOS, duration of surgery, and pancreas resection-related outcomes.
Conclusions:
The current RCT evidence suggests potential better outcomes in LPD in comparison with RPD and OPD. However, few studies demonstrated robust statistical significance in outcome measures, suggesting an underpowered evidence base and possible selection bias. Hence, with current equivocal data, there is a need for ongoing RCTs to validate the role of minimally invasive approaches in PD.
INTRODUCTION
Pancreatoduodenectomy (PD) is a technically challenging and complex procedure with high mortality and morbidity. Advances in minimally invasive surgery including laparoscopic and robotic platforms have been associated with some improved outcomes in pancreatic surgery.1 Randomised controlled trials (RCTs) of these minimally invasive surgical approaches will provide a higher level evidence base to support more widespread adoption. There must additionally be a cost–benefit demonstration for pressurised healthcare systems, and definitive evidence of patient benefit.2
Since the first RCT was published in 2017,3 several RCTs have been completed for the use of minimally invasive surgery. Laparoscopic and robotic approaches have significant differences compared with the open approach, the latter of which is the current standard of care in most centres worldwide. While laparoscopic pancreatoduodenectomy (LPD) is now considered safe and feasible in high-volume centres with vast experience in minimally invasive techniques, robotic systems are thought to have benefits for overcoming restrictions to challenging laparoscopic ergonomics.2 The robotic systems are considered particularly valuable during the reconstruction stages of PD and when a vascular resection may be required.4 Several retrospective studies have compared OPD to LPD and robotic pancreatoduodenectomy (RPD)1,5–8 and previous meta-analyses do not incorporate the latest evidence.9–11 Furthermore, there are no RCTs comparing LPD to RPD. The more recent RCTs predominantly compared open with the robotic approach for PD and are likely to be the most frequently used approaches for PD over LPD, particularly in Europe.
Given the emergence of new RCTs in this otherwise controversial evidence base, comparison of all available evidence is therefore prudent. We aimed to assess RCTs comparing open, laparoscopic, and robotic approaches to PD and compare their outcomes and synthesise the existing trial evidence. We further aimed to undertake a network meta-analysis (NMA) with trial sequential analysis to compare the 3 operative strategies given the absence of direct evidence comparing LPD with RPD.
METHODS
This review adhered to the recommendations of the Cochrane Handbook for Systematic Reviews and Interventions and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.12 A review protocol was developed a priori and submitted for registration to PROSPERO (ID: 522756).
The inclusion criteria were patients over 18 years old with benign, premalignant, or malignant indications for PD.
Literature Search
A systematic search of MEDLINE (OVID), EMBASE, EMBASE Classic, and the Cochrane Controlled Register of Trials (CENTRAL) was conducted from their date of inception to May 24, 2024, and filtered for only “randomised controlled trials (RCTs)” and all ”clinical trials.” The following query key-words and MeSH terms were employed: laparoscop*, ‘Minimally Invasive Surgical Procedure’, Robotic Surgical Procedures pancreatoduodenectomy, pancrea* surg*, pancrea* resection, Whipple. The full search is presented in Supplemental Appendix A, http://links.lww.com/AOSO/A417. An additional hand search was conducted and content experts in the field of pancreatic surgery were consulted to identify any other possible studies.
Study Selection and Data Points and Extraction
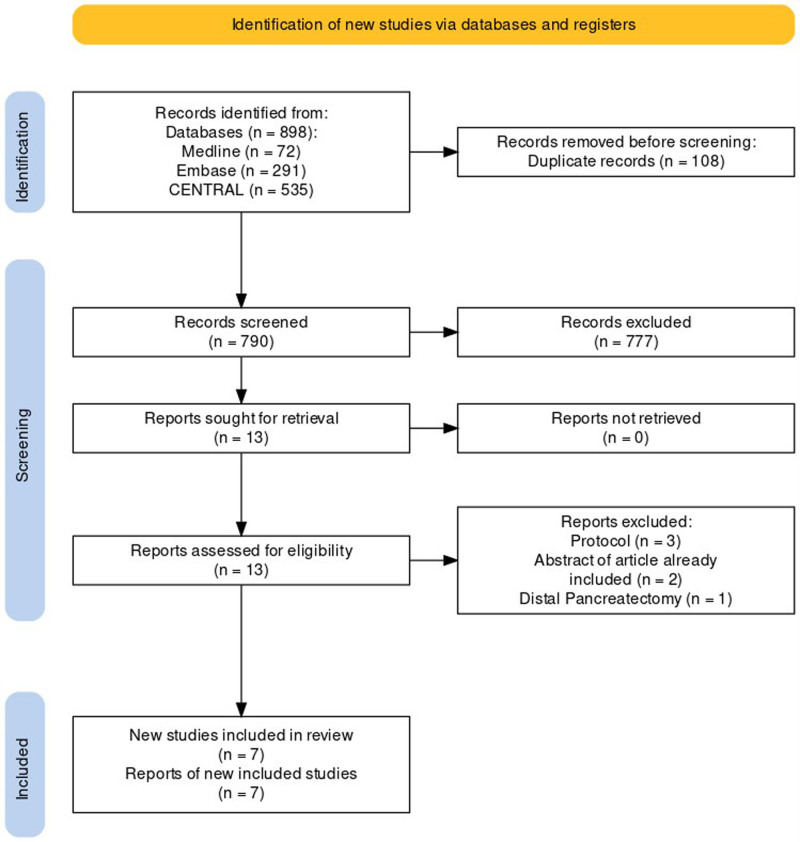
Title and abstract screening were conducted independently by 2 reviewers (N.J. and J.L.) following deduplication. In cases where there was disagreement regarding the suitability of trials for full-text review, a third party (C.V.) was consulted to resolve any discrepancies. Subsequently, the full texts of shortlisted articles were reviewed independently by the same 2 reviewers. A comprehensive overview of the screening process is provided in the PRISMA flow diagram (Fig. 1).
FIGURE 1.
PRISMA flow diagram of study selection.
The data were extracted using a predefined extraction sheet, which can be provided upon reasonable request. Two reviewers (N.J. and C.V.) conducted this independently of each other. Following extraction, the reviewers compared the data, addressing any inconsistencies through discussion. If necessary, a third party (J.L.) was consulted to resolve any discrepancies and reach a consensus.
Data was sought for general information (eg, year of publication, country, number of institutions); study participant characteristics (eg, age, disease, American Society of Anesthesiologists status), and inclusion and also exclusion criteria; type of intervention (laparoscopic, robotic-assisted, open, technique used); surgeon experience in both techniques used; and clinical outcomes (length of stay [LOS], postoperative pancreatic fistula [POPF], postpancreatoduodenectomy haemorrhage [PPH], delayed gastric emptying [DGE], bile leak).
Outcome Measures, Terminology, and Definitions
The primary outcome measure was LOS. This was because LOS is the most commonly reported primary outcome in RCTs published to date comparing OPD to LPD or RPD (4 of 7 RCTs). Secondary outcome measures were estimated intraoperative blood loss, oncological outcomes, and pancreatic-specific outcomes; DGE, POPF, biliary fistula, and PPH as per the International Study Group for Pancreatic Surgery (ISGPS).13–16
Classification of the different minimally invasive approaches was done using the Brescia European Guidelines on Minimally Invasive Pancreatic Surgery (EGUMIPS).17 In the current study, “Robotic PD” denoted operations considered “purely robotic” and “robot-assisted” as per the EGUMIPS. Purely RPD is characterised by the placement of 3 to 4 robotic ports and 1 or more laparoscopic ports. The procedure is performed through 3 to 4 robotic ports and 1 or more laparoscopic ports. The robot is docked at the beginning of the surgery. Resection and reconstructive phases are carried out with robotic instrumentation. By comparison, the “robot-assisted” variant used the same port setup but with the resection phase carried out using both robotic and laparoscopic instruments. Hybrid procedures involving mini-laparotomies or extensive laparoscopic surgery were not considered in this group. “Laparoscopic PD” denoted “laparoscopic” and “single-port laparoscopic” procedures as defined by EGUMIPs were considered LPD in the current study.17
Risk of Bias Assessment
The Cochrane Collaboration’s Risk-of-bias tool 2.0 was used to assess study design.18
Statistical Analysis
R (R Foundation for Statistical Computing, Vienna, Austria) was used for this analysis using the meta package. In studies that reported medians for continuous variables, mean and SD estimates were calculated from the methods of Wan et al19 and Luo et al.20 We first conducted a random-effects meta-analysis for primary outcomes. The relative risk and 95% confidence interval (CI) were estimated.
Subsequently, we performed a trial sequential analysis (TSA) to assess the reliability of the effect size based on cumulative aggregation of included trials per the recommendations of Pogue and Yusuf21 (Supplemental Methods, http://links.lww.com/AOSO/A417). If corrected trial sequential monitoring boundaries were crossed before meeting the required information size, meta-analyses were considered reliable, and if not, further RCTs are required.22
A random-effects NMA, with 3 nodes for each of the approaches studies, was performed using GeMTC in R.23 GeMTC employs a Bayesian framework with noninformative priors. Network maps were generated to visualise all direct comparisons made. Line thickness corresponded with the number of studies assessing a particular direct comparison and the size of nodes correlated with the number of participants receiving a particular intervention. Odds ratios were used for categorical outcome data, and mean differences (MDs) for continuous data, both accompanied by 95% credibility intervals (CRIs). OPD was the comparator arm. Rankogram plots visualised the relative effectiveness of each intervention per outcome represented as stacked bar plots of the probability of each intervention achieving each rank. Sum under the cumulative ranking scores were used to rank interventions where a score of 1 meant the intervention was the best ranked 100% of the time, and a score of 0 where it ranked as the worst intervention 100% of the time.24 Heterogeneity was assessed by assessing the random-effects SDs.25 Transitivity was assessed by collecting and comparing demographic data, surgical approach, and cointerventions across direct comparisons. Comparison-adjusted funnel plots were constructed and visually inspected for asymmetry to indicate publication bias.
RESULTS
Study Characteristics
The search results and study selection are summarised in Figure 1. The final 7 studies included 1336 patients (range: 64–656) and were conducted in 5 different countries (India, Spain, The Netherlands, China, and Germany). Three studies were single-centre trials,3,9,26 a further 4 were multicentre trials.11,27–29 One study was a multicentre patient-blinded study.29 The PLOT trial exclusively included patients with periampullary cancers,3 whereas the Wang et al only included patients who underwent PD for pancreatic ductal adenocarcinoma.28 The remaining RCTs included patients with benign, premalignant, and malignant conditions of the pancreatic and periampullary regions. The conversion rate from LPD to OPD and from RPD to OPD varied from 3% to 23.5% and 3.7% to 19%, respectively. The summated rate of conversion from a minimally invasive approach to open was 6.2% (32/513) in LPD and 8.8% (9/110) in RPD. Study characteristics are presented in Table 1. Age, gender mix, and body mass index were generally comparable between direct comparisons. Study population characteristics, operative characteristics, and histological findings are presented in Table 2.
TABLE 1.
Study Characteristics of RCTs Included
| Palanivelu et al(3) | Poves et al(26) | van Hilst et al(29) | Wang et al(27) | Wang et al(28) | Klotz et al(9) | Liu et al(11) | |
|---|---|---|---|---|---|---|---|
| Year | 2017 | 2018 | 2019 | 2021 | 2023 | 2024 | 2024 |
| Country | India | Spain | The Netherlands | China | China | Germany | China |
| Design | Single-centre, open-label RCT | Single-centre, open-label RCT | Multicentre, patient-blinded RCT | Multicentre, open-label RCT | Multicentre, open-label RCT | Single-centre, open-label RCT | Multicentre, open-label RCT |
| Primary outcome | Length of hospital stay | Length of hospital stay | |||||
| Diseases included | Periampullary cancers | Benign, premalignant, malignant conditions of the pancreatic and periampullary region | Benign, premalignant, malignant conditions of the pancreatic and periampullary region | Benign, premalignant, malignant conditions of the pancreatic and periampullary region | PDAC | Benign, premalignant, malignant conditions of the pancreatic and periampullary region | Benign, premalignant, malignant conditions of the pancreatic and periampullary region |
| Patients randomised | 64 | 66 | 105 | 656 | 200 | 81 | 164 |
| Patients analysed | 64 | 61 | 99 | 594 | 200 | 62 | 161 |
| Conversion rate (%) | 3 | 23.5 | 20 | 11.1 | 2 | 23 | 3.7 |
| Method of analysing | ITT | mITT | mITT | mITT | mITT | mITT | mITT |
| Inclusion criteria | (i) Unresectable disease at the outset of the procedure (ii) Unresectable disease at a later stage of the procedure |
(i) Metastatic disease (ii) Locally advanced tumour requiring preplanned major vascular resection (iii) Rescue surgery after neoadjuvant treatment (iv) Eastern Cooperative Oncology Group (ECOG) 22 score >2 (v) Severe chronic hepatic, renal, pulmonary or cardiac disease (vi) Clearly hostile abdomen for the laparoscopic approach (v) Pregnancy (vi) No consent from patient |
(i) Suspicion of tumour involvement of major vasculature (portal vein, superior mesenteric vein, superior mesenteric artery or hepatic artery) based on CT (ii) Body mass index >35 kg/m2 (iii) Neoadjuvant radiotherapy for pancreatic cancer |
(i) Distant metastases (ii) Pancreatectomy or palliative surgery other than pancreatoduodenectomy (iii) ASA >3 (iv) Synchronous malignancy (v) Pregnancy (vi) Neoadjuvant chemotherapy |
(i) Vascular invasion and requiring vascular resection (ii) Distant metastases (iii) Lesions that required left, central or total pancreatectomy or another form of palliative surgery (iv) ASA >3 |
(i) Borderline or unresectable carcinoma of the pancreatic head—defined in the National Comprehensive Cancer Network guidelines (ii) Presence of distant metastases (iii) ASA >3 (iv) Participation in another interventional clinical trial (v) Anticipated language barrier or lack of compliance |
(i) Distant metastases (ii) Severe cardiac, pulmonary, hepatic or renal diseases (iii) Synchronous tumours (iv) Neoadjuvant therapy (v) Pregnant |
| Exclusion criteria | (i) 30–70 yr of age (ii) Resectable periampullary cancer (iii) No radiological involvement of the superior mesenteric vein and portal vein, and preserved fat planes between the tumour and coeliac axis, hepatic artery and superior mesenteric artery (iv) Initial surgery without previous chemotherapy (v) No previous chemotherapy (vi) No metastatic disease after staging laparoscopy |
(i) Consecutive patients scheduled for elective PD to treat benign condition, premalignant condition and malignant condition (ii) >18 yr (iii) Informed consent |
(i) Benign, premalignant or malignant indications for elective pancreaticoduodenectomy (ii) Fit to undergo procedure (iii) Written informed consent before randomization |
(i) 18–75 yr (ii) Benign, premalignant and malignant resectable tumours of the pancreatic and periampullary region eligible for pancreaticoduodenectomy |
(i) Histologically confirmed PDAC (ii) 18–75 yr |
(i) Adult patients eligible for PD for benign, premalignant and malignant indications (ii) Consent |
(i) Resectable benign, premalignant or malignant tumour in the pancreatic head or periampullary region (ii) 18–75 yr (iii) Eastern Oncology Cooperative Group (ECOG) performance status of 0–1 (iv) ASA score <4 |
| No. surgeons | Two (both performed LPDs and OPDs) | Two (1 surgeon operated on all LPDs and the other surgeon performed OPDs) | Nine surgeons (all performed both LPDs and OPDs) | 14 | NA | Two performed OPD and RPD, 13 performed OPD only | 5 |
| Required and existent surgeon experience in minimally invasive PD | Each ≥25 LPDs | 15 previous LDPs performed at the centre from 2006 to 2013 (beginning of the trial) | ≥ LPDs | ≥ 104 | 104 (20 annually) | ≥40 | >40 |
ASA indicates American Society of Anesthesiologists; CT, computed tomography; ITT, intention-to-treat; mITT, modified intention-to-treat; NA, not applicable; PDAC, pancreatic ductal adenocarcinoma.
TABLE 2.
Study Population Characteristics
| References | Approach | Age (yr) | Men (%) | BMI (mean) | ASA 1 status (%) | ASA 2 status (%) | ASA 3 status (%) | Classic (%) | Pylorus preserving (%) | Vascular resection (%) | Soft pancreatic consistency (%) | Tumour size (mm) | Ampullary carcinoma (%) | Pancreatic carcinoma (%) | Biliary carcinoma (%) | Duodenal carcinoma (%) | IPMN (%) | Other (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Palanivelu et al(3) | LPD | 57.8 (mean) | 56 | 24.9 | 41 | 53 | 6 | 31 | 69 | 3 | Not assessed | 33 (mean) | 47 | 9 | 13 | 31 | 0 | 0 |
| Palanivelu et al(3) | OPD | 58.6 (mean) | 69 | 22.4 | 34 | 56 | 9 | 19 | 81 | 9 | Not assessed | 36 (mean) | 34 | 25 | 19 | 22 | 0 | 0 |
| Poves et al(26) | LPD | 69 (mean) | 41 | 24 | 3 | 56 | 41 | 19 | 78 | 13 | 66 | 24 (median) | 16 | 47 | 13 | 0 | 3 | 22 |
| Poves et al(26) | OPD | 70 (mean) | 69 | 26 | 3 | 45 | 52 | 38 | 62 | 14 | 41 | 29 (median) | 7 | 72 | 7 | 0 | 3 | 10 |
| van Hilst et al(29) | LPD | 67 (median) | 40 | 25 | 10 | 64 | 26 | 16 | 76 | 10 | 66 | 26 (mean) | 24 | 28 | 10 | 6 | 16 | 16 |
| van Hilst et al(29) | OPD | 66 (median) | 51 | 26 | 14 | 53 | 33 | 16 | 80 | 4 | 39 | 26 (mean) | 12 | 31 | 16 | 8 | 18 | 14 |
| Wang et al(27) | LPD | 61 (median) | 58 | 22.4 | 17 | 60 | 23 | 82 | 19 | 3 | 61 | 2.4 (mean) | 8 | 31 | 18 | 20 | 2 | 22 |
| Wang et al(27) | OPD | 60 (median) | 65 | 22.1 | 17 | 64 | 19 | 86 | 15 | 3 | 58 | 2.5 (mean) | 8 | 37 | 16 | 17 | 3 | 19 |
| Wang et al(28) | LPD | 61.9 (mean) | 61 | 22.9 | 32 | 41 | 27 | NA | NA | 0 | 44 | 2.9 | 0 | 97 | 0 | 0 | 0 | 3 |
| Wang et al(28) | OPD | 60.7 (mean) | 61 | 22.2 | 22 | 54 | 24 | NA | NA | 0 | 39 | 2.9 | 0 | 98 | 0 | 0 | 0 | 2 |
| Klotz et al(9) | RPD | 64.7 (mean) | 58.6 | 26.9 | 0 | 62.1 | 37.9 | 6.9 | 17.2 | 17.2 | 44.8 | NA | 6.3 | 75 | 6.3 | 0 | 0 | 6.3 |
| Klotz et al(9) | OPD | 62.6 (mean) | 48.5 | 26.6 | 0 | 60.6 | 39.4 | 0 | 54.5 | 15.2 | 36.4 | NA | 11.1 | 55.6 | 11.1 | 0 | 16.7 | 6.7 |
| Liu et al(11) | RPD | 62 (median) | 57 | 23.3 | 11 | 82 | 7 | NA | NA | NA | 53 | 25 (median) | 15 | 33 | 12 | 7 | 12 | 20 |
| Liu et al(11) | OPD | 60 (median) | 60 | 23.3 | 10 | 80 | 10 | NA | NA | NA | 58 | 27 (median) | 10 | 38 | 20 | 11 | 6 | 15 |
ASA indicates American Society of Anesthesiologists; BMI, body mass index; IPMN, intraductal papillary mucinous neoplasm; NA, not applicable.
Pairwise Meta-Analysis
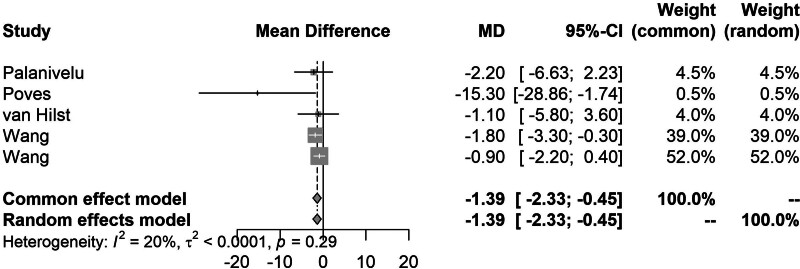
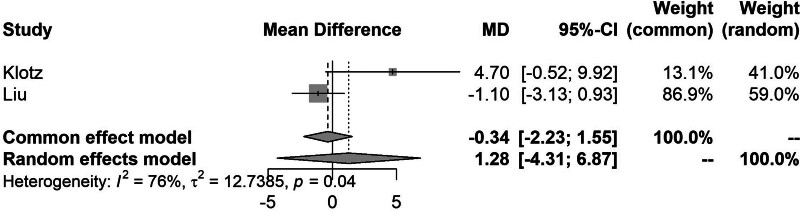
Pairwise meta-analysis of LPD to OPD demonstrated that LPD was significantly associated with a shorter LOS (MD, −1.39; 95% CI, − 2.33 to −0.45) (Fig. 2) and lower intraoperative blood loss compared with OPD (MD, −131; 95% CI, −146 to −117). However, LPD was associated with significantly longer operative duration (MD, 39.5; 95% CI, 34–45). Direct comparisons between RPD and OPD did not show any significant differences in any of the outcomes analysed (Fig. 3). The full results of the pairwise meta-analysis are presented in the Supplemental Appendix, http://links.lww.com/AOSO/A417.
FIGURE 2.
Forest plot showing the meta-analysis of LOS comparing LPD to OPD.
FIGURE 3.
Forest plot showing the meta-analysis of LOS comparing LPD to OPD.
Outcomes of the Network Meta-Analysis
Primary Outcome Measure
Length of Stay
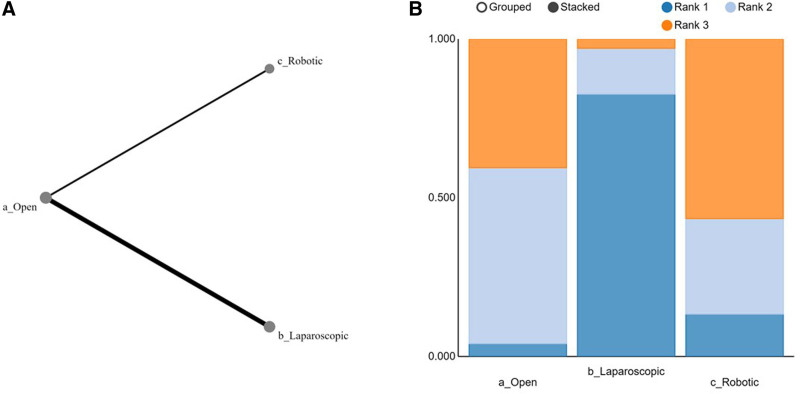
Table 2 summarised the results of the NMA from the direct comparisons of LOSs between the different surgical approaches. OPD compared with the LPD had the most direct comparisons (n = 5). Rankograms showed that LPD was associated with the shortest LOS, followed by RPD (Fig. 4). LPD, RPD, and OPD were ranked the best approach in 82%, 13%, and 5% of all comparisons, respectively.
FIGURE 4.
Network graph and Rankogram comparing LOS. A, Rankogram showing relative effectiveness of each intervention. B, Network graph showing direct comparisons. Line thickness corresponded with the number of studies assessing a particular direct comparison and the size of nodes correlated with the number of participants receiving a particular intervention.
Secondary Outcome Measures
Intraoperative Blood Loss
All 7 trials reported estimated intraoperative blood loss and made direct comparisons of the specific approaches. Open versus laparoscopic approach was the most frequent comparison. LPD was associated with a significant reduction in estimated intraoperative blood loss compared with OPD (MD [CRI]: −130 [−250 to −37]). LPD had the lowest estimated blood loss in 71.1% of all comparisons followed by RPD in 28.7% of all comparisons. Open surgery was associated with the lowest blood loss in 0.02% of all comparisons.
Duration of Surgery
All 7 trials made direct comparisons of the duration of surgery between either open and robotic or open and laparoscopic approaches. LPD was associated with significantly longer operating time than OPD (MD [CRI]: 67 [5.4–130]). Open surgery had the shortest operating time in 54% of all comparisons. This was followed by RPD and LPD in 45% and 1% of all comparisons, respectively.
Ninety-Day Mortality
The overall 90-day mortality reported across all 7 trials was 2.4% (30/1240). For patients undergoing OPD, the 90-day mortality was 2.2% (14/623). In the LPD and RPD groups, it was 2.9% (15/511) and 0.90% (1/110), respectively. In 86.6% of all comparisons, robotic-assisted PD was associated with the lowest rate of 90-day mortality. In comparison to this, laparoscopic and open approaches were associated with the lowest 90-day mortality rates in 5.4% and 8.1% of all comparisons.
Ninety-Day Readmission
Overall, 5.6% (35/623) of patients who had OPD were readmitted to the hospital in the 90 days following discharge after their index operation. The 90-day readmission rate was 5.3% (27/511) for the LPD group and 10% (11/110) for the RPD group. There were no significant differences in readmission rates between the different surgical approaches. Among all comparisons made, LPD was associated with the lowest rates of 90-day readmission in 40% of all comparisons, followed by RPD and OPD in 30% of all comparisons each.
Ninety-Day Reoperation
All 7 trials reported direct comparisons of 90-day reoperations between either RPD and OPD or between LPD and OPD. The rate of 90-day reoperation in patients undergoing OPD was 4.8% (30/623), 4.1% (21/511) in LPD, and 5.5% (8/110) in RPD. LPD was associated with the lowest rates of 90-day reoperation in 54.2% of all comparisons, this was followed by RPD in 31.7% and OPD in 14.1% of all comparisons.
Pancreas-Specific Postoperative Complications
Postoperative Pancreatic Fistula
Overall POPF rates were reported in all 7 trials. The overall incidence of clinically significant (grade B and C) POPF was 13.5% (84/623) in OPD; 11.1% (57/511) in LPD; and 20% in RPD (22/110). Open compared with the laparoscopic approach had the most number of direct comparisons. Rankograms showed that LPD was associated with the lowest rates of POPF in 78% of all comparisons; this was followed by 14% in OPD and then 8% in RPD.
Delayed Gastric Emptying
All 7 trials reported the incidence of DGE. Six trials only reported clinically relevant DGE and 1 trial reported all incidents of DGE without further stratification. The incidence of DGE was 13.8% (86/623) in OPD; 14% (72/511) in LPD, and 19.0% (21/110) in RPD. The most number of direct comparisons of postoperative DGE was made between open and laparoscopic surgical approaches. Rankogram showed that the laparoscopic approach was best in minimizing postoperative DGE in 58% of cases. Open surgery was best in 32% of cases and the robotic approach was the best technique only in 9% of cases.
Bile Leak
Five studies reported clinically relevant (grade B/C) bile leak, with an incidence of 5.6% (62/1116). One study did not specify the grade of the bile leak.3 Poves and colleagues reported biliary fistula without again specifying the grade of bile leak. The rate of bile leak was 5.5% (34/620) for OPD; for LPD it was 5.7% (29/511), and 7.2% (8/110) in RPD. As all previous outcomes, the most direct comparisons were between OPD and LPD. RPD had the lowest rates of bile leak in 40% of all comparisons. OPD and LPD had the lowest rates of bile leak in 30% of all comparisons made each.
Postpancreatectomy Haemorrhage
Direct comparisons of the rate of clinically relevant PPH between the OPD and LPD or OPD and robotic-assisted PD were reported in 6 trials. The overall rate of clinically relevant PPH postoperatively was 8.1% (39/479) in LPD; 10% (11/110) in RPD, and 9.0% (53/588) in OPD. The laparoscopic approach was associated with the lowest rate of clinically relevant PPH in 56% of all comparisons. Robotic-assisted and open approaches were associated with the lowest rate of clinically relevant postoperative PPH in 23% and 21% of all comparisons, respectively.
Oncological Outcomes
Number of Lymph Nodes Resected
The number of nodes resected in the operation was reported by all 7 studies, with 5 direct comparisons between LPD and OPD and 2 direct comparisons between OPD and RPD. In 50% of all comparisons, RPD was associated with the greatest number of lymph nodes resected. This was followed by LPD and OPD in 45% and 5% of all comparisons, respectively.
Trial Sequential Analysis
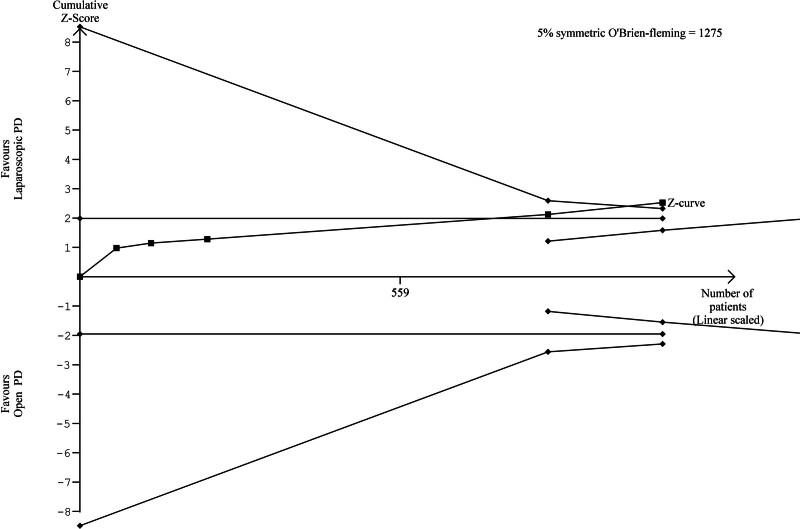
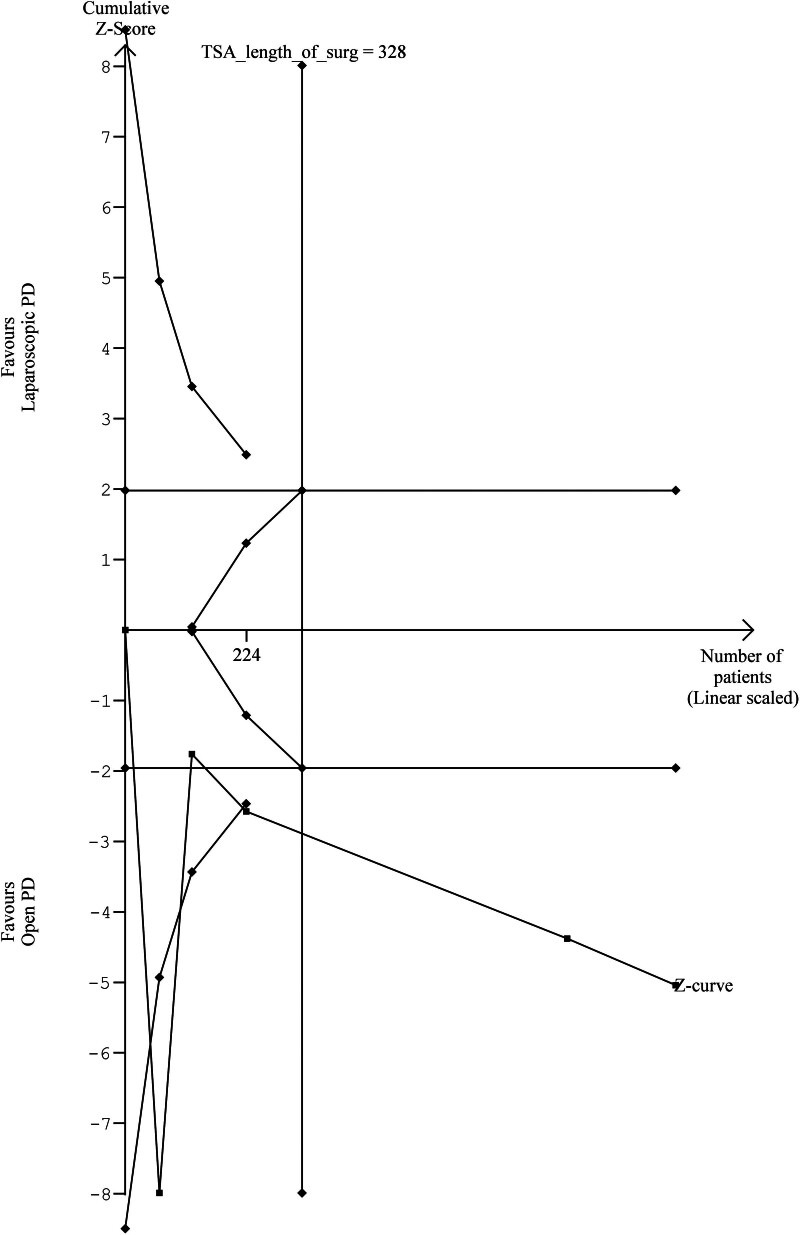
TSA of LOS demonstrated that the cumulative Z-curve crossed the trial sequential monitoring boundary, which suggested an advantage of LPD over OPD (Fig. 5). For length of surgery, the cumulative Z-score crossed the TSMB in favour of OPD compared with LPD (Fig. 6). The cumulative Z-score for 90-day mortality and POPF, did not cross the conventional boundary or the futility boundary which indicates insufficient evidence for the effect of LPD versus OPD. TSA of the remaining outcomes investigating the effectiveness of LPD and OPD was not statistically tenable due to limited information.
FIGURE 5.
TSA figure for LOS (LPD vs OPD).
FIGURE 6.
TSA figure for length of operation (LPD vs OPD).
For comparisons of RPD to OPD, the cumulative Z-score for 90-day mortality and the number of lymph nodes retrieved from the dissection did not cross the TSMB or futility boundaries, again suggesting insufficient evidence for the effect of RPD over OPD. TSA was not able to be performed for the remaining outcomes comparing RPD to OPD due to limited information (Supplemental Appendix, http://links.lww.com/AOSO/A417).
Transitivity Analysis
Definitions of outcomes analysed were largely similar, however, one study defined LOS as the total number of nights spent in the hospital following admission.29 All other trials defined it as the number of nights spent in the hospital after the index operation. All 7 studies used the ISGPS definition for pancreatic-specific complications (POPF, DGE, bile leak, PPH). All trials made admissions of technical differences in operative technique including: the decision to employ the pylorus-preserving technique; enteric anastomotic configuration; extent of lymph node dissection, and other techniques. This was described to be often left to the discretion of the primary surgeon. While there were no concomitant techniques described with any approach, Klotz and colleagues did report a higher proportion of patients undergoing OPD having pylorus preservation. Ages, gender mix, and other patient characteristics were generally comparable between direct comparisons. Most trials were conducted in Asia (60%) with the remaining trials being carried out in Europe (40%).
Risk of Bias
Risk of bias assessments are demonstrated in the Supplemental Appendix, http://links.lww.com/AOSO/A417. Only one study was determined to have a low overall risk of bias. Four (57.1%) trials had an overall high risk of bias, while a further 2 trials (28.6%) had some concerns. Most studies demonstrated clear and efficacious randomization, appropriate intention-to-treat analysis, and transparent outcome reporting.
Heterogeneity and Publication Bias Analysis
Some heterogeneity was found for the outcomes: postoperative LOS, and estimated intraoperative blood loss. Postoperative 90-day mortality, DGE, biliary leak, and the number of nodes resected. No significant heterogeneity was identified in the other outcome data. Heterogeneity analysis is summarised in the appendix. Comparison-adjusted funnel plots showed an even distribution of studies adjacent to the pooled estimate line for most outcomes (Supplemental Appendix, http://links.lww.com/AOSO/A417).
DISCUSSION
This NMA and trial sequential analysis presents the cumulative evidence from RCTs comparing the outcomes of open, laparoscopic, and robotic approaches for PD. Pairwise comparisons between OPD and LPD, as well as OPD and RPD, revealed significantly improved outcomes for LPD, while no significant differences were observed between OPD and RPD. However, few outcome differences reach statistical significance suggesting an underpowered evidence-base from current trials to make firm conclusions.30 The rank scores of the network analysis suggest that LPD is superior to the other approaches for many outcomes. Furthermore, it should be noted that the absolute differences in rates of postoperative outcomes were often minimal, making the ranks from the network analysis less informative. Trial sequential analysis confirms that while the LPD reduces hospital stay, it is associated with longer operative duration compared with OPD, inadequate data exists to determine differences in pancreas-specific complications, oncological outcomes, health-system outcomes, and patient-reported outcomes (PROs). Therefore, there is an ongoing need for more randomised evidence to define the value of minimally invasive pancreatic surgery platforms.
The findings of the present systematic review and meta-analysis validate 2 important conclusions made by previous meta-analyses and trials.2,31 The duration of surgery for OPD was significantly shorter compared with LPD, while LPD resulted in a reduced LOS. These consistent findings are further strengthened by the results of the TSA which shows that the collated trials to date have provided enough statistical evidence to make these conclusions valid. However, operative time can be influenced by factors such as advancements in technology, surgeon training, and evolving surgical paradigms, and therefore may remain a relevant clinical endpoint in future trials. This study did not confirm purported lower rates of DGE after either LPD or RPD when compared with OPD,32 as well as higher numbers of lymph nodes resected after RPD and LPD over OPD.31 Furthermore, when compared with earlier studies our results show outcomes after RPD appear less favourable against laparoscopic approaches and even OPD in some outcome parameters. This is likely due to the paucity of RCT-grade evidence available for the robotic approach and the obligatory learning curves which vary between centres. Moreover, the results of the 2 RCTs were divergent. The EUROPA trial showed a trend for poorer postoperative outcomes after RPD when compared with OPD.9 In comparison, Liu et al11 showed improved intraoperative blood loss and intraoperative transfusion rate for RPD over OPD. Notably, the conversion rate of RPD to OPD in the EUROPA trial was 23% compared with the 3% reported in the trial by Liu and colleagues. An explanation for the reported difference was that a threshold of 40 was used to determine proficiency in both EUROPA and the study by Liu and colleagues, yet 2 surgeons in the former trial did not meet this threshold. It would be reasonable to conclude that the quantity of evidence is therefore currently insufficient and likely to be disproportionately influenced by these potentially anomalous findings to confirm the efficacy of RPD.
Despite these contradictory findings robotic surgery still offers clear advantages to what are some of the intrinsic limitations of laparoscopy, including 3-dimensional visualization, better instrument dexterity, and ergonomics. However, its adoption in pancreatic surgery has not been as widespread due to the technical complexity of the procedure, relevant costs (not systematically assessed yet in literature), and the low caseload of PD themselves. It is, therefore, perhaps not surprising that the few comparative studies to date have shown conflicting clinical outcomes. One previous systematic review and meta-analysis identified RPD was associated with significantly lower rates of conversion to open surgery and transfusions. However, it was composed entirely of nonrandomised comparative studies.33 The current RCT evidence, indirectly compared in the NMA, is underpowered to conclusively determine the superiority of any single approach.
Research in pancreatic surgery demonstrates that concentrating procedures in high-volume centres among smaller numbers of surgeons with greater case experience results in improved patient outcomes.34,35 The definition of learning curves and its stages in pancreatic surgery is heterogeneous. The number of procedures required to surpass the learning curve for PD, based on a statistical calculation, varied: 30 for OPD (range: 20–50), 39 for LPD (range: 11–60), and 25 for RPD (range: 8–100).36 However, factors such as the number of previous different minimally invasive procedures performed as well as institutional case volume likely confound the learning curve.37 This creates additional heterogeneity between the studies in the current meta-analysis, even when the minimum number of cases required to be done were comparable between the trials. The interpretation of results is likely therefore influenced by different stages of operative technique mastery. In fact, a recent systematic review demonstrated variation in the evolution of both intraoperative and postoperative outcome parameters across the different phases of the learning curve.36 The first outcome parameters to show improvement were intraoperative variables such as LOS and operative time. Postoperative parameters such as complications decreased at a later stage of the learning curve. This idea, taken together with the fact the current RCT evidence is limited warrants further investigation.
The Idea, Development, Exploration, Assessment, and Long-term monitoring (IDEAL) framework is a step-by-step approach to the development and implementation of new surgical technologies.38 This was developed by an international interdisciplinary consensus process. In this framework, RCTs have a role in facilitating definitive conclusions regarding system efficacy to be made. The RCTs investigating both LPD and RPD in this systematic review were identified to have significant methodological bias. This was most commonly due to inappropriate outcome selection and measurement. The outcome measures are poorly defined or measured in most of the trials and often rely on variables subject to surgeon preference/discretion such as LOS. A core set of outcomes should be defined through international and interdisciplinary collaboration to best determine the clinical efficacy of minimally invasive PD. Furthermore, there has been no incorporation of PROs to assess both LPD and RPD. This will further aid in understanding of potential strengths and weaknesses of the different approaches. PROs are also an opportunity to determine the acceptability of different minimally invasive approaches to patients and will enhance patient participation in the implementation and innovation of minimally invasive surgery.39 In addition, new-generation outcomes and measures such as robotic kinematics, haptic sensors, and video data have yet to be reported in the current RCT literature.40 This data can be useful to assess and score proficiency of surgical technique which can then be investigated for association with clinical outcomes.
The present analysis has several limitations. To date, there has been an accumulation of only low quality and quantity of evidence for minimally invasive approaches for PD. This limitation reduces the statistical power and precludes making definitive conclusions of superiority or inferiority of any approach. The primary outcomes in the trials varied, including LOS, and postoperative inflammatory markers. This complicates comparisons because each trial is powered to detect differences in its designated primary outcome. Consequently, the statistical power and relevance of results are tailored to these unique endpoints, making direct comparisons challenging. Furthermore, there were no direct comparisons between LPD and RPD. The EUROPA trial was a phase 2b trial, which looked primarily at assessing the safety of the proposed intervention before conducting larger trials to assess efficacy. Notably, this was one of the 2 trials investigating RPD, reducing the ability of the current analysis to provide conclusive evidence. Three previous NMAs comparing open, laparoscopic, and robotic approaches have been performed to date.31,32,41 However, previous NMAs have primarily analysed nonrandomised observational studies and therefore were encumbered by significant methodological biases, particularly in NMA where the robustness of indirect comparisons relies on the unbiased estimates provided by existing direct comparisons in the absence of confounding. It is also important to acknowledge that following the results of the LEOPARD-2 trial, the use of robotic platforms for PD is increasing. Data from The Netherlands after the aftermath of the LEOPARD-2 trial with the implementation of a proctored RPD has shown safe implementation of the RPD program with comparable morbidity and mortality to OPD. It is therefore very likely future randomised trials will predominantly compare RPD with OPD to further confirm or refute the purported benefits of RPD shown in observational studies. A further limitation is that it is not possible to assess consistency in the NMA comparisons between LPD and RPD because only indirect comparisons are available.
Future trials should consider avoiding using postoperative LOS or operative duration as primary endpoints to measure the comparative value of RPD, LPD, or OPD and should instead focus on investigating oncological, PROs, pancreas-specific complications, costs, and more importantly should include surgeons that have surmounted the learning curve for minimally invasive approaches. Trials should also involve incorporating independent proctoring into the methodology to more accurately determine the superiority of any surgical approach. While this may reduce generalizability to smaller centres where the learning curve may not be surmounted, it ameliorates a major design flaw that reduces the comparability of different trials.
CONCLUSIONS
The current RCT evidence suggests potential better outcomes in LPD in comparison with RPD and OPD. However, few studies demonstrated robust statistical significance in outcome measures, suggesting an underpowered evidence base and possible selection bias. Hence, with current equivocal data, there is a need for ongoing RCTs to validate the role of minimally invasive approaches in PD.
ACKNOWLEDGMENTS
N.J.: formal analysis, software, methodology, writing—original draft, project administration. C.V.: formal analysis, software, methodology, writing—original draft. J.L.: data curation, methodology, writing—original draft. M.J.M.: visualization, writing—original draft. S.T.: visualization, software, writing—review and editing. G.M., K.S., M.A.-H., M.B., and S.W.: writing—review and editing. J.S.: writing—review and editing, conceptualization. S.P.: writing—original draft, conceptualization, supervision.
Supplementary Material
Footnotes
Disclosure: The authors declare that they have nothing to disclose. C.V. is supported by the HRC Clinical Research Training Fellowship and NZSG Janssen Fellowship. S.T. contributed to this work while funded by a MRC CRTF (MR/Y000676/1).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810–819. [DOI] [PubMed] [Google Scholar]
- 2.Sattari SA, Sattari AR, Makary MA, et al. Laparoscopic Versus Open Pancreatoduodenectomy in Patients With Periampullary Tumors: A Systematic Review and Meta-analysis. Ann Surg. 2023;277:742–755. [DOI] [PubMed] [Google Scholar]
- 3.Palanivelu C, Senthilnathan P, Sabnis SC, et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg. 2017;104:1443–1450. [DOI] [PubMed] [Google Scholar]
- 4.Beane JD, Zenati M, Hamad A, et al. Robotic pancreatoduodenectomy with vascular resection: Outcomes and learning curve. Surgery. 2019;166:8–14. [DOI] [PubMed] [Google Scholar]
- 5.Lai ECH, Yang GPC, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy--a comparative study. Int J Surg. 2012;10:475–479. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Chen JZ, Zhan Q, et al. Robot-assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid-term follow-up study. Surg Endosc. 2015;29:3698–3711. [DOI] [PubMed] [Google Scholar]
- 7.Stauffer JA, Coppola A, Villacreses D, et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: long-term results at a single institution. Surg Endosc. 2017;31:2233–2241. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe SM, Talamonti MS, Wang CE, et al. Early national experience with laparoscopic pancreaticoduodenectomy for ductal adenocarcinoma: a comparison of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy from the National Cancer Data Base. J Am Coll Surg. 2015;221:175–184. [DOI] [PubMed] [Google Scholar]
- 9.Klotz R, Mihaljevic AL, Kulu Y, et al. Robotic versus open partial pancreatoduodenectomy (EUROPA): a randomised controlled stage 2b trial. Lancet Reg Health Eur. 2024;39:100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickel F, Haney CM, Kowalewski KF, et al. Laparoscopic Versus Open Pancreaticoduodenectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann Surg. 2020;271:54–66. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Li M, Gao Y, et al. Effect of robotic versus open pancreaticoduodenectomy on postoperative length of hospital stay and complications for pancreatic head or periampullary tumours: a multicentre, open-label randomised controlled trial. Lancet Gastroenterol Hepatol. 2024;9:428–437. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761–768. [DOI] [PubMed] [Google Scholar]
- 14.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. [DOI] [PubMed] [Google Scholar]
- 15.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. [DOI] [PubMed] [Google Scholar]
- 16.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584–591. [DOI] [PubMed] [Google Scholar]
- 17.Abu Hilal M, van Ramshorst TME, Boggi U, et al. ; Collaborators. The Brescia Internationally Validated European Guidelines on Minimally Invasive Pancreatic Surgery (EGUMIPS). Ann Surg. 2024;279:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 [cited 2022 Sep 24]. Available at: https://www.bmj.com/content/366/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–1805. [DOI] [PubMed] [Google Scholar]
- 21.Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta-analysis. Control Clin Trials. 1997;18:580–93; discussion 661. [DOI] [PubMed] [Google Scholar]
- 22.Thorlund K, Engstrøm J, Wetterslev J, et al. Copenhagen Trial Unit. 2017. [cited 2024]. User Manual for Trial Sequential Analysis (TSA) [pdf]. Available at: https://ctu.dk/tsa/learn-more/. Accessed June 2024. [Google Scholar]
- 23.gemtc.drugis.org [Internet]. [cited 2024 Jun 12]. Bayesian evidence synthesis. Available at: https://gemtc.drugis.org/#!/analyses [Google Scholar]
- 24.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. [DOI] [PubMed] [Google Scholar]
- 25.de Keijser J van. VGBSEORDSC. gemtc.drugis.org. 2016. Available at: https://gemtc.drugis.org/signin.html#!/analyses. Accessed XXX [Google Scholar]
- 26.Poves I, Burdío F, Morató O, et al. Comparison of Perioperative Outcomes Between Laparoscopic and Open Approach for Pancreatoduodenectomy: The PADULAP Randomized Controlled Trial. Ann Surg. 2018;268:731–739. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Li D, Chen R, et al. ; Minimally Invasive Treatment Group in the Pancreatic Disease Branch of China's International Exchange and Promotion Association for Medicine and Healthcare (MITG-P-CPAM). Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:438–447. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Pan S, Qin T, et al. Short-Term Outcomes Following Laparoscopic vs Open Pancreaticoduodenectomy in Patients With Pancreatic Ductal Adenocarcinoma: A Randomized Clinical Trial. JAMA Surg. 2023;158:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Hilst J, de Rooij T, Bosscha K, et al. ; Dutch Pancreatic Cancer Group. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. 2019;4:199–207. [DOI] [PubMed] [Google Scholar]
- 30.Ricci C, Stocco A, Ingaldi C, et al. Trial sequential meta-analysis of laparoscopic versus open pancreaticoduodenectomy: is it the time to stop the randomization? Surg Endosc. 2023;37:1878–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamarajah SK, Bundred JR, Marc OS, et al. A systematic review and network meta-analysis of different surgical approaches for pancreaticoduodenectomy. HPB. 2020;22:329–339. [DOI] [PubMed] [Google Scholar]
- 32.Kabir T, Tan HL, Syn NL, et al. Outcomes of laparoscopic, robotic, and open pancreatoduodenectomy: A network meta-analysis of randomized controlled trials and propensity-score matched studies. Surgery. 2022;171:476–489. [DOI] [PubMed] [Google Scholar]
- 33.Kamarajah SK, Bundred J, Marc OS, et al. Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:6–14. [DOI] [PubMed] [Google Scholar]
- 34.Krautz C, Nimptsch U, Weber GF, et al. Effect of Hospital Volume on In-hospital Morbidity and Mortality Following Pancreatic Surgery in Germany. Ann Surg. 2018;267:411–417. [DOI] [PubMed] [Google Scholar]
- 35.van Heek NT, Kuhlmann KFD, Scholten RJ, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–8, discussion 788–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller PC, Kuemmerli C, Cizmic A, et al. Learning Curves in Open, Laparoscopic, and Robotic Pancreatic Surgery: A Systematic Review and Proposal of a Standardization. Ann Surg Open. 2022;3:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shyr BU, Chen SC, Shyr YM, et al. Learning curves for robotic pancreatic surgery-from distal pancreatectomy to pancreaticoduodenectomy. Medicine (Baltimore). 2018;97:e13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcus HJ, Ramirez PT, Khan DZ, et al. ; IDEAL Robotics Colloquium. The IDEAL framework for surgical robotics: development, comparative evaluation and long-term monitoring. Nat Med. 2024;30:61–75. [DOI] [PubMed] [Google Scholar]
- 39.Snyder CF, Jensen RE, Segal JB, et al. Patient-reported outcomes (PROs): putting the patient perspective in patient-centered outcomes research. Med Care. 2013;51(8 Suppl 3):S73–S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guni A, Varma P, Zhang J, et al. Artificial Intelligence in Surgery: The Future is Now. Eur Surg Res. 2024;11. doi: 10.1159/000536393. [DOI] [PubMed] [Google Scholar]
- 41.Aiolfi A, Lombardo F, Bonitta G, et al. Systematic review and updated network meta-analysis comparing open, laparoscopic, and robotic pancreaticoduodenectomy. Updates Surg. 2021;73:909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]