Summary:
Hard-to-heal wounds represent a global and growing medical and economic burden. Skin autografting is a useful treatment option but is often limited by donor site morbidity, logistical considerations, and grafting success in compromised wound beds. Combining autologous skin cell suspension (ASCS) technology with minced dermal grafts can allow for dermal elements and epithelial healing as well as closed donor sites. This study explores the combination of minced autografting with ASCS in healing recalcitrant wounds. Two patients with diabetic foot ulcers (DFUs) whose previous skin grafting had failed were included. Under local anesthesia, donor skin was harvested as a full-thickness ellipse and divided into a superficial split-thickness graft (STSG) and a dermal autograft. ASCS was prepared from the STSG, and the dermal component was finely minced using a scalpel. Prepared wound beds were directly dressed with Telfa clear and compression dressings. Patients included a 62-year-old man with a DFU (15 cm2) on the left plantar heel present for 2 years, and a 53-year-old man with a DFU (10 cm2) on the plantar surface of the first metatarsal head present for 2 years. After combination treatment, complete closure was achieved by days 27 and 24, respectively. There was evidence of continued remodeling and skin thickening for the following 4 months. Combining dermal mincing with ASCS promotes healing of both dermal and epidermal layers while enabling primary closure of donor sites. These initial cases are encouraging, and ongoing studies are validating outcomes in more patients with various hard-to heal wounds.
Hard-to-heal wounds are a major healthcare challenge.1,2 This burden is expected to increase alongside the rise in obesity, diabetes, and the aging population.2 Rapid wound closure minimizes morbidity, but hard-to-heal wounds often require additional reconstructive techniques (standard-of-care treatment with debridement, advanced dressings, and topical antimicrobials are typically insufficient).3 Autologous split-thickness skin grafts (STSGs) have traditionally been recommended, but in some circumstances can suffer high rates of graft failure, donor site morbidity, and technological/logistical challenges.3
The autologous skin cell suspension (ASCS) technology is a new addition to the reconstructive toolkit and involves disaggregation of a donor STSG into its constituent regenerative cells by enzymatic digestion which are then distributed to a wound bed as a spray. The technology reduces donor tissue requirements4 and facilitates re-epithelialization, likely mediated by the regenerative basal keratinocytes that undergo loss of contact inhibition and thereby become “activated” during skin disaggregation.5 Restoration of dermal elements and tissue volume with ASCS, however, is limited. Pixel grafting is another donor-sparing autografting technique, where harvested skin grafts are minced into morsels (~300–600 µm) and applied to a woundbed.6 This technique enhances healing and granulation tissue formation, but re-epithelialization occurs more slowly.7
To maximize the strengths of these different autografting expansion techniques, we co-applied ASCS of the superficial portion, along with mincing of the dermal portion, of a full-thickness skin graft. We explore the potential of this combination treatment in healing diabetic foot ulcers (DFUs), among the most challenging recalcitrant wounds, in 2 complex patients.
METHODS
Two patients with long-duration nonhealing DFUs whose previous skin grafting had failed were included. Procedures took place in the office setting with local anesthesia. Donor skin was harvested as both a split-thickness ellipse and dermal graft from the same area using a #15 scalpel. The superficial STSG was processed using the RECELL System (AVITA Medical, Valencia, CA), as per manufacturer instructions. The dermis graft was finely minced with the scalpel. The donor site was closed primarily. (See Video [online], which outlines the steps taken for the combination autografting procedure in patient 1: [1] preparation of the treatment site; [2] preparation of the donor site with planning of the elliptical incision and injecting local anesthesia; [3] harvest of the superficial STSG using a #15 scalpel; [4] preparation of the ASCS using the RECELL System; [5] harvest of the dermal graft by using the #15 scalpel to complete the elliptical incision through the full-thickness of the dermis; [6] closure of the donor site primarily and mincing of the dermal graft [sharply, using scissors and the #15 scalpel]; [7] sharp debridement of the wound bed to create pinpoint bleeding; [8] grafting of the minced dermis; [9] application of the ASCS; and [10] dressing of the treatment site.)
Video 1. outlines the steps taken for the combination autografting procedure in patient 1: [1] preparation of the treatment site; [2] preparation of the donor site with planning of the elliptical incision and injecting local anesthesia; [3] harvest of the superficial STSG using a #15 scalpel; [4] preparation of the ASCS using the RECELL System; [5] harvest of the dermal graft by using the #15 scalpel to complete the elliptical incision through the full-thickness of the dermis; [6] closure of the donor site primarily and mincing of the dermal graft [sharply, using scissors and the #15 scalpel]; [7] sharp debridement of the wound bed to create pinpoint bleeding; [8] grafting of the minced dermis; [9] application of the ASCS; and [10] dressing of the treatment site.
Wound beds and edges were sharply debrided. The freshly minced autologous dermal grafts were applied to the wound, followed by the ASCS as an aspirated solution via syringe. Treatment sites were dressed with Telfa clear, followed by Kerlix, gauze, and foam dressings. Patients received perioperative oral antibiotics and were instructed to avoid weight-bearing until closure. Weekly clinic visits continued until durable re-epithelialization was achieved. Wound closure (100% re-epithelialization) was confirmed on clinical assessment, defined as no drainage and no need for further dressing changes.
RESULTS
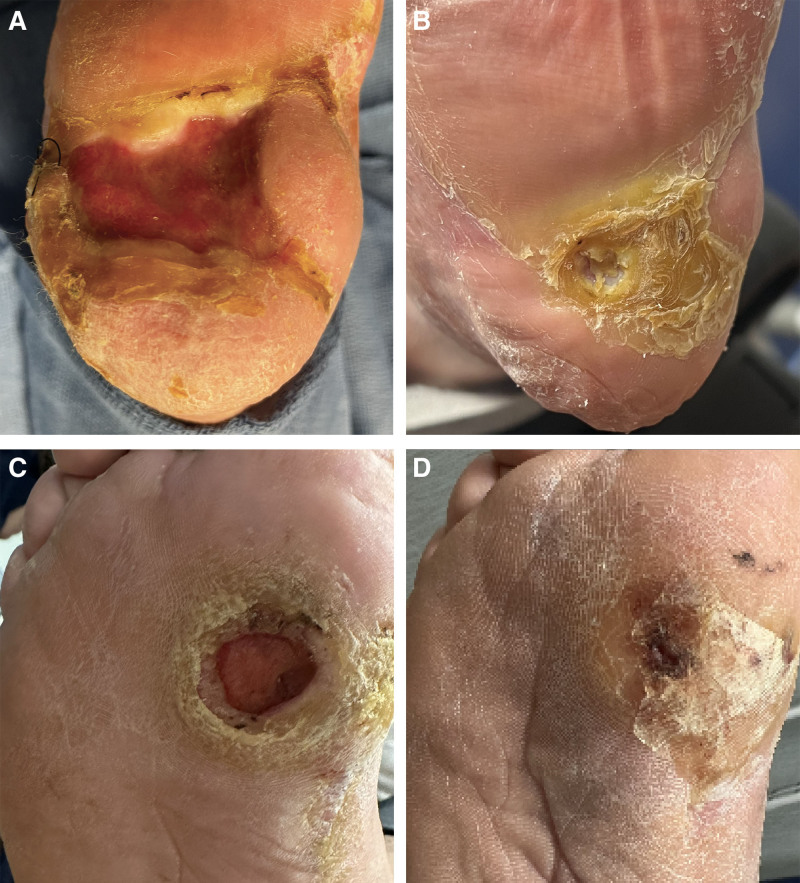
Patient 1 was a 56-year-old man with peripheral vascular disease and type II diabetes and a DFU (15 cm2) present for more than 5 years on the left plantar heel who had 2 failed prior STSGs and 2 rotation flaps, alongside more than 6 months off-loading and advanced dressings. Post procedure, there was some loss of the dermal autograft component, but complete wound closure was achieved by day 27. The neoepidermis continued to thicken over the following 4 months with development of hyperkeratotic glabrous plantar skin (Fig. 1). (See figure, Supplemental Digital Content 1, which displays the diabetic foot ulcer [DFU, 15 cm2] on the left plantar heel of patient 1, a 62-year-old man, shown 14 days after autografting [A] and once complete re-epithelialization was noted on postoperative day 27 [B]. The DFU [10 cm2] on the plantar surface of the first metatarsal head of patient 2, a 53-year-old man, shown 11 days after autografting where a 50% reduction in wound size was apparent [C], and on postoperative day 24 once complete re-epithelialized was achieved [D], http://links.lww.com/PRSGO/D681.)
Fig. 1.
The DFU (15 cm2) on the left plantar heel of patient 1, a 62-year-old man, before autografting (A) and at final follow-up (B). Complete re-epithelialization was noted by postoperative day 27 with continued thickening and formation of the plantar glabrous epidermis up until final follow-up on postoperative day 130 (4 months and 8 days). B, The DFU (10 cm2) on the plantar surface of the first metatarsal head of patient 2, a 53-year-old man, shown from before autografting (C) and at final follow-up (D). After combination autografting, the wound re-epithelialized by postoperative day 24, and continued to contract and thicken up until final follow-up on postoperative day 100 (3 months and 8.5 days).
Patient 2 was a 56-year-old man with type II diabetes who had a DFU (5 cm2) present for 6 years on the medial plantar surface of the first metatarsal head which had failed to heal with pressure off-loading and dressings for 6 months. Post procedure, the ulcer achieved 50% closure by day 11. Reopening was observed on day 40 due to nonadherence with pressure off-loading, but complete closure was achieved by day 100 (Fig. 1; Supplemental Digital Content 1, http://links.lww.com/PRSGO/D681). The wound also developed hyperkeratosis, but no further wound breakdown occurred despite full weight-bearing for 2 months.
DISCUSSION
Hard-to-heal wounds are a growing global public health challenge.2 Autografting is a standard treatment option to reconstruct chronic wounds that are not amenable to primary or secondary closure and have failed nonsurgical options.3 However, STSGs are often associated with high failure rates and donor site morbidity in certain situations.8 ASCS technology has been shown to augment the healing properties of other types of skin grafts. To address the limitations of skin grafts in difficult wounds, we describe the novel co-application of 2 autografting techniques: dermal mincing and ASCS. We demonstrate that the 2 techniques used in combination are effective in healing 2 DFUs where previous STSGs have failed. Complete re-epithelialization was achieved by 1 month and continued remodeling, and epidermal thickening occurred throughout the 4-month follow-up, producing thickened skin able to resist ambulatory pressure.
These autografting techniques appeared to offer advantages in combination. Treated wounds exhibited both re-epithelialization and formed dermal tissue formation, circumventing the limitations of ASCS alone in restoring tissue volume and dermal elements,5 and the slower epithelialization seen with dermal mincing.7 On gross inspection, wounds also appeared to undergo contraction, suggesting an effect of paracrine signaling. There were also benefits noted at the donor site. Although both autografting techniques are donor-sparing compared with standard STSG, the combination treatment allowed for donor sites to be closed primarily, reducing donor site morbidity.3 Finally, the simple and minimally invasive nature of the combination treatment makes it suitable for administration under local anesthesia, which has medicoeconomic advantages for patients, providers, and institutions.
The conclusions of this work are limited to 2 individual case reports, and ongoing work is being conducted to validate outcomes with more patients with a diversity of chronic wounds. Longer follow-up is crucial to assess durability of closure over time. The healing course of the second patient also highlights the importance of postoperative wound care, particularly off-loading for wounds on pressure-bearing areas such as the plantar surface.
CONCLUSIONS
There is a clear and paramount need to develop better strategies for managing difficult-to-treat wounds. In our limited study, combining dermal mincing with ASCS promotes healing of both dermal and epidermal layers and enables primary closure donor sites. Initial results are promising, and ongoing studies are being undertaken to confirm in additional patients and hard-to-heal wounds.
DISCLOSURES
Dr. Borrelli assisted with the writing of this article and is employed by AVITA Medical as a Medical Science Liaison. The other authors have no financial interest to declare in relation to the content of this article. The 2 RECELL Autologous Cell Harvesting Devices used in this study were donated for trial purposes.
Supplementary Material
Footnotes
Published online 20 December 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen. 2000;8:347–352. [PubMed] [Google Scholar]
- 2.Sun H, Pulakat L, Anderson DW. Challenges and new therapeutic approaches in the management of chronic wounds. Curr Drug Targets. 2020;21:1264–1275. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson E, Liu PY, Schultz GS, et al. Chronic wounds: treatment consensus. Wound Repair Regen. 2022;30:156–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood FM, Giles N, Stevenson A, et al. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell® kit. Burns. 2012;38:44–51. [DOI] [PubMed] [Google Scholar]
- 5.Roshan A, Murai K, Fowler J, et al. Human keratinocytes have two interconvertible modes of proliferation. Nat Cell Biol. 2016;18:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuutila K, Varon DE, Broomhead M, et al. 19 minced skin grafts can be expanded up to 500 times to re-epithelialize full-thickness burns. J Burn Care Res. 2022;43:S16. [DOI] [PubMed] [Google Scholar]
- 7.Boggio P, Tiberio R, Gattoni M, et al. Is there an easier way to autograft skin in chronic leg ulcers? “Minced micrografts”, a new technique. J Eur Acad Dermatol Venereol. 2008;22:1168–1172. [DOI] [PubMed] [Google Scholar]
- 8.Brusselaers N, Pirayesh A, Hoeksema H, et al. Skin replacement in burn wounds. J Trauma. 2010;68:490–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.