Abstract
BACKGROUND:
A proposed new global definition of ARDS seeks to update the Berlin definition and account for nonintubated ARDS and ARDS diagnoses in resource-variable settings.
RESEARCH QUESTION:
How do ARDS epidemiologic characteristics change with operationalizing the new global definition of ARDS in a resource-limited setting?
STUDY DESIGN AND METHODS:
We performed a real-use retrospective cohort study among adult patients meeting criteria for the Berlin definition of ARDS or the global definition of ARDS at ICU admission in two public hospitals in the KwaZulu-Natal Department of Health, South Africa, from January 2017 through June 2022.
RESULTS:
Among 5,760 adults (aged ≥ 18 years) admitted to the ICU, 2,027 patients (35.2%) met at least one ARDS definition, including 1,218 patients meeting the Berlin definition of ARDS (60.1% of all ARDS diagnoses) and 809 new diagnoses of the global definition of ARDS that were not captured by the Berlin definition alone (39.9% of all ARDS diagnoses and 14.0% of all ICU admissions). After adjustment for hospital-level factors, patients who met only the global definition of ARDS criteria (ie, who would not have been captured by the Berlin definition) showed no statistically significant ICU mortality difference vs patients with ARDS according to the Berlin definition (21.7% [95% CI, 18.9%−24.4%] vs 23.8% [95% CI, 21.5%−26.2%]; OR, 0.88 [95% CI, 0.70–1.10]; P = .25). In prespecified exploratory subgroup analyses, patients without COVID-19 who met only the criteria for the global definition of ARDS showed reduced ICU mortality (14.2% [95% CI, 11.6%−16.9%] vs 22.2% [95% CI, 19.8%−24.6%]; OR, 0.58 [95% CI, 0.45–0.75]; P < .0005) compared with patients without COVID-19 who met the Berlin definition for ARDS.
INTERPRETATION:
The new global definition of ARDS captures a significant proportion of patients who would not have been included by the Berlin definition alone. These additional patients with ARDS may have heterogenous patterns of outcomes among diagnostic subgroups, including by COVID-19 status, compared with patients with ARDS according to the Berlin definition.
Keywords: ARDS, COVID-19, global health, low-income and middle-income countries, resource-limited settings
The Berlin definition has governed ARDS since 2012.1 A diagnosis of ARDS under this definition required < 1 week since an acute etiologic insult or new or worsening symptoms, bilateral opacities on chest imaging not fully explained by other causes, respiratory failure not fully explained by cardiac failure or fluid overload, and a Pao2 to Fio2 ratio of ≤ 300 mm Hg. In a strict application of this definition, an ARDS diagnosis necessarily required an arterial blood gas measurement (for Pao2) and treatment with mechanical ventilation (aside from a small carveout for the patients with the lowest severity of disease).
Two important motivating critiques to the Berlin definition have emerged. First, with a large burden of disease now recognized in resource-limited and resource-variable settings, the requirement of access to arterial blood gas measurements and mechanical ventilation risked excluding a large group of patients with physiologic ARDS, but without access to the requisite laboratory or respiratory support capabilities.2–7 Second, the Berlin definition, strictly applied, potentially would miss a large population of patients across resource levels treated intentionally with noninvasive respiratory support methods—such as noninvasive ventilation (NIV) or high-flow nasal oxygen (HFNO)—but with otherwise compatible physiologic features.7–9 This later critique was heightened during the COVID-19 pandemic, when large numbers of patients were managed noninvasively either in the context of resource limitations or as part of evolving critical care management strategies.10–12
These critiques motivated the proposal of a new global definition of ARDS7,8,13–15 that would allow for a range of respiratory support strategies and cohort entry by either Pao2 to Fio2 ratio or more accessible peripheral oxygen saturation (Spo2) to Fio2 ratio cutoffs. Although this proposed update promises to bring into the ARDS fold patients with compatible physiologic features managed noninvasively or without blood gas measurements, it also potentially risks jeopardizing longitudinal ARDS epidemiologic evaluations and further exacerbating the known heterogeneity of the ARDS syndrome diagnosis.7,9,16 Particular concerns exist that patients with lower acuity of disease and superior outcomes would be added disproportionately to the ranks of patients with ARDS7,12,17,18 and that use of Spo2 would introduce bias related to skin tone disparities.7,19,20
As part of the South Africa Intensive Care Unit Capacity Strain Study Group, we performed a retrospective cohort study to examine operationalizing the new global definition of ARDS and to analyze the resultant impact on ARDS epidemiology in a resource-limited setting. We hypothesized that patients in a global definition-only ARDS cohort (ie, newly added patients with ARDS according to the global definition who would not have been included by the Berlin definition alone) would demonstrate lower ICU mortality compared with patients meeting the Berlin definition for ARDS.
Study Design and Methods
Study Setting and Data Source
The study data source was the Integrated Critical Care Electronic Database,21 which has been the source for multiple prior publications from the South Africa Intensive Care Unit Capacity Strain Study Group.22–26 The ICU database includes all referrals and admissions for ICU care at two public hospitals within the KwaZulu-Natal Department of Health. Hospital and ICU organizational characteristics and longitudinal capacity strain at these facilities have been described previously.22,26 Briefly, ICU capabilities include nine ICU beds among 530 hospital beds (1.7%) and 11 ICU beds among 900 hospital beds (1.2%) with a pooled median ICU occupancy of 76.4% before the pandemic and 100% during the pandemic26 and approximately 50% of ICU referrals declined for ICU admission.22
The study protocol was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (“Class Approval for a Critical Care Database”; October 21, 2019; protocol no. BCA211/14; Durban, South Africa), Harry Gwala Regional Hospital (formerly Edendale Hospital; “Characteristics and Outcomes of Patients Admitted With COVID-19 to a South African ICU”; March 16, 2022; Pietermaritzburg, South Africa), and Greys Hospital (“Characteristics and Outcomes of Patients Admitted With COVID-19 to South African Regional and Tertiary ICUs”; November 25, 2020; protocol no. 00002156; Pietermaritzburg, South Africa), and by the institutional review board of the University of Pennsylvania (“Association of ICU Capacity Strain and Mortality in a Resource-Limited Setting”; July 29,2020; protocol no. 824688; Philadelphia, PA). The procedures followed were in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for reporting observational studies.27
Study Population
The study included all adult patients (aged ≥ 18 years) meeting at least one ARDS definition at the time of ICU admission at the study hospitals from January 1, 2017, through June 30, 2022, including approximately 3 years before the pandemic and 2.5 years during the COVID-19 pandemic. Various subgroups of these patients have been described and studied previously.23–26
ARDS Cohort Definitions
ARDS was defined as meeting the Berlin definition of ARDS or the global definition of ARDS as assessed at the time of ICU referral and admission.1,13 The Berlin definition of ARDS was defined by a nonmissing Pao2 to Fio2 ratio of ≤ 300 mm Hg and the use of invasive mechanical ventilation, with subcategorizations of mild (200 mm Hg < Pao2 to Fio2 ratio ≤ 300 mm Hg), moderate (100 mm Hg < Pao2 to Fio2 ratio ≤ 200 mm Hg), and severe (Pao2 to Fio2 ratio ≤ 100 mm Hg).1 The global definition of ARDS was defined by a nonmissing Pao2 to Fio2 ratio of ≤ 300 mm Hg or Spo2 to Fio2 ratio of ≤ 315 (if Spo2 ≤ 97%) without regard to respiratory support, with subcategorizations of mild (200 < Pao2 to Fio2 ratio ≤ 300 or 235 < Spo2 to Fio2 ratio ≤ 315), moderate (100 < Pao2 to Fio2 ratio ≤ 200 or 148 < Spo2 to Fio2 ratio ≤ 235), and severe (Pao2 to Fio2 ratio ≤ 100 or Spo2 to Fio2 ratio ≤ 148; if discrepancies in severity levels resulted from Pao2 to Fio2 ratio vs Spo2 to Fio2 ratio differences, patients were categorized by the higher severity level).3,13,15 e-Appendix 1 includes details on the global definition of ARDS subcohorts: intubated patients with ARDS, nonintubated patients with ARDS, and patients with ARDS using a modified definition for resource-limited settings. For our primary analytic comparison with the Berlin definition of ARDS cohort, we defined a global definition-only ARDS cohort as patients meeting the global definition of ARDS criteria and specifically not meeting Berlin definition criteria (ie, newly added patients with ARDS according to the global definition who would not have been included by the Berlin definition alone).
In all cases, ARDS diagnoses were made based on data at the time of ICU referral and admission and required a hospital length of stay before ICU admission of < 7 days (to approximate an acute onset or worsening of hypoxemia within 1 week of a predisposing risk factor or trigger) and the absence of an acute cardiac disease diagnosis at the time of ICU admission, as assessed and recorded in the database in real time by the admitting ICU team (to exclude patients with cardiogenic pulmonary edema as a primary driver of pulmonary opacities and hypoxemia). These exclusion criteria were interrogated in sensitivity analyses. Because of local resource limitations (ie, lack of digital radiographic records and primary team clinician-interpreted images without standardized radiology reports), chest imaging results were not available feasibly for adjudication (< 10% documentation in a highly ARDS-enriched subsample) (e-Appendix 2). Therefore, we operationalized the ARDS cohort definitions described herein without chest imaging criteria.
Exposure Variables, Outcomes, and Modeling Strategies
We performed a retrospective cohort study comparing the global definition-only ARDS cohort (ie, newly added patients with ARDS according to the global definition who would not have been included by the Berlin definition alone) with the comparator group of patients meeting the Berlin definition of ARDS. We measured descriptive statistics of the ARDS cohorts including demographics, ICU referral and admission details, acute diagnosis and physiologic features, and chronic comorbidities; descriptive univariate relationships were analyzed using logistic and linear regression and χ2 tests where appropriate. The primary analysis measured the association between meeting global definition-only ARDS criteria as compared with meeting the Berlin definition of ARDS with a primary outcome of ICU mortality, defined as a death in the ICU or a palliative discharge from the ICU, modeled with multivariable logistic regression.26 Models were adjusted for hospital-level factors including facility, the peripandemic period, and five capacity strain metrics (ICU occupancy, ICU referral burden, ICU turnover, ICU acuity, and national 7-day rolling mean of incident SARS-CoV-2 cases per 1 million residents), all as previously defined and studied.22,26 Because the study’s objective was to describe the clinically visible epidemiologic characteristics of the new global definition of ARDS in real use, we a priori intentionally did not adjust for patient-level characteristics such as acute physiologic features and comorbidities, so as not to adjust away important potential differences between the cohorts or to create only theoretical analytic cohorts of similar patients except for meeting different cohort definitions.
Missing Data
Respiratory support and supplemental oxygen data were not recorded in 12.9% of ICU admissions. Because the global definition resource-limited settings modification ARDS subcohort (e-Appendix 1) is agnostic to respiratory support, these patients would not be excluded from an ARDS diagnosis, but might be misclassified in the resource-limited settings modification subcohort, rather than in an intubated or nonintubated (ie, NIV or HFNO) subcohort. For oxygenation variables, Pao2 was not recorded in 10.3% of ICU admissions, Spo2 was not recorded in 6.7% of ICU admissions, and Fio2 was not recorded in 12.1% of ICU admissions; lack of a useable Pao2 to Fio2 ratio or Spo2 to Fio2 ratio was 16.3% (after applying the Spo2 ≤ 97% criteria). Nonrecording could be a combination of lack of measurement in real clinical care (eg, no blood gas drawn) or true data missingness, and we would expect nonrecorded respiratory variables to skew toward lower degrees of acuity. Outcome and adjustment variables were missing < 1%. Because of the study goal to evaluate real use in a resource-limited setting where data missingness is a reality, we did not impute missing Pao2 to Fio2 ratio or Spo2 to Fio2 ratio values in the primary analysis. In a secondary imputation analysis to evaluate the potential range of impact of this missingness, we repeated the primary analysis assuming that the 16.3% of patients missing both Pao2 to Fio2 ratio and Spo2 to Fio2 ratio, who therefore were excluded from an ARDS diagnosis in the primary analysis, instead had values meeting oxygenation criteria for ARDS.
Subgroup Sensitivity Analyses
Prespecified exploratory subgroups, analyzed again with the same modeling, adjustment strategy, and outcome, included: COVID-19 status, before pandemic vs pandemic periods, ICU admitting diagnoses and other acute diagnoses present at ICU admission (eg, trauma, infection or sepsis, and nontrauma and noninfection), medical vs surgical patients, and HIV status. For subgroups analyses, we continued to compare the global definition of ARDS only vs the Berlin definition of ARDS within subgroups; we did not compare across subgroups. To assess the impact of excluding patients with a hospital length of stay before ICU admission of ≥ 7 days and an acute cardiac disease diagnosis at the time of ICU admission, we repeated our primary analyses removing these exclusion criteria.
Oxygenation Severity Secondary Analyses
In secondary analyses, we evaluated the prognostic usefulness of oxygenation severity categories (ie, mild, moderate, and severe) across ARDS cohorts using the same modeling, adjustment strategy, and outcome as above. To interrogate the role of the COVID-19 population in contributing to emerging severity-mortality patterns, in a post hoc analysis, we stratified the above oxygenation severity analyses by COVID-19 status.
Results
ARDS Cohort Patient Characteristics and Outcomes
Five thousand seven hundred sixty adults (aged ≥ 18 years) were admitted to the study hospital ICUs from January 1, 2017, through June 30, 2022. Two thousand twenty-seven patients (35.2%) met the criteria for at least one definition for ARDS: 1,218 patients met the Berlin definition of ARDS (60.1% of all ARDS diagnoses and 21.1% of all ICU admissions) and 809 patients met only the global definition of ARDS (ie, new diagnoses of the global definition of ARDS that were not captured by the Berlin definition alone; 39.9% of all ARDS diagnoses and 14.0% of all ICU admissions). In univariate comparisons with the Berlin definition of ARDS cohort (descriptive threshold, P < .05), patients in the global definition of ARDS only cohort were older, were more often female, were less often Black, were less likely to have trauma and more likely to have infection as the primary admitting diagnosis, were more often had COVID-19, showed higher Pao2 to Fio2 and Spo2 to Fio2 ratios, showed different distributions of disease severity by ARDS oxygenation severity and Quick Sequential Sepsis Organ Failure Assessment score, and showed higher rates of chronic cardiovascular disease and diabetes (Table 1, e-Tables 1–2). The global definition-only ARDS cohort received heterogenous oxygen support including mechanical ventilation (16.0%; compared with definitionally 100% in the Berlin definition of ARDS cohort), NIV or CPAP (14.6%), HFNO (1.6%), face mask (25.8%), and low-flow nasal oxygen (14.1%). e-Appendix 3 describes the characteristics of patients in the global definition of ARDS subcohort and e-Tables 1–4 report patient characteristics, chronic comorbidities, and ICU admission acute diagnoses and end-organ dysfunction in the global definition of ARDS subcohorts and by COVID-19 status.
TABLE 1 ].
Patient Characteristics and Observed Outcomes Across the Berlin Definition of ARDS and Global Definition-Only ARDS Cohorts
| Patient Characteristics at ICU Admission | Berlin Definition of ARDS | Global Definition-Only ARDSa |
|---|---|---|
| No. of patients | 1218 | 809 |
| Age, y | 39.2 (15.3) | 44.1 (16.1) |
| Male sex | 746 (61.3%) | 434 (53.7%) |
| Black race | 1095 (92.2%) | 651 (83.9%) |
| Primary ICU admitting diagnosisb | ||
| Trauma | 462 (38.6%) | 221 (28.7%) |
| Infection | 238 (19.9%) | 260 (33.8%) |
| Otherc | 496 (41.5%) | 288 (37.5%) |
| Referring service | ||
| Surgical | 904 (74.5%) | 490 (61.0%) |
| Medical | 310 (25.5%) | 314 (39.1%) |
| Pandemic cohort | ||
| Before pandemic without COVID-19 | 727 (59.7%) | 410 (50.7%) |
| Pandemic without COVID-19 | 443 (36.4%) | 248 (30.7%) |
| Pandemic with COVID-19 | 48 (3.9%) | 151 (18.7%) |
| Respiratory support at ICU admission | ||
| Mechanical ventilation | 1218 (100.0%) | 129 (16.0%) |
| Noninvasive ventilation or CPAP | N/A | 118 (14.6%) |
| High-flow nasal oxygen | N/A | 13 (1.6%) |
| Face mask | N/A | 209 (25.8%) |
| Low-flow nasal oxygen | N/A | 26 (3.2%) |
| Oxygen not administered (room air) | N/A | 114 (14.1%) |
| Not recorded or missing | N/A | 200 (24.7%) |
| Admission Pao2 to Fio2 ratio, mm Hg | 158 (73) | 192 (149) |
| Admission Spo2 to Fio2 ratio | 155 (81) | 194 (111) |
| ARDS oxygenation severityd | ||
| Mild | 386 (31.7%) | 200 (24.7%) |
| Moderate | 509 (41.8%) | 294 (36.3%) |
| Severe | 323 (26.5%) | 315 (38.9%) |
| Quick Sequential/Sepsis Organ Failure Assessment Score, points | ||
| 0–1 | 766 (64.5%) | 594 (75.7%) |
| 2 | 355 (29.9%) | 172 (21.9%) |
| 3 | 67 (5.6%) | 19 (2.4%) |
| Serum lactate, mM/L | 4.2 (4.8) | 2.9 (2.9) |
| ICU mortality | 278 (22.8%) | 187 (23.1%) |
| ICU length of stay, calendar days | 5 (3–9) | 4 (2–7) |
Data are presented as No. (%), mean (SD), or median (interquartile range). Variables are reported as complete cases unless noted. N/A = not applicable by definition; Spo2 = peripheral oxygen saturation.
Patients meeting at least one global definition cohort criteria (ie, global definition intubated, nonintubated, or resource-limited settings modification) and specifically not meeting Berlin definition criteria (ie, newly added patients with ARDS who would not have been included by the Berlin definition alone).
The primary indication for ICU admission as determined by the admitting ICU team as part of real-time routine care.
The most common ICU admission acute diagnoses and end-organ dysfunctions reported among patients with a nontrauma and noninfection primary indication for ICU admission included: infection (68.2% and 63.5%), acute metabolic derangements (58.3% and 69.4%), sepsis (42.7% and 36.5%), acute perioperative support (35.1% and 22.9%), acute neurologic dysfunction (30.2% and 12.5%), acute gastrointestinal processes (22.8% and 21.9%), and acute kidney injury (10.1% and 12.2%) for the Berlin definition of ARDS and the global definition-only ARDS cohorts, respectively.
Mild (200 < Pao2 to Fio2 ratio ≤ 300 or 235 < Spo2 to Fio2 ≤ 315), moderate (100 < Pao2 to Fio2 ratio ≤ 200 or 148 < Spo2 to Fio2 ≤ 235), and severe (Pao2 to Fio2 ratio ≤ 100 or Spo2 to Fio2 ≤ 148).
In the primary pooled analysis adjusting for hospital-level factors including capacity strain and peripandemic period (Fig 1, Table 2), patients who met global definition of ARDS criteria only showed no statistically significant different ICU mortality (21.7% [95% CI, 18.9%−24.4%] vs 23.8% [95% CI, 21.5%−26.2%]; OR, 0.88 [95% CI, 0.70–1.10]; P = .25) vs patients meeting the Berlin definition of ARDS criteria. e-Table 5 reports unadjusted analyses and e-Appendix 4 reports similar results after imputation for missing oxygenation data.
Figure 1 –
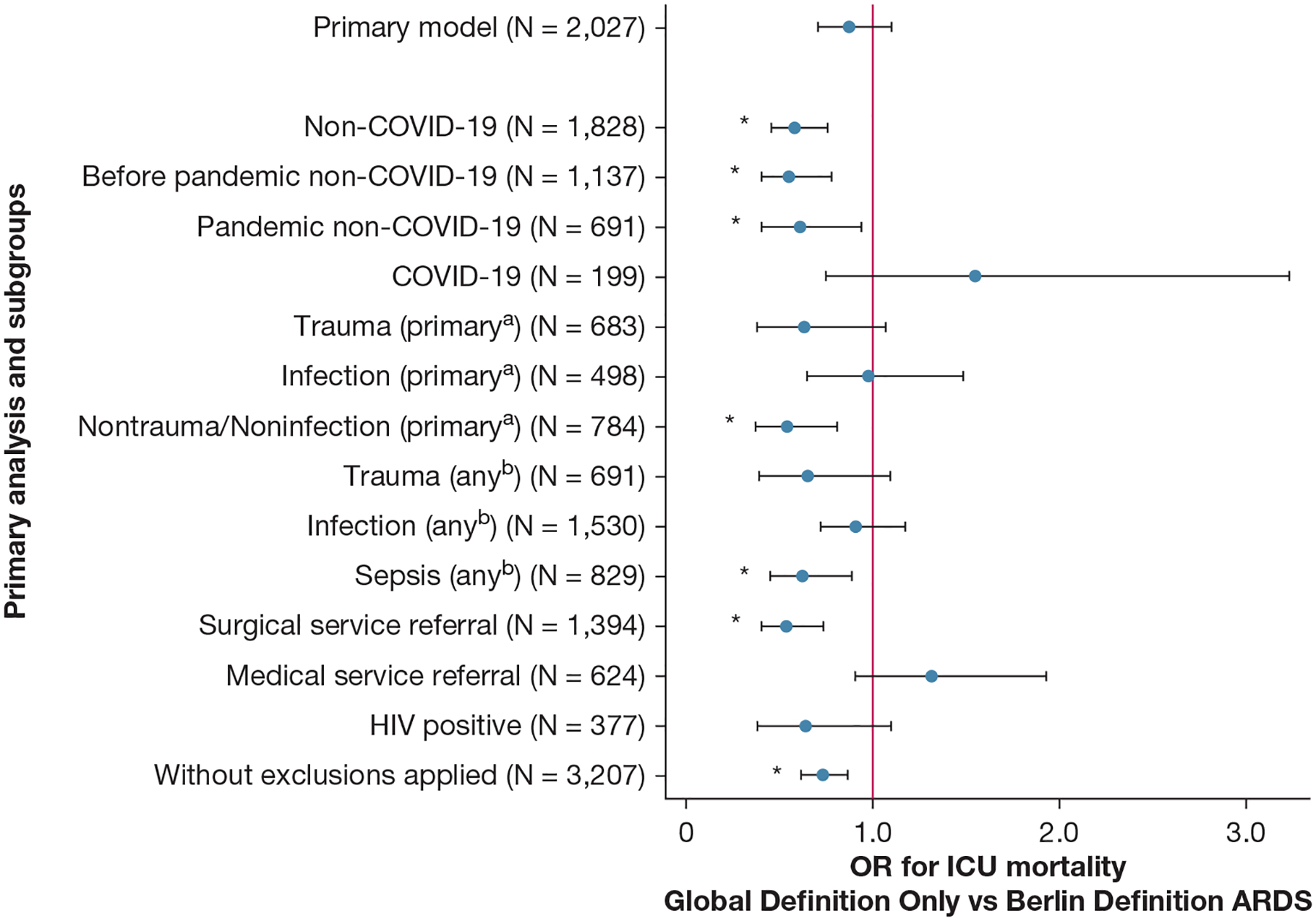
Forest plot showing ICU mortality according to only the global definition ARDS vs the Berlin definition of ARDS by subgroups. After adjustment for hospital-level factors, patients who met only the global definition of ARDS criteria (ie, who would not have been captured by the Berlin definition) showed no statistically significant different ICU mortality vs patients meeting the Berlin definition of ARDS criteria (21.7% [95% CI, 18.9%−24.4%] vs 23.8% [95% CI, 21.5%−26.2%]; OR, 0.88 [95% CI, 0.70–1.10]; P = .25). However, prespecified exploratory subgroup analyses revealed heterogeneity. Patients without COVID-19 meeting only the global definition of ARDS criteria showed reduced ICU mortality (14.2% [95% CI, 11.6%−16.9%] vs 22.2% [95% CI, 19.8%−24.6%]; OR, 0.58 [95% CI, 0.45–0.75]; P < .0005) compared with patients without COVID-19 and meeting the Berlin definition of ARDS, whereas patients with COVID-19 meeting only the global definition of ARDS criteria showed a suggestion of increased ICU mortality that did not reach statistical significance (58.6% [95% CI, 51.0%−66.2%] vs 48.7% [95% CI, 34.1%−63.3%]; OR, 1.55 [95% CI, 0.74–3.24]; P = .25) compared with patients with COVID-19 meeting the Berlin definition of ARDS. aPrimary refers to the primary indication for ICU admission as determined by the admitting ICU team as part of real-time routine care. bAny refers to acute active diagnoses and processes present at the time of ICU admission (but not necessarily the primary indication for ICU admission) as determined by the admitting ICU team as part of real-time routine care. *P < .05.
TABLE 2 ].
ICU Mortality for the Global Definition-Only ARDS Cohort vs the Berlin Definition of ARDS Cohort, Adjusted for Hospital-Level Factors
| Subgroup | No. | Berlin Definition ICU Mortality | Global Definition-Onlya ICU Mortality | Global Definition-Onlya for ICU Mortality Compared With Berlin Definition | |
|---|---|---|---|---|---|
| OR (95% CI) | P Value | ||||
| Primary analysis | 2,027 | 23.8 (21.5–26.2) | 21.7 (18.9–24.4) | 0.88 (0.70–1.09) | .25 |
| Subgroups | |||||
| Peripandemic era | |||||
| Non-COVID-19 | 1,828 | 22.2 (19.8–24.6) | 14.2 (11.6–16.9) | 0.58 (0.45–0.75) | < .0005b |
| Before pandemic without COVID-19c | 1,137 | 22.2 (19.2–25.2) | 13.6 (10.3–16.9) | 0.55 (0.40–0.77) | < .0005b |
| Pandemic without COVID-19 | 691 | 22.3 (18.5–26.2) | 15.2 (10.8–19.5) | 0.61 (0.40–0.93) | .022 |
| COVID-19 | 199 | 48.7 (34.1–63.3) | 58.6 (51.0–66.2) | 1.55 (0.74–3.24) | .25 |
| Ancestral strain erad | 44 | 35.2 (1.8–68.6) | 62.7 (46.9–78.6) | 4.09 (0.46–36.26) | .21 |
| Beta variant erad | 63 | 49.1 (20.5–77.6) | 62.9 (50.0–75.8) | 1.82 (0.47–7.11) | .39 |
| Delta variant erad | 66 | 59.9 (32.7–87.0) | 64.3 (53.0–75.7) | 1.27 (0.27–6.00) | .77 |
| Omicron variant erad | 26 | 27.5 (11.7–43.2) | 26.2 (6.0–46.4) | 0.84 (0.02–36.36) | .93 |
| Primary admitting diagnosise | |||||
| Trauma | 683 | 14.4 (11.2–17.6) | 9.7 (5.9–13.6) | 0.64 (0.38–1.07) | .09 |
| Infection | 498 | 41.2 (34.9–47.5) | 40.8 (34.9–46.6) | 0.98 (0.65–1.49) | .92 |
| Nontrauma and noninfection | 784 | 24.5 (20.7–28.2) | 15.2 (11.1–19.3) | 0.54 (0.37–0.80) | .002b |
| Diagnosis present at ICU admissionf | |||||
| Trauma | 691 | 14.5 (11.3–17.7) | 10.0 (6.1–13.9) | 0.65 (0.39–1.08) | .10 |
| Concern for infectiong | 1,530 | 26.6 (23.8–29.5) | 25.1 (21.7–28.5) | 0.92 (0.72–1.17) | .49 |
| Sepsish | 829 | 31.7 (27.7–35.7) | 22.8 (18.2–27.5) | 0.63 (0.45–0.88) | .007b |
| Referring service | |||||
| Surgical | 1,394 | 21.8 (19.1–24.4) | 13.2 (10.2–16.1) | 0.54 (0.40–0.74) | < .0005b |
| Medical | 624 | 29.1 (24.0–34.0) | 34.5 (29.4–39.5) | 1.32 (0.91–1.93) | .15 |
| HIV positive | 377 | 27.9 (22.3–33.6) | 20.7 (14.5–26.8) | 0.64 (0.38–1.09) | .10 |
| Without exclusion criteria | 3,207 | 27.2 (25.2–29.2) | 21.6 (19.4–23.8) | 0.73 (0.62–0.87) | .0005b |
Data are presented as percentage (95% CI) unless otherwise indicated. Data are reported among complete cases and adjusted for facility, peripandemic period (before pandemic and during pandemic strain-dominant periods), and five capacity strain metrics (ICU occupancy, ICU referral burden, ICU turnover, ICU acuity, and national 7-day rolling mean of incident SARS-CoV-2 cases per 1 million residents).
Patients meeting at least one global definition cohort criteria (ie, global definition intubated, nonintubated, or resource-limited settings modification) and specifically not meeting Berlin definition criteria (ie, newly added patients with ARDS who would not have been included by the Berlin definition alone).
P < .05.
Patients without COVID-19 before the pandemic not adjusted for pandemic era.
COVID-19 subgroups stratified on, but not adjusted for, pandemic variant era.
The primary indication for ICU admission as determined by the admitting ICU team as part of real-time routine care.
Acute active diagnoses and processes present at the time of ICU admission (but not necessarily the primary indication for ICU admission) as determined by the admitting ICU team as part of real-time routine care.
Concern for infection includes infection as the primary indication for ICU admission or admission sepsis flag (both as determined by the admitting ICU team as part of real-time routine care) or receipt of antimicrobials at the time of ICU admission.
Sepsis includes concern for infection, as above, and meeting criteria by Quick Sequential Organ Failure Assessment score or Systemic Inflammatory Response Syndrome score, or admission sepsis flag (as determined by the admitting ICU team as part of real-time routine care).
Subgroup and Sensitivity Analyses
Figure 1 and Table 2 report results of prespecified exploratory subgroup and sensitivity analyses, adjusted for hospital-level factors (e-Table 5 reports unadjusted analyses). Although the primary analysis showed no statistically significant difference in mortality between the global definition-only ARDS cohort and the Berlin definition of ARDS cohort, exploratory subgroup analyses revealed heterogeneity. Patients without COVID-19 meeting only the global definition of ARDS criteria showed reduced ICU mortality (14.2% [95% CI, 11.6%−16.9%] vs 22.2% [95% CI, 19.8%−24.6%]; OR, 0.58 [95% CI, 0.45–0.75]; P < .0005) compared with patients without COVID-19 meeting the Berlin definition of ARDS, whereas patients with COVID-19 meeting only the global definition of ARDS criteria showed a suggestion of increased ICU mortality that did not reach statistical significance (58.6% [95% CI, 51.0%−66.2%] vs 48.7% [95% CI, 34.1%−63.3%]; OR, 1.55 [95% CI, 0.74–3.24]; P = .25) compared with patients with COVID-19 meeting the Berlin definition of ARDS. Also statistically significant decreases in ICU mortality were noted in the global definition-only ARDS cohort, as compared with the Berlin definition of ARDS cohort, in subgroups admitted for noninfection and nontrauma primary ICU admission diagnoses, admitted with sepsis, and referred from a surgical service, and signals toward decreased ICU mortality (point estimate OR, < 1) in the global definition-only ARDS cohort subgroups admitted for or with trauma and concern for infection and with HIV infection. To examine further the impact of the population with COVID-19, in a post hoc analysis, we repeated our primary model comparing global definition-only ARDS and the Berlin definition of ARDS cohorts now including an interaction term between ARDS cohort and COVID-19 status and adjusted for COVID-19 status. In this model, patients who met global definition-only ARDS criteria showed reduced ICU mortality (18.9% [95% CI, 16.3%−21.4%] vs 24.1% [95% CI, 21.6%−26.7%]; OR, 0.58 [95% CI, 0.45–0.76]; P < .0005) vs patients meeting the Berlin definition of ARDS criteria with a statistically significant interaction (OR, 3.70 [95% CI, 1.79–7.66;] P < .0005) for ICU mortality in the global definition-only ARDS cohort compared with the Berlin definition of ARDS cohort if COVID-19 status is positive. e-Appendix 5, Figure 1, Table 2, and e-Table 5 report results after removing exclusion criteria of < 7 days hospital length of stay before ICU admission and no acute cardiac disease diagnosis at the time of ICU admission.
ARDS Oxygenation Severity Secondary Analyses
Figure 2 and e-Table 6 report ICU mortality across ARDS cohorts and by oxygenation severity levels, adjusted for hospital-level factors. Among the Berlin definition of ARDS cohort, and consistent with prior literature,4 worsening ARDS oxygenation severity classification was associated with stepwise increased ICU mortality: mild, 15.9% (95% CI, 12.2%−19.5%); moderate, 23.7% (95% CI, 20.1%−27.4%; with OR, 1.67 [95% CI, 1.18–2.35]; P = .004 compared with mild); and severe, 29.6% (95% CI, 24.6%−34.6%; with OR, 2.27 [95% CI, 1.56–3.29]; P < .0005 compared with mild). The global definition-only ARDS cohort showed a notable decrease and narrowing of ICU mortality outcomes between the mild and moderate levels and a widening and worsening of the severe level ICU mortality outcomes: mild, 12.9% (95% CI, 8.0%−17.8%); moderate, 13.1% (95% CI, 9.1%−17.1%; with OR, 1.02 [95% CI, 0.57–1.83]; P = .95 compared with mild); and severe, 36.9% (95% CI, 31.6%−42.1%; with OR, 4.39 [95% CI, 2.61–7.38]; P < .0005 compared with mild). (e-Table 7 reports unadjusted analyses.)
Figure 2 –
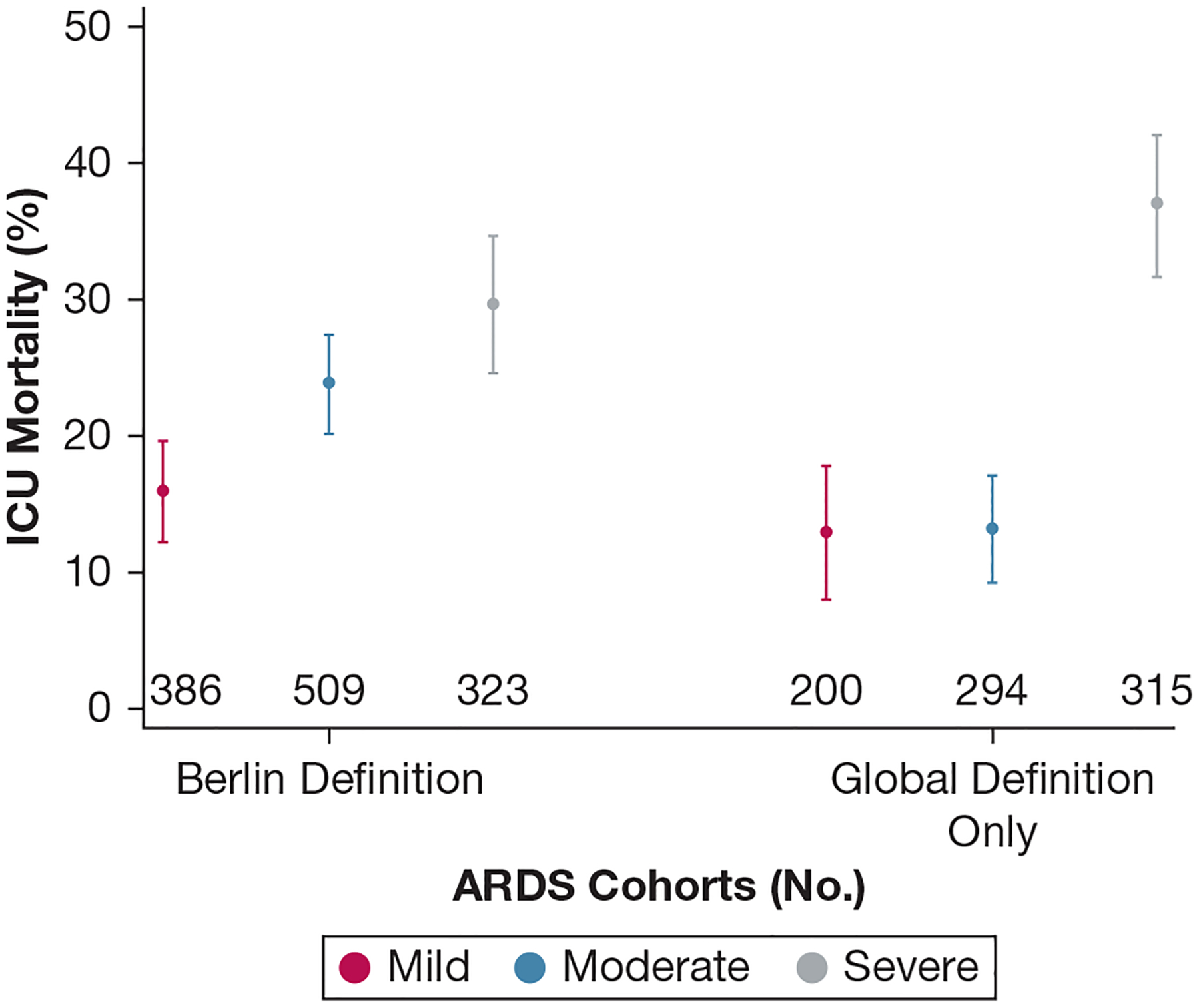
Graph showing ICU mortality across ARDS cohorts and oxygenation severity levels. In the Berlin definition of ARDS cohort, and consistent with prior literature, worsening ARDS oxygenation severity classification was associated with increased ICU mortality after adjustment for hospital-level factors. ICU mortality across ARDS classifications showed a narrowing between mild and moderate levels and a worsening in the severe level in the global definition-only ARDS cohort. Figure symbols represent point estimates and 95% CIs.
Figure 3 shows results stratified by the global definition of ARDS subcohorts and COVID-19 status. The intubated patients in the global definition ARDS cohort, similar to the highly overlapping Berlin definition of ARDS cohort, showed worsening ARDS oxygenation severity classification associated with increased ICU mortality, and the global definition resource-limited settings modification ARDS cohort, similar to the substantially overlapping global definition-only ARDS cohort, showed a decrease and narrowing of ICU mortality outcomes between the mild and moderate levels and a widening and worsening of the severe level ICU mortality outcomes (Fig 3A). The nonintubated patients in the global definition ARDS cohort was too small for meaningful precision. Stratification by COVID-19 status shows the increased mortality of the severe level driven by patients with COVID-19 (Fig 3B) and the decreased mortality in the mild and moderate levels driven by patients without COVID-19 (Fig 3C).
Figure 3 –
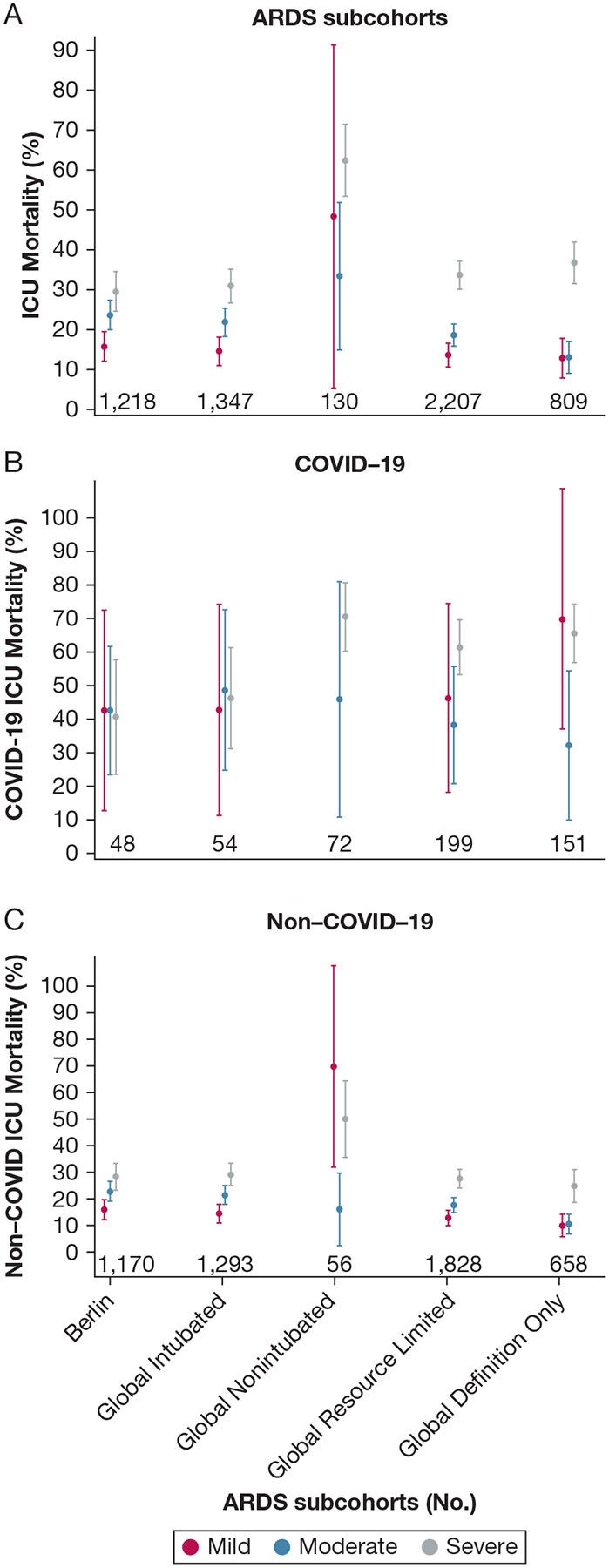
A-C, Graphs showing ICU mortality by the global definition of ARDS subcohorts, oxygenation severity levels, and COVID-19 status. A, Intubated patients in the global definition of ARDS cohort, similar to the highly overlapping the Berlin definition of ARDS cohort, showed worsening ARDS oxygenation severity classification associated with increased ICU mortality, and the global definition resource-limited settings modification ARDS cohort, similar to the substantially overlapping global definition-only ARDS cohort, showed a decrease and narrowing of ICU mortality outcomes between the mild and moderate levels and a widening and worsening of the severe level ICU mortality outcomes. Nonintubated patients in the global definition of ARDS cohort were too few for meaningful precision. B, C, Stratification by COVID-19 status showed the increased mortality of the severe level driven by patients with COVID-19 (B) and the decreased mortality in the mild and moderate levels driven by patients without COVID-19 (C).
Discussion
ARDS remains a challenging syndrome across multiple axes including, but not limited to, definition, diagnosis, epidemiology, and therapeutics.9,16 Recognition of a heavy global burden of disease across resource levels2–5 and insights from the COVID-19 pandemic10–12 have led to enthusiasm for an expanded definition of ARDS that allows for the inclusion of nonintubated patients and of patients for whom resource access, but not physiologic characteristics, may preclude traditional cohort inclusion.7 This two-hospital real-use retrospective cohort study in the South African public health system sought to examine operationalizing the new global definition of ARDS in one such resource-limited setting and to analyze the resultant impact on ARDS epidemiology.
The primary findings of this study include that: (1) the new global definition of ARDS captures a significant proportion of patients, primarily nonintubated or without high supplemental oxygen support, who would not have been captured by the Berlin definition alone (40% of all ARDS diagnoses in this analysis); (2) pooled findings of similar outcomes between global definition-only ARDS and the Berlin definition of ARDS, here with a nonstatistically significant point estimate suggesting possible lower mortality, may hide important subgroup differences such as decreased ICU mortality for patients without COVID-19 and potentially increased ICU mortality for patients with COVID-19 in the global definition of ARDS cohort; and (3) long-standing ARDS severity classifications may diverge in part because of these subgroup effects.
Our results add further diversity to the ARDS literature. The study population from the South African public health system, as previously reported,22,26 is younger (mean age, 39.2 years for the Berlin definition of ARDS cohort), more predominantly Black (92.2%), and more often admitted for a primary trauma indication (38.6%) than many ARDS studies in higher-resourced settings. For instance, in the Dexamethasone Treatment for the Acute Respiratory Distress Syndome (DEXA-ARDS) study in ICUs in Spain, the intervention arm reported a mean age of 56 years with 8% trauma-induced ARDS.28 The present younger, trauma-enriched ARDS study population also showed a lower observed mortality (22.8%) than ARDS studies from higher-resourced settings and populations (eg, 39.4% in one large meta-analysis29).
Patients with COVID-19 and their outcomes clearly impacted the examined data. South Africa in general, and the study hospitals in particular, saw high COVID-19 mortality and major critical care practice changes—such as new use of noninvasive respiratory support strategies and extreme efforts to avert intubation—occur during the pandemic and specifically for these patients with COVID-19.26 Together, these phenomena made the nonintubated patients (ie, NIV or HFNO) in the global definition ARDS cohort, 56.5% with COVID-19, stand out, and added noteworthy subgroup heterogeneity important in its own right and also critical to recognize in interpreting pooled primary results. Although the global definition-only ARDS cohort showed no statistically significant ICU mortality reduction in pooled analyses compared with the Berlin definition of ARDS cohort, the point estimate trended toward reduced mortality and nearly every subgroup without COVID-19 met or trended toward statistical significance for reduced mortality for the global definition-only ARDS cohort (Fig 1). In contrast, the patients with COVID-19 in the global definition-only ARDS cohort trended toward an ICU mortality increase and likely were responsible for the null primary result. Absent the patients with COVID-19, the addition of the global definition-only ARDS cohort likely will reduce overall observed ARDS mortality, consistent with an overall lower disease acuity cohort (ie, with lower intensity of respiratory support), a primary critique of the more expansive global definition criteria.7,12,17,18 Of note, this finding is vulnerable to bias because of the lack of chest imaging findings that may have excluded a greater proportion of patients with global definition-only ARDS of lower acuity.
ARDS severity classifications also diverged likely in part because of these subgroup effects, consistent with emerging evidence.20 In the global definition of ARDS cohorts, our results demonstrated increased mortality of the severe level driven by patients with COVID-19 (Fig 3B) and decreased mortality in the mild and moderate levels driven by patients without COVID-19 newly included via the lower-threshold global definition of ARDS (Fig 3C). This high-severity or higher-mortality COVID-19 (and nonintubated patients with global definition of ARDS) phenomenon could represent either patients who would have benefited from earlier intubation, allocation of scarce life support resources, or more frequent end-of-life discussions during the pandemic; simply worse COVID-19 outcomes; or a combination thereof. In contrast, the low-severity and lower-mortality non-COVID-19 phenomenon could represent patients who would have never required mechanical ventilation. Subject to continued discussion is whether these patients (predominantly without COVID-19) with lower severity of disease and with even lower observed mortality have sufficiently similar physiologic and biopathologic characteristics to warrant the formal ARDS label.
Notable strengths of our study include examining operationalizing of the new global definition of ARDS in a real-use but well-constructed clinical database and an assessment of the resultant updated epidemiologic characteristics in a resource-limited setting, including the identification of important heterogeneity such as being the result of COVID-19.
The results of this study should be interpreted in the context of important limitations. Chest imaging findings were not readily available and therefore were not included in cohort definitions and eligibility, and subsequently could have influenced findings in particular in the lower acuity global definition-only ARDS cohort, where lack of compatible chest imaging, if available, would have excluded additional patients. In our study, the Berlin definition of ARDS criteria, without chest radiography but after excluding primary cardiac admissions, identified 21.1% of all adult ICU admissions, and 32.7% of those mechanically ventilated, as ARDS. This is somewhat higher than the 10.4% of ICU admissions and 23.4% of mechanically ventilated patients in a large global prevalence study,4 but lower than the 70.6% of mechanical ventilated patients in the ICU in an ICU study in Uganda.15 True rates of radiographically consistent ARDS among patients meeting a Pao2 to Fio2 ratio of ≤ 300 are unknown because of low expert interobserver agreement, with ranges as wide as 36% to 71%.30 In total, our study, agnostic to chest radiography, likely overdiagnoses ARDS in some patients, but to an unknown degree.
The ICU database is limited to data at the time of ICU referral or admission and selected end-ICU outcomes, and does not contain information on longitudinal physiologic features and interventions during the ICU stay or after the ICU hospital course and outcomes. Additionally, in this and similar resource-limited settings and in particular during the COVID-19 pandemic, patients with or at risk of ARDS may be cared for to a greater extent on general medical wards alone (with nonrandom selection) and would not have been captured in the ICU database; future work is planned to investigate this non-ICU subgroup whose data are not captured currently. The ICU database is assembled as part of routine clinical care by the ICU teams, which carries the risks of unknown entry error rates and the absence of information that occurs in a busy clinical setting. The requirement of hospital length of stay before ICU admission of < 7 days may exclude some patients with hospital-onset ARDS (eg, postoperative aspiration pneumonia) and the exclusion of acute cardiac diagnoses may exclude erroneously some patients with cardiac diagnoses unrelated to or nondominant in their acute respiratory physiologic features. Important issues related to skin tone disparities with Spo2 measurement7,19,20 are not addressed directly in this predominantly Black South African population. This is a two-hospital, single-country study; the new global definition of ARDS and related research questions should continue to be investigated in diverse global settings.
Interpretation
When operationalized, the new global definition of ARDS captures a significant proportion of patients, primarily nonintubated or without high supplemental oxygen support or data, who would not have been captured by the Berlin definition alone. These additional patients with ARDS may have heterogenous patterns of outcomes among diagnostic subgroups, including COVID-19 status, compared with patients with the Berlin definition of ARDS.
Supplementary Material
Take-home Points.
Study Question:
What are the epidemiologic characteristics of ARDS in resource-limited settings after operationalizing the new global definition of ARDS?
Results:
The new global definition of ARDS captures a significant proportion of patients (40% in this study), predominantly nonintubated or without high supplemental oxygen support or data, who would not have been captured by the Berlin definition alone. These additional patients with ARDS may have different patterns of outcomes among diagnostic subgroups, such as COVID-19 status, compared with patients with ARDS according to the Berlin definition.
Interpretation:
We found that the new global definition of ARDS captures additional patients, likely including many cared for in resource-limited settings, who have variable risk of poor ICU outcomes.
Acknowledgments
Author contributions: Conception and design of study: G. L. A., A. R., J. I., S. M. S., Z. F., and M. T. D. S. Data acquisition: G. L. A., A. R., J. I., S. M. S., R. D. W., and M. T. D. S. Analysis and data interpretation: G. L. A., A. R., J. I., S. M. S., R. D. W., Z. F., and M. T. D. S. Drafting and revision of the manuscript: G. L. A., A. R., J. I., S. M. S., R. D. W., Z. F., and M. T. D. S.
South Africa Intensive Care Unit Capacity Strain Study Group collaborator: Douglas P. K. Wilson, MBChB, PhD, Department of Internal Medicine, School of Clinical Medicine, University of KwaZulu-Natal, Pietermaritzburg, South Africa.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the collaborative South Africa Intensive Care Unit Capacity Strain Study Group, a longitudinal partnership of the University of Pennsylvania Perelman School of Medicine (Philadelphia, PA), the University of KwaZulu-Natal School of Clinical Medicine, and the KwaZulu-Natal Department of Public Health (KwaZulu-Natal, South Africa).
Additional information: The e-Appendixes and e-Tables are available online under “Supplementary Data.”
Funding/Support
This study was supported by the Leonard Davis Institute of Health Economics at the University of Pennsylvania Perelman School of Medicine (G. L. A.), the Thomas B. McCabe and Jeannette E. Laws McCabe Fund at the University of Pennsylvania Perelman School of Medicine (G. L. A.), and the National Institutes of Health [Grant K23HL161353 (G. L. A.)].
ABBREVIATIONS:
- HFNO
high-flow nasal oxygen
- NIV
noninvasive ventilation
- Spo2
peripheral oxygen saturation
Footnotes
Financial/Nonfinancial Disclosures
The authors have reported to CHEST Critical Care the following: G. L. A. reports payments for authoring chapters for UpToDate and for expert witness consulting, and reports that his spouse is employed by the US Food and Drug Administration. None declared (A. R., J. I., S. M. S., R. D. W., Z. F., M. T. D. S.).
References
- 1.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 2.Kwizera A, Dunser MW. A global perspective on acute respiratory distress syndrome and the truth about hypoxia in resource-limited settings. Am J Respir Crit Care Med. 2016;193(1):5–7. [DOI] [PubMed] [Google Scholar]
- 3.Riviello ED, Kiviri W, Twagirumugabe T, et al. Hospital incidence and outcomes of the acute respiratory distress syndrome using the Kigali modification of the Berlin definition. Am J Respir Crit Care Med. 2016;193(1):52–59. [DOI] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. [DOI] [PubMed] [Google Scholar]
- 5.Santa Cruz R, Matesa A, Gomez A, et al. Mortality due to acute respiratory distress syndrome in Latin America. Crit Care Med. 2024;52(8):1275–1284. [DOI] [PubMed] [Google Scholar]
- 6.Beltramo F, Khemani RG. Definition and global epidemiology of pediatric acute respiratory distress syndrome. Ann Transl Med. 2019;7(19):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smit MR, Brower RG, Parsons PE, Phua J, Bos LDJ. The global definition of acute respiratory distress syndrome: ready for prime time? Am J Respir Crit Care Med. 2024;209(1):14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthay MA, Thompson BT, Ware LB. The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included? Lancet Respir Med. 2021;9(8): 933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G, Calfee CS, Camporota L, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49(7): 727–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreyro BL, Angriman F, Munshi L, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perkins GD, Ji C, Connolly BA, et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022;327(6):546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranieri VM, Tonetti T, Navalesi P, et al. High-flow nasal oxygen for severe hypoxemia: oxygenation response and outcome in patients with COVID-19. Am J Respir Crit Care Med. 2022;205(4): 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthay MA, Arabi Y, Arroliga AC, et al. A new global definition of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2024;209(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alipanah-Lechner N, Cavalcanti AB, Diaz J, Ferguson ND, Myatra SN, Calfee CS. From Berlin to global: the need for syndromic definitions of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2024;209(1):21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwizera A, Kateete DP, Ssenyonga R, et al. Acute respiratory distress syndrome in an African intensive care unit setting: a prospective study of prevalence and outcomes. Ann Am Thorac Soc. 2022;19(4):691–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson JG, Calfee CS. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit Care. 2020;24(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alrawashdeh M, Klompas M, Rhee C. The impact of common variations in sequential organ failure assessment score calculation on sepsis measurement using Sepsis-3 criteria: a retrospective analysis using electronic health record data. Crit Care Med. 2024;52(9):1380–1390. [DOI] [PubMed] [Google Scholar]
- 18.Qian F, van den Boom W, See KC. The new global definition of acute respiratory distress syndrome: insights from the MIMIC-IV database. Intensive Care Med. 2024;50(4):608–609. [DOI] [PubMed] [Google Scholar]
- 19.Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. N Engl J Med. 2020;383(25):2477–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong AI, Charpignon M, Kim H, et al. Analysis of discrepancies between pulse oximetry and arterial oxygen saturation measurements by race and ethnicity and association with organ dysfunction and mortality. JAMA Netw Open. 2021;4(11): e2131674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allorto NL, Wise RD. Development and evaluation of an integrated electronic data management system in a South African metropolitan critical care service. South Afr J Anaesth Analg. 2015;21:31–35. [Google Scholar]
- 22.Anesi GL, Gabler NB, Allorto NL, et al. Intensive care unit capacity strain and outcomes of critical illness in a resource-limited setting: a 2-hospital study in South Africa. J Intensive Care Med. 2020;35(10): 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishop LA, Wilson DPK, Wise RD, Savarimuthu SM, Anesi GL. Prognostic value of the Quick Sepsis-related Organ Failure Assessment (qSOFA) score among critically ill medical and surgical patients with suspected infection in a resource-limited setting. Afr J Thorac Crit Care Med. 2021;27(4). 10.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn S, Wise R, Savarimuthu SM, Anesi GL. Association between pre-ICU hospital length of stay and ICU outcomes in a resource-limited setting. SAJCC. 2021;37(3):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savarimuthu SM, Cairns C, Allorto NL, Weissman GE, Kohn R, Wise RD, Anesi GL. qSOFA as a predictor of ICU outcomes in a resource-limited setting in KwaZulu-Natal Province, South Africa. SAJCC. 2020;36(2):92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anesi GL, Savarimuthu SM, Invernizzi J, et al. ICU mortality across prepandemic and pandemic cohorts in a resource-limited setting: a critical care resiliency analysis from South Africa. Chest Crit Care. 2023;1(1):100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. [DOI] [PubMed] [Google Scholar]
- 28.Villar J, Ferrando C, Martinez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. [DOI] [PubMed] [Google Scholar]
- 29.Sadana D, Kaur S, Sankaramangalam K, et al. Mortality associated with acute respiratory distress syndrome, 2009–2019: a systematic review and meta-analysis. Crit Care Resusc. 2022;24(4):341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116(5): 1347–1353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


