Abstract
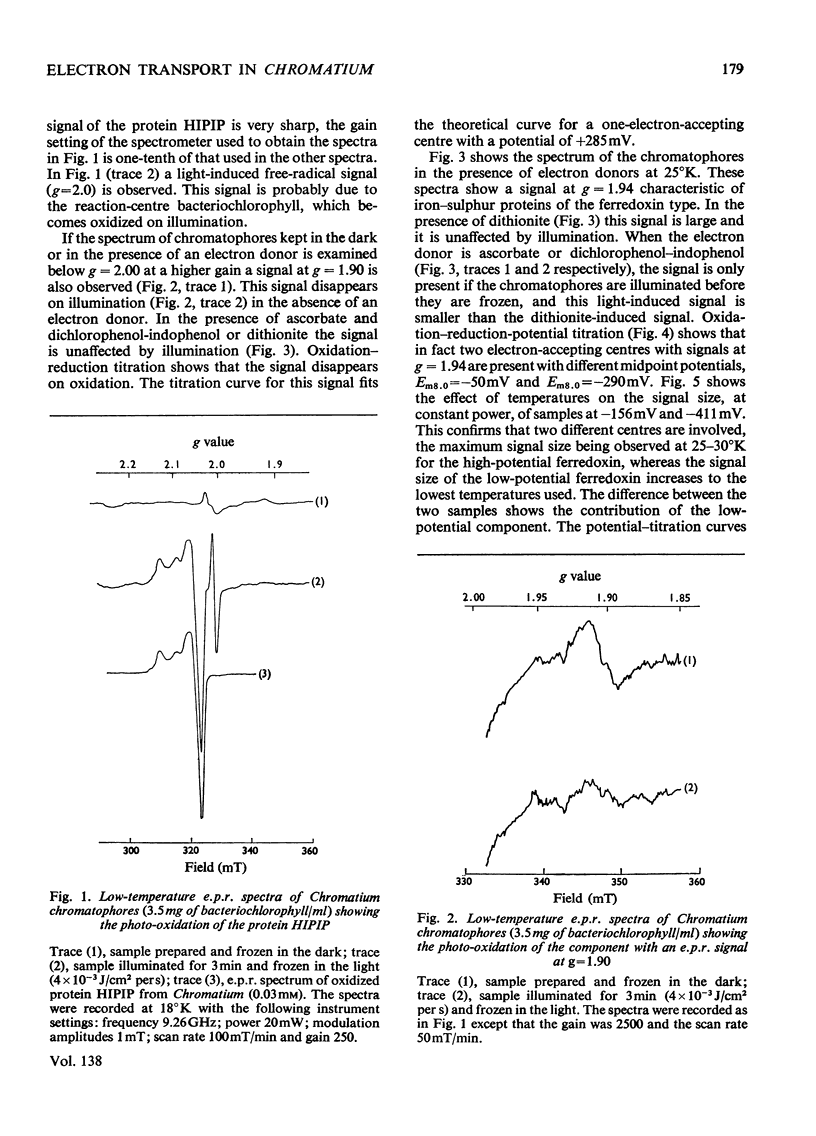
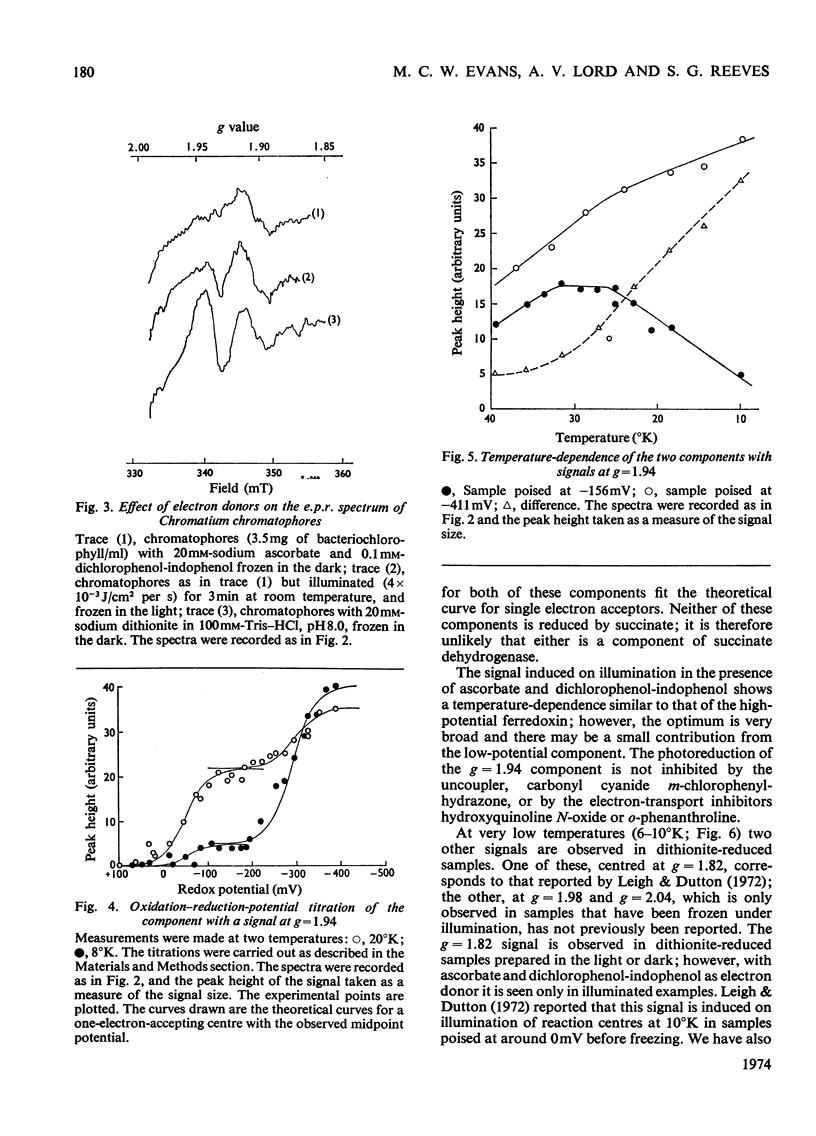
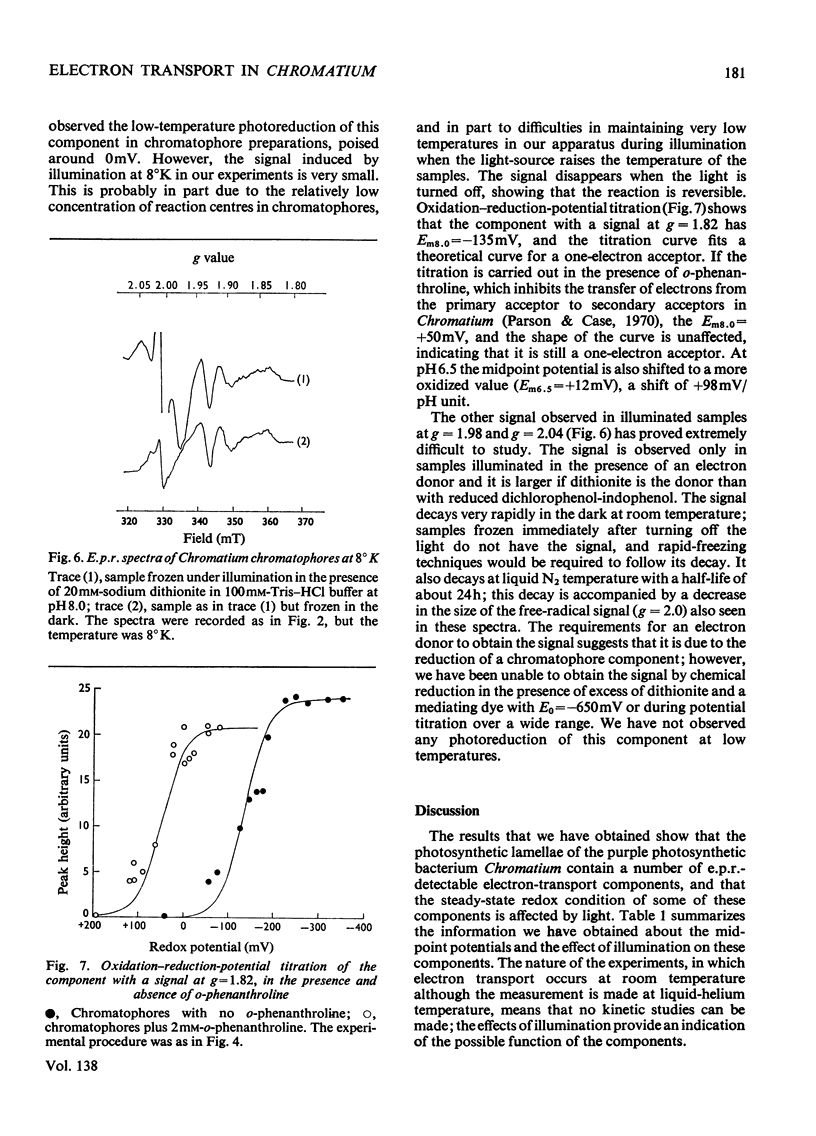
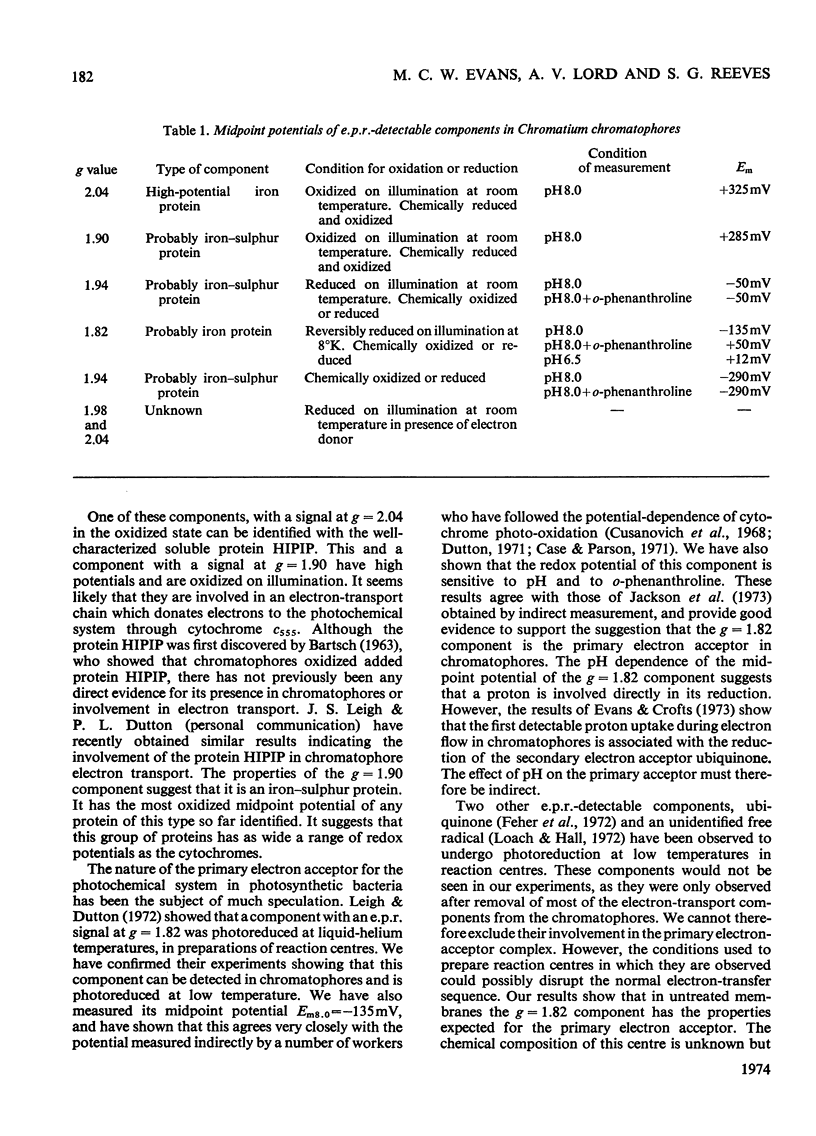
Low-temperature e.p.r. (electron-paramagnetic-resonance) spectroscopy was used to detect electron-transport components in Chromatium chromatophores with e.p.r. signals in the g=2.00 region. High-potential iron protein (Em8.0=+325mV, where Em8.0 is the midpoint potential at pH8) and a second component (g=1.90, Em8.0=+285mV) are oxidized in illuminated chromatophores. Two iron–sulphur proteins (g=1.94) with Em8.0=−290mV and Em8.0=−50mV are present. One (Em8.0=−50mV) is reduced on illumination. A component (g=1.82) with Em8.0=−135mV is photoreduced at 10°K. The midpoint potential of this component is altered by o-phenanthroline and pH. The properties of this component suggest that it is the primary electron acceptor of a photochemical system. Another component (g=1.98) also has some of the properties of a primary electron acceptor, but its function cannot be completely defined. These results show that iron–sulphur proteins are present in the electron-transport system of Chromatium and indicate their role in electron transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan B. B., Arnon D. I. Ferredoxins: chemistry and function in photosynthesis, nitrogen fixation, and fermentative metabolism. Adv Enzymol Relat Areas Mol Biol. 1970;33:119–176. doi: 10.1002/9780470122785.ch3. [DOI] [PubMed] [Google Scholar]
- Case G. D., Parson W. W. Thermodynamics of the primary and secondary photochemical reactions in Chromatium. Biochim Biophys Acta. 1971 Nov 2;253(1):187–202. doi: 10.1016/0005-2728(71)90244-1. [DOI] [PubMed] [Google Scholar]
- Cusanovich M. A., Bartsch R. G., Kamen M. D. Light-induced electron transport in Chromatium strain D. II. Light-induced absorbance changes in Chromatium chromatophores. Biochim Biophys Acta. 1968 Feb 12;153(2):397–417. doi: 10.1016/0005-2728(68)90083-2. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Leigh J. S., Jr, Reed D. W. Primary events in the photosynthetic reaction centre from Rhodopseudomonas spheroides strain R26: triplet and oxidized states of bacteriochlorophyll and the identification of the primary electron acceptor. Biochim Biophys Acta. 1973 Apr 5;292(3):654–664. doi: 10.1016/0005-2728(73)90013-3. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Buchanan B. B. Photoreduction of ferredoxin and its use in carbon dioxide fixation by a subcellular system from a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1420–1425. doi: 10.1073/pnas.53.6.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Telfer A., Lord A. V. Evidence for the role of a bound ferredoxin as the primary electron acceptor of photosystem I in spinach chloroplasts. Biochim Biophys Acta. 1972 Jun 23;267(3):530–537. doi: 10.1016/0005-2728(72)90181-8. [DOI] [PubMed] [Google Scholar]
- Feher G., Okamura M. Y., McElroy J. D. Identification of an electron acceptor in reaction centers of Rhodopseudomonas spheroides by EPR spectroscopy. Biochim Biophys Acta. 1972 Apr 20;267(1):222–226. doi: 10.1016/0005-2728(72)90155-7. [DOI] [PubMed] [Google Scholar]
- Garcia A., Vernon L. P., Ke B., Mollenhauer H. Some structural and photochemical properties of Rhodopseudomonas species NHTC 133 subchromatophore particles obtained by treatment with Triton X-100. Biochemistry. 1968 Jan;7(1):326–332. doi: 10.1021/bi00841a041. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Cogdell R. J., Crofts A. R. Some effects of o-phenanthroline on electron transport in chromatophores from photosynthetic bacteria. Biochim Biophys Acta. 1973 Jan 18;292(1):218–225. doi: 10.1016/0005-2728(73)90266-1. [DOI] [PubMed] [Google Scholar]
- Leigh J. S., Jr, Dutton P. L. The primary electron acceptor in photosynthesis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):414–421. doi: 10.1016/s0006-291x(72)80154-2. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Hall R. L. The question of the primary electron acceptor in bacterial photosynthesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):786–790. doi: 10.1073/pnas.69.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Case G. D. In Chromatium, a single photochemical reaction center oxidizes both cytochrome C552 and cytochrome C555. Biochim Biophys Acta. 1970;205(2):232–245. doi: 10.1016/0005-2728(70)90253-7. [DOI] [PubMed] [Google Scholar]
- Tagawa K., Arnon D. I. Oxidation-reduction potentials and stoichiometry of electron transfer in ferredoxins. Biochim Biophys Acta. 1968 Apr 2;153(3):602–613. doi: 10.1016/0005-2728(68)90188-6. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Valentine R. C. Ferredoxins and flavodoxins of bacteria. Annu Rev Microbiol. 1972;26:139–162. doi: 10.1146/annurev.mi.26.100172.001035. [DOI] [PubMed] [Google Scholar]


