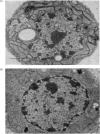
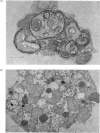
Abstract
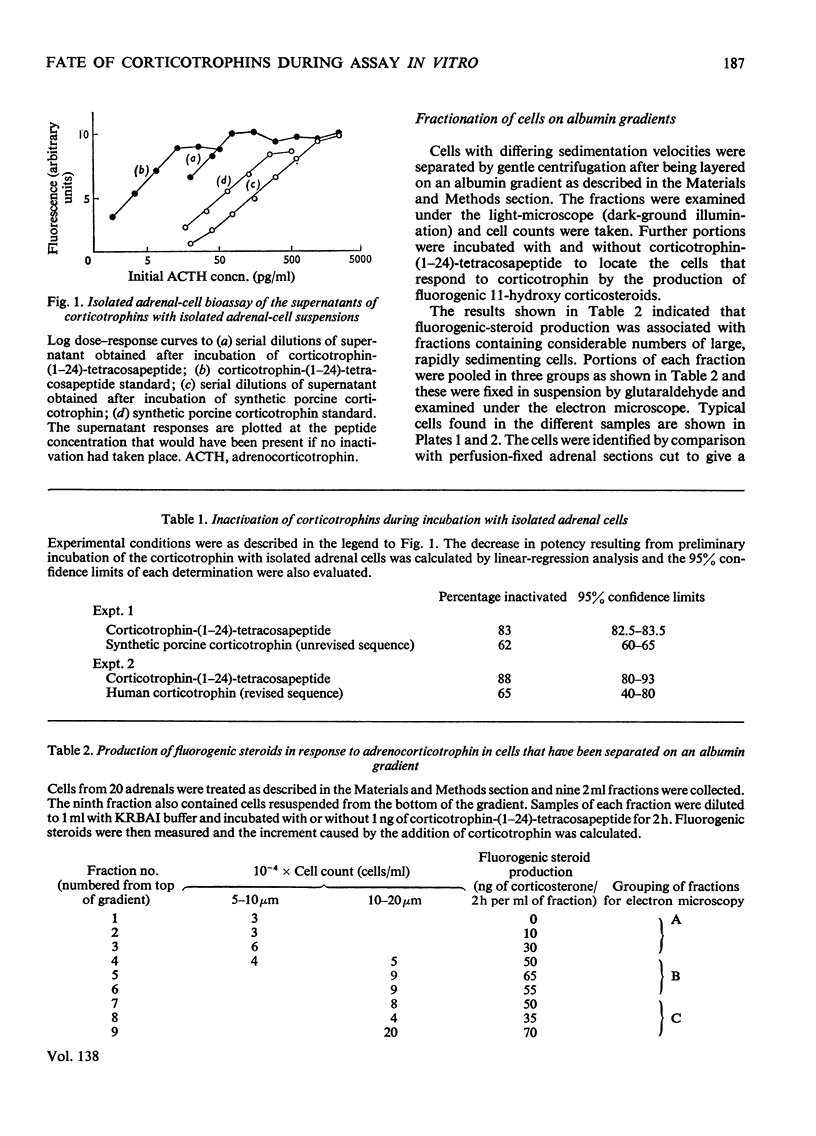
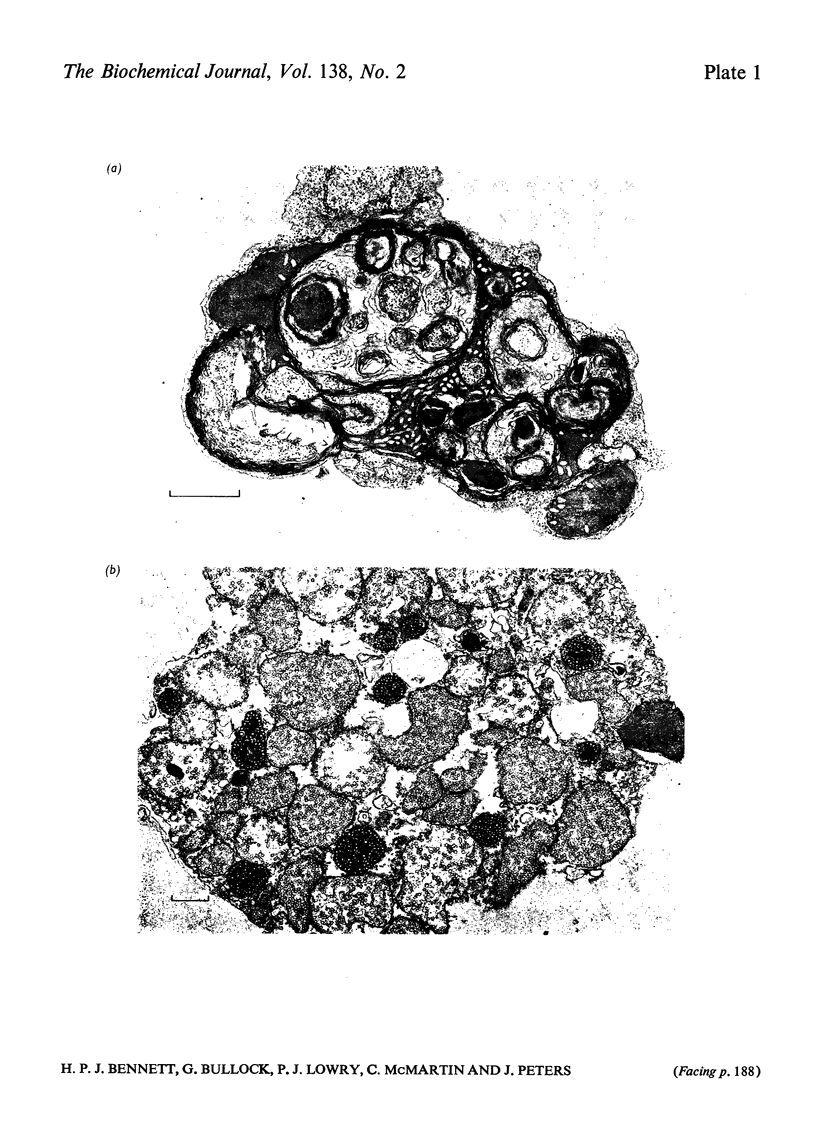
1. The fate of corticotrophins in a trypsin-dispersed rat adrenal-cell assay system was investigated with a view to establishing whether differences in the rate of inactivation might contribute to potency differences observed between analogues. 2. Corticotrophin-(1–24)-tetracosapeptide and to a lesser extent synthetic 1–39 corticotrophins were found to be inactivated during incubation with cell suspension. 3. Peptide fragments were isolated by using [[3H2]Tyr23]corticotrophin-(1–24)- tetracosapeptide as a marker. The fragments indicate a peptidase with a predominantly tryptic specificity. 4. The peptidase is present in the extracellular fluid and is released from cells when they are damaged. 5. Cells were fractionated on an albumin gradient. Cells from the zona fasciculata and the zona intermedia or reticularis were present in fractions which produced fluorogenic steroids in response to corticotrophin. 6. Purification of the cells by centrifugation through albumin decreased degradation by peptidases, so that if the assay is carried out with a dilute suspension of purified cells peptide breakdown should not affect the observed potencies of adrenocorticotrophin analogues. 7. No binding of [[3H2]Tyr23]corticotrophin-(1–24)- tetracosapeptide to cells could be detected at low concentrations of the peptide. This indicated that less than 120 receptors/cell are occupied during stimulation by a dose that would elicit approx. 80% of the maximal response.
Full text
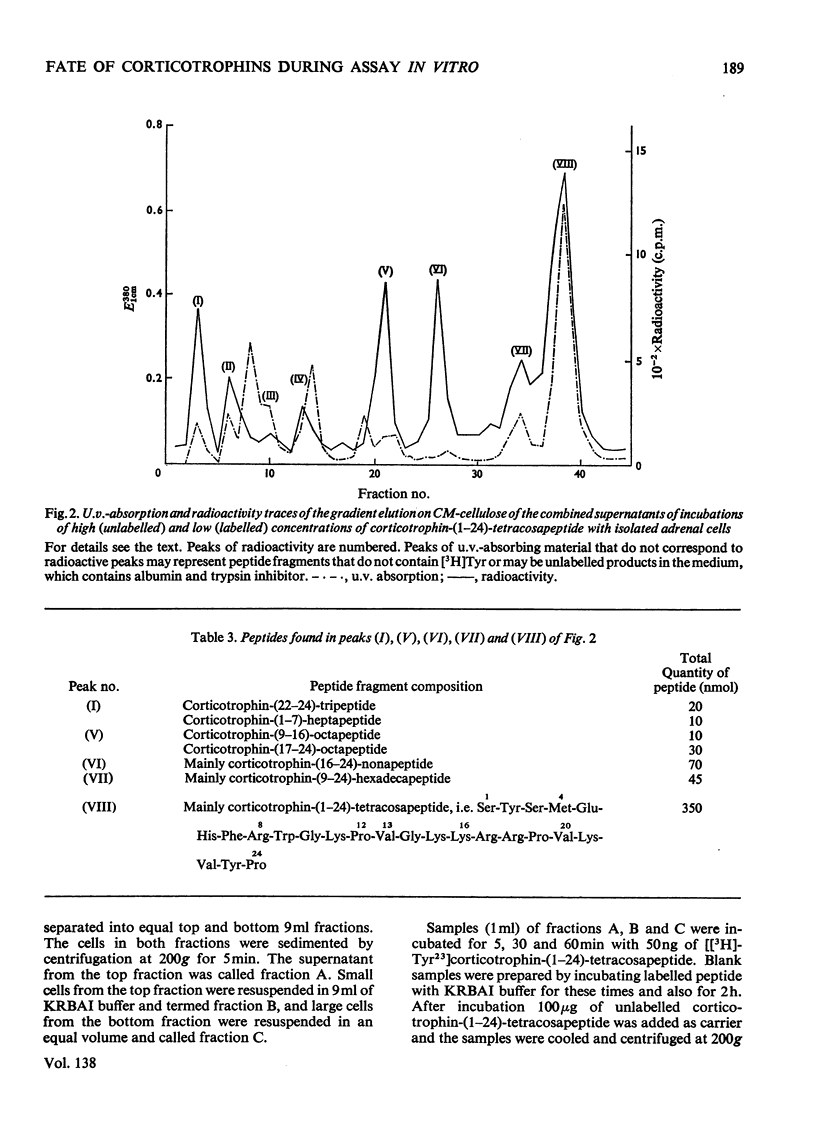
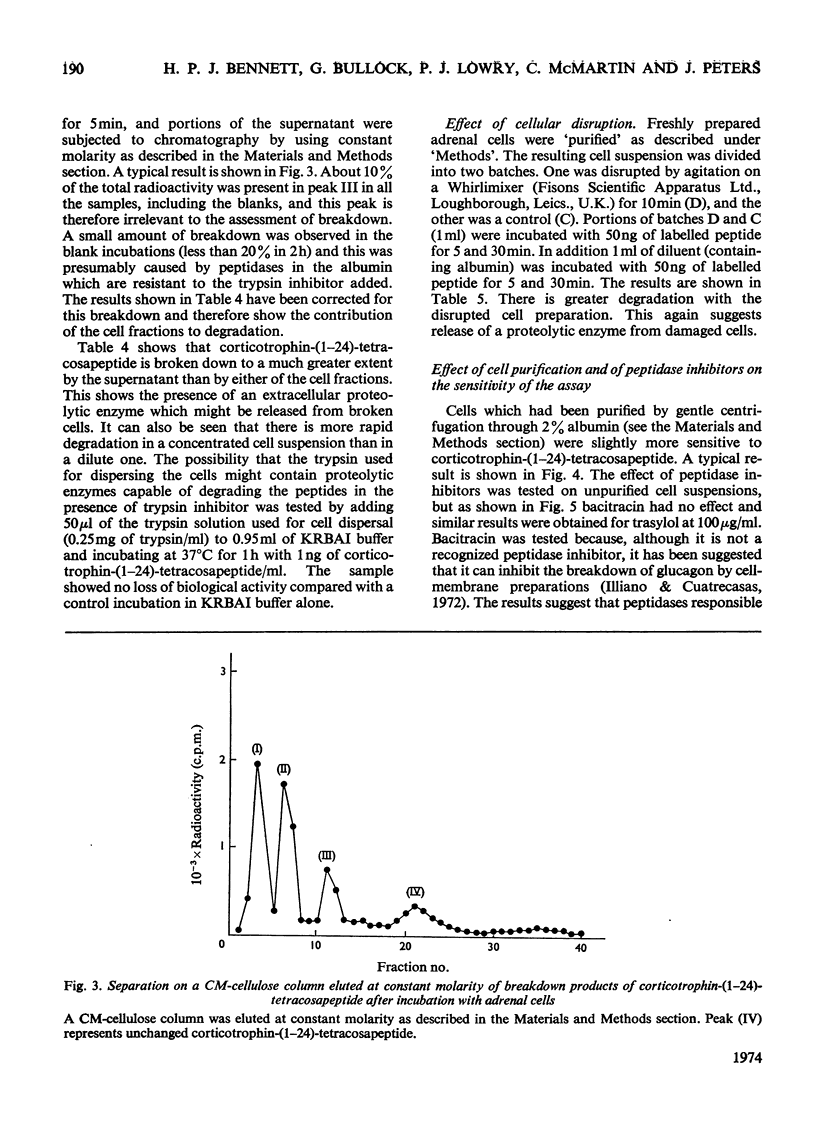
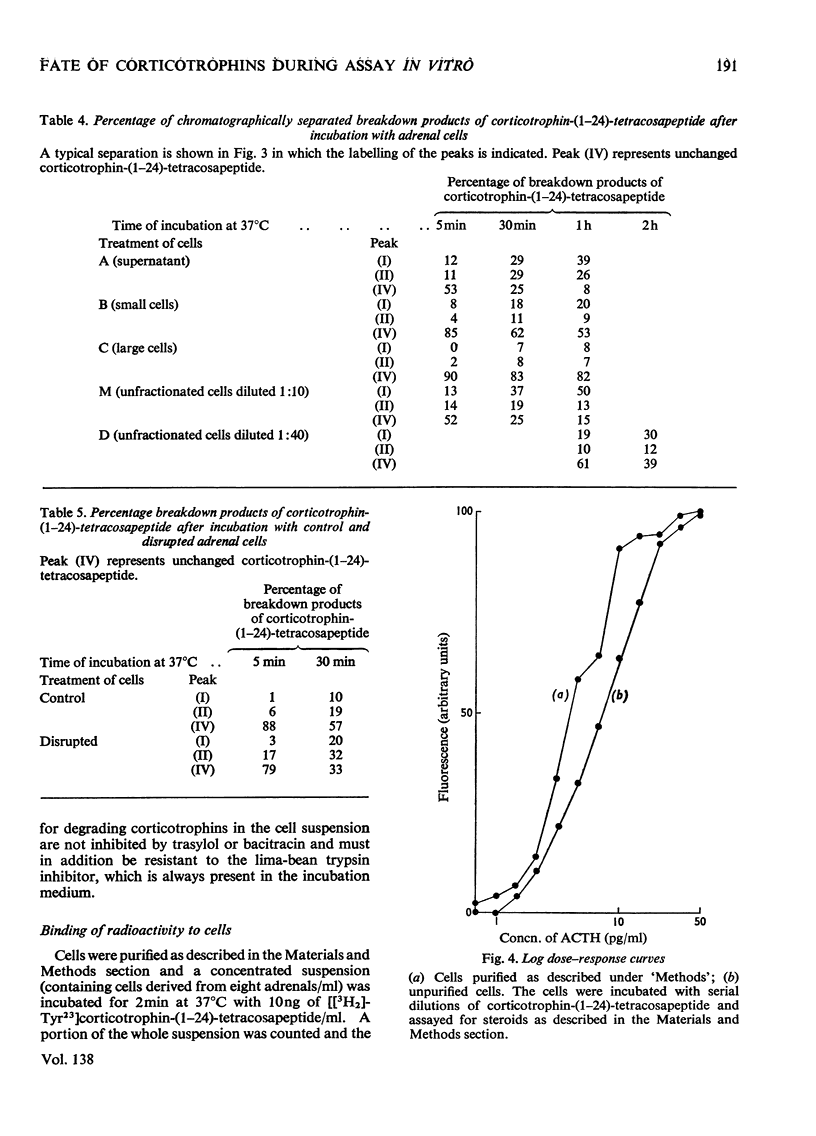
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett H. P., Lowry P. J., McMartin C. Confirmation of the 1-20 amino acid sequence of human adrenocorticotrophin. Biochem J. 1973 May;133(1):11–13. doi: 10.1042/bj1330011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illiano G., Cuatrecasas P. Modulation of adenylate cyclase activity in liver and fat cell membranes by insulin. Science. 1972 Feb 25;175(4024):906–908. doi: 10.1126/science.175.4024.906. [DOI] [PubMed] [Google Scholar]
- Kitabchi A. E., Sharma R. K. Corticosteroidogenesis in isolated adrenal cells of rats. I. Effect of corticotropins and 3',5'-cyclic nucleotides on corticosterone production. Endocrinology. 1971 May;88(5):1109–1116. doi: 10.1210/endo-88-5-1109. [DOI] [PubMed] [Google Scholar]
- Lowry P. J., McMartin C., Peters J. Properties of a simplified bioassay for adrenocorticotrophic activity using the steroidogenic response of isolated adrenal cells. J Endocrinol. 1973 Oct;59(1):43–55. doi: 10.1677/joe.0.0590043. [DOI] [PubMed] [Google Scholar]
- Riniker B., Sieber P., Rittel W., Zuber H. Revised amino-acid sequences for porcine and human adrenocorticotrophic hormone. Nat New Biol. 1972 Jan 26;235(56):114–115. doi: 10.1038/newbio235114b0. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., DE ROBERTIS E. D. Ultrastructural zonation of adrenocortex in the rat. J Biophys Biochem Cytol. 1961 Jan;9:105–119. doi: 10.1083/jcb.9.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULER W., SCHAR B., DESAULLES P. [On the pharmacology of an ACTH-active, fully synthetic polypeptide, beta1-24 corticotropin, Ciba 30920-Ba, Synacthen]. Schweiz Med Wochenschr. 1963 Jul 27;93:1027–1030. [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers G., Ma R. M., Giordano N. D. Isolated adrenal cells: corticosterone production in response to cyclic AMP (adenosine-3',5'-monophosphate). Proc Soc Exp Biol Med. 1971 Feb;136(2):619–622. doi: 10.3181/00379727-136-35325. [DOI] [PubMed] [Google Scholar]
- Swallow R. L., Sayers G. A technic for the preparation of isolated rat adrenal cells. Proc Soc Exp Biol Med. 1969 May;131(1):1–4. doi: 10.3181/00379727-131-33789. [DOI] [PubMed] [Google Scholar]