Abstract
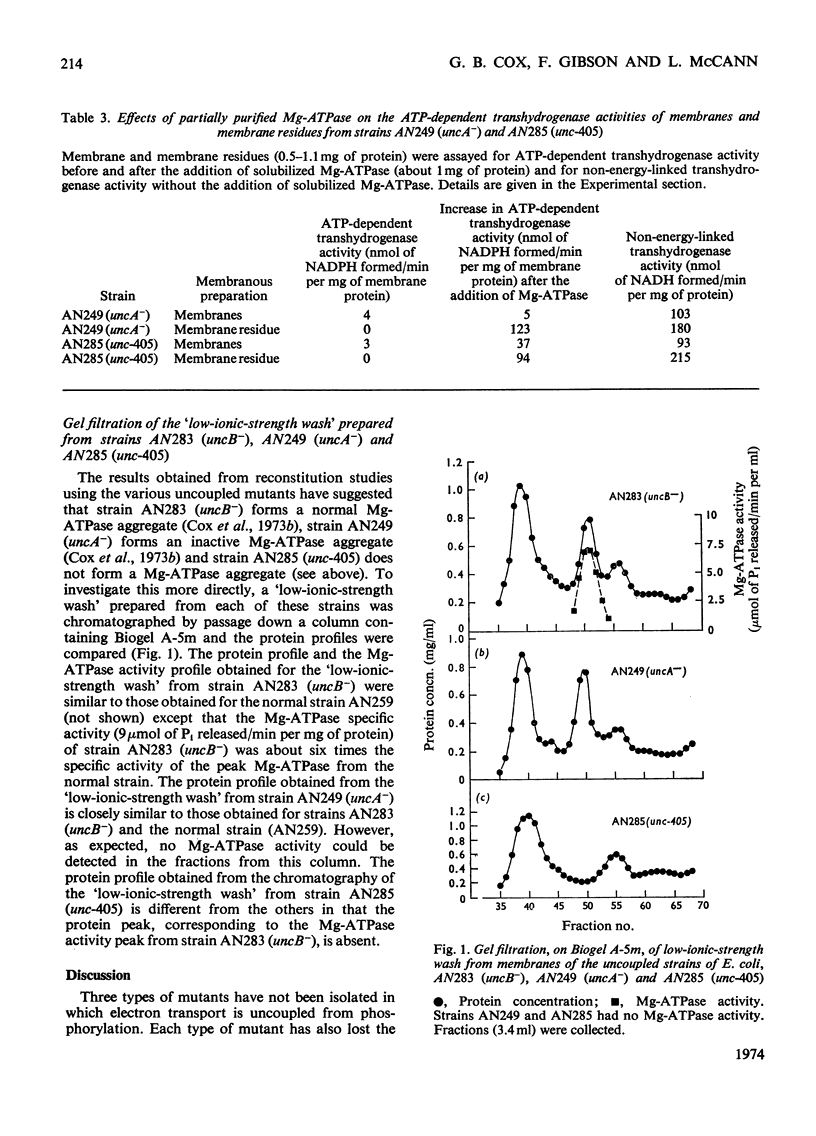
1. A new mutant strain (AN228) of Escherichia coli K12, unable to couple phosphorylation to electron transport, has been isolated. The mutant allele (unc-405), in strain AN228, was found to map near the uncA and uncB genes at about minute 74 on the E. coli genome. 2. A transductant strain (AN285) carrying the unc-405 allele is similar to the uncA and uncB mutants described previously in that it is unable to grow on succinate, gives a low aerobic yield on limiting concentrations of glucose, has a normal rate of electron transport, is unable to couple phosphorylation to electron transport, and lacks ATP-dependent transhydrogenase activity. 3. Strain AN285 (unc-405) is similar to an uncA mutant, but different from an uncB mutant, in that it is unable to grow anaerobically in a glucose–mineral-salts medium, and membrane preparations do not have Mg2+-stimulated adenosine triphosphatase activity. 4. Strain AN285 (unc-405) does not form an aggregate analogous to the membrane-bound Mg2+-stimulated adenosine triphosphatase aggregate found in normal cells. In this respect it differs from strain AN249 (uncA−), which forms an inactive membrane-bound Mg2+-stimulated adenosine triphosphatase aggregate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. M., Butlin J. D., Crane F. L. Reconstitution of the energy-linked transhydrogenase activity in membranes from a mutant strain of Escherichia coli K12 lacking magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1973 Apr;132(4):689–695. doi: 10.1042/bj1320689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. Reconstitution of oxidative phosphorylation and the adenosine triphosphate-dependent transhydrogenase activity by a combination of membrane fractions from unCA- and uncB- mutant strains of Escherichia coli K12. Biochem J. 1973 Aug;134(4):1015–1021. doi: 10.1042/bj1341015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Butlin J. D., Gibson F. The energy-linked transhydrogenase reaction in respiratory mutants of Escherichia coli K12. Biochem J. 1971 Nov;125(2):489–493. doi: 10.1042/bj1250489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. J., Sanadi D. R. Energy-linked nicotinamide adenine dinucleotide transhydrogenase in membrane particles from Escherchia coli. Biochim Biophys Acta. 1971 Aug 6;245(1):34–41. doi: 10.1016/0005-2728(71)90005-3. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Energy linked nicotinamide adenine dinucleotide transhydrogenase in a mutant of Escherichia coli K12 lacking membrane Mg(2+)&z.sbnd;Ca(2+)-activated adenosine triphosphatase. FEBS Lett. 1972 May 1;22(2):197–199. doi: 10.1016/0014-5793(72)80043-7. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Use of neomycin in the isolation of mutants blocked in energy conservation in Escherichia coli. J Bacteriol. 1972 Jul;111(1):287–289. doi: 10.1128/jb.111.1.287-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- PITTARD J. EFFECT OF INTEGRATED SEX FACTOR ON TRANSDUCTION OF CHROMOSOMAL GENES IN ESCHERICHIA COLI. J Bacteriol. 1965 Mar;89:680–686. doi: 10.1128/jb.89.3.680-686.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer H. U., Haddock B. A. -Galactoside accumulation in a Mg 2+ -,Ca 2+ -activated ATPase deficient mutant of E.coli. Biochem Biophys Res Commun. 1972 Aug 7;48(3):544–551. doi: 10.1016/0006-291x(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Shallenberger M. K. Coupling of energy to active transport of amino acids in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2663–2667. doi: 10.1073/pnas.69.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]


