Abstract
The intestinal microbiota plays a critical role in host immunity and might contribute to the significant variation between individuals’ vaccine responses. A systematic search was done using MEDLINE and Embase to identify original human studies investigating the association between intestinal microbiota composition and humoral and cellular vaccine responses. In total, 30 publications (26 studies, 14 in infants, 12 in adults), were included. Of these, 26 publications found an association between intestinal microbiota composition and vaccine responses. A beneficial effect of Actynomycetota (particularly Bifidobacterium) and a detrimental effect of Pseudomonadota (particularly Gammaproteobacteria) were observed across studies. Study designs were highly heterogenous, with variation in vaccine type, outcome measure, timing of stool analysis and analysis methods. Overall, studies support the concept that the composition of the intestinal microbiota influences vaccine responses. Further adequately powered studies are needed to confirm this association and inform potential microbiota-targeted interventions to optimise vaccine responses.
Subject terms: Medical research, Health care
Introduction
Vaccines save ~2–3 million lives per year1. Despite their effectiveness, vaccine responses are highly variable between individuals2,3. This has been documented for many types of vaccines, including conjugated pneumococcal (PCV), hepatitis B (HepB), inactivated influenza (IIV) and Bacillus Calmette–Guérin (BCG) vaccines4–6. Factors contributing to this variability include age (lower responses in infants and elderly)7–9, geographic location (lower responses in low-middle income countries (LMICs) compared with high-income countries (HICs))9–13 and comorbidities9. Variability in vaccine responses has been observed after both oral and parental vaccines and encompasses both humoral and cellular responses14. Understanding host factors that influence vaccine responses is key to implementing strategies to increase vaccine efficacy.
The intestinal microbiota has emerged as a key host factor that may modulate vaccine responses14,15. As with vaccine responses, the composition of the intestinal microbiota also varies with age, geographic location and comorbidities3. The intestinal microbiota is less stable and diverse during infancy and old age compared with late childhood and adulthood16. Several studies have reported important differences between those in LMICs and HICs in the diversity of intestinal microbiota and relative abundance of specific bacteria17–20.
In a systematic review in 2018, only four studies were identified that assessed the relationship between the composition of intestinal microbiota and vaccine responses. Despite the sparsity of studies, a higher relative abundance of Actynomycetota (previously known as Actinobacteria) was consistently associated with higher vaccine responses and a higher relative abundance of Bacteroidota (previous Bacteroidetes) with lower responses, while the association between the relative abundance of the phyla Bacillota (previously Firmicutes) and Pseudomonadota (previous Proteobacteria) and vaccine responses varied for different genera and species15. As there have now been many more studies on this topic, we present here an updated review of studies that investigated the association between the intestinal microbiota and both humoral and cellular vaccine responses.
Results
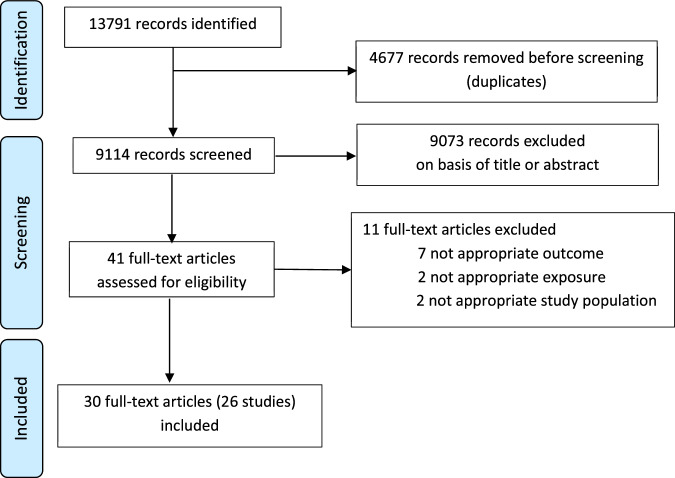
In total, 9114 records were identified. Of these, 30 (accounting for 26 studies) fulfilled the inclusion criteria and were included in the final analysis21–50 (Fig. 1). The characteristics of the studies are summarised in Table 1. Fourteen studies (16 publications) were done in infants and 12 (14 publications) in adults. Nine publications included less than 50 participants, 16 between 50 and 200 and 5 more than 200 participants. Most publications were from studies done in Asia (16 publications), mainly India, Bangladesh, and China, followed by Europe (6 publications), Africa (5 publications), North America (5 publications), New Zealand (2 publications), and South America (1 publication). Most of the studied vaccines were oral: rotavirus (ORV, 8 publications), poliovirus (OPV, 4 publications), cholera (OCV, 2 publications) and Salmonella typhi (1 publication). The remainder were parenteral: SARS-CoV-2 (7 publications), PCV, (3 publications), IIV (2 publications), and meningococcus C polysaccharide vaccine (MenC), poliovirus (IPV), tetanus, pertussis, DTaP/Hib (all together), hepatitis B (HBV) vaccines (1 publication each). To assess vaccine responses, most studies measured serum or plasma immunoglobulin (Ig) G (18 publications) or IgA (10 publications, all to ORV or OCV), followed by T-cell responses (5 publications), intestinal IgA (2 publications, OPV) and saliva IgG (1 publication). Stool samples were collected at different time points, but all studies included at least one sample before the first vaccine dose. Studies in infants collected the first stool sample between birth and 6 weeks of age (except one study investigating OPV in which stool samples were collected at 6 months of age). The stool analysis techniques used included 16S rRNA gene sequencing (18 publications), bacteria-specific polymerase chain reaction (PCR) (7 publications), shotgun metagenomic sequencing (10 publications), Human Intestinal Tract Chip (2 publications) and bacterial culture (1 publication). Seven publications used more than one technique.
Fig. 1. Selection of studies.
Flow diagram of identified, screened and included studies and articles, according to PRISMA guidelines.
Table 1.
Summary of findings of studies investigating the influence of the intestinal microbiota on vaccine responses (significant findings are indicated in bold)
| n | Study design# Level of evidence## |
Vaccine and schedule Age (adult studies) |
Time stool collected | Stool analysis technique | Time vaccine response measured | Main findings | Author Country Publication year |
|---|---|---|---|---|---|---|---|
| Children | |||||||
| 341 |
Multicentre birth cohort Level 4 |
ORV (pentavalent) 6, 10 w |
6, 10 w | Shotgun metagenomic sequencing, NovaSeq 6000 (Illumina), | 6, 14 w |
ORV responders (serum IgA at ≥20 IU/ml or >4x increase of titre): • lower alpha (Shannon index, p < 0.05) and beta diversity (Bray–Curtis distance, p < 0.005) among seronegative infants No differences in prevalence or abundance of individual genera |
Cunningham‑Oakes42 India, Malawi 2023 |
| 72 |
Multicentre (2 primary care clinics) birth cohort Level 4 |
DTaP/Hib and PCV13 2, 4, 6, 12 m |
2 m | Untargeted metagenomic sequencing HiSeq (Illumina) | 12 m |
DTaP/Hib and PCV plasma or serum IgG. Responders if >4x titre values protective thresholds (categorical) or as continuous • lower alpha diversity (evenness of genera, p = 0.04) for continuous IgG (for DTaP/Hib) No differences in prevalence or abundance of individual genera |
Shaffer43 USA 2023 |
| 472 |
Multicentre cohort Level 4 |
IPV or OPV, TT and pertussis Country-specific schedules |
1, 2, 3 m | qPCR for Bifidobacterium infantis and B. longum | 15 m |
Serum neutralising IgG log2 titres for poliovirus, tetanus and pertussis: • no association between absolute abundance and early colonisation of B. infantis or longum with tetanus and pertussis IgG titres • negative association between abundance of B. infantis and polio IgG titre (p < 0.05) |
Colston23 Bangladesh, Pakistan, Tanzania 2022 |
| 120 |
Single centre cohort Level 4 |
PCV10: 2, 3, 4, 11 m MenC: 12 m |
0, 7, 14 d 1, 2, 4, 6, 9, 12 m |
qPCR for E. coli, Klebsiella, Enterococcus plus VetMAX™ MastiType Multi Kit (samples at 7 d) 16S rRNA gene sequencing, V4, MiSeq (Illumina), RDP and SILVA v119 Subset (n = 20 at 7 d): shotgun metagenomic sequencing, NovaSeq (Illumina), MetaPhlAn2 |
PCV:12 m MenC: 18 m |
Saliva MenC-specific IgG levels (n = 66). High responses associated with' : • no association with alpha diversity (Shannon index), overall composition (Bray–Curtis index) and observed number of species • higher relative abundance of Lachnospiraceae at 2 m and Pseudobutyrivibrio, Lachnospira and Roseburia at 12 m (adjusted p < 0.05). • lower relative abundance of Bifidobacteriaceae, Veillonella and Klebsiella at 2 m (adjusted p < 0.05). Saliva pneumococcal 6B serotype-specific IgG (n = 101). High response associated with (stools from 2 m): • no association with alpha (Shannon index), or beta diversity (Bray–Curtis index), inverse correlation with number of species. • higher relative abundance of Esch/Shigella, Bifidobacterium, Bacteroides, several Ruminococcaceae OTUs, and Streptococcus bovis (adjusted p < 0.05) • lower relative abundance of several Bacillota OTUs, Enterobacteriaceae, Prevotella, Bifidobacterium bifidum and Garnerella (adjusted p < 0.05). |
deKoff24 Netherlands 2022 |
| 155 |
Multicentre cohort Level 4 |
PCV13 and TT 2, 4, 6 m |
6 w |
16S rRNA gene sequencing, V4, V5, MiSeq (Illumina), SILVA Subset (n = ?): shotgun metagenomic sequencing, NextSeq (Ilumina), MetaPhlAn3 |
12 m |
Above median responses to PCV (serum IgG) • no association with beta diversity (Bray–Curtis dissimilarity) • no association with abundance of specific taxa Above median responses to TT (serum IgG) • no association with beta diversity (Bray–Curtis dissimilarity). • lower abundance Aeriscardovia aeriphila (adjusted p < 0.05) |
Moroishi33 USA 2022 |
| 158 |
Single centre cohort, nested in RCT (hygiene and feeding measures) Level 4 |
ORV (monovalent)6, 10 w | 4, 12 w | Shotgun metagenomic sequencing, HiSeq 2500 or HiSeqX (Illumina), MetaPhlAn3 | 4, 12 w (median 43 d) |
ORV responders (serum IgA > 20 UI/mL) • no difference in alpha diversity (Shannon index and number observed species), beta diversity (Bray–Curtis distance), and functional composition • higher relative abundance of Bacteroides thetaiotaomicron and Slackia isoflavoniconvertens (p < 0.05) |
Robertson39 Zimbabwe 2021 |
| 86 |
Multicentre birth cohort Level 4 |
ORV (pentavalent) India, Malawi: 6, 10 w UK: 8, 12 w |
6, 10 w 8, 12 w |
16S rRNA gene sequencing, V3-V4, HiSeq or MiSeq (Illumina), SILVA v132 |
6, 14 w 8, 16 w |
ORV responders (serum IgA at ≥20 IU/ml or >4x increase of titre): • UK: no difference in alpha diversity (Shannon index) or beta diversity (Bray–Curtis distance) • India & Malawi: lower alpha (Shannon index, p < 0.05) and beta diversity (Bray–Curtis distance, p < 0.005) among seronegative infants No differences in prevalence or abundance of individual genera |
Parker36 UK, India, Malawi 2021 (same as42) |
| 45 |
Single centre cohort Level 4 |
ORV (pentavalent) 2 m |
2 m | 16S rRNA gene sequencing, V4, MiSeq (Illumina), Greengenes | 3 m |
ORV responders (serum IgA 4-fold increase): • no association with alpha diversity (Shannon index) or richness • higher relative abundance of Eggerthella (p = 0.02, FDR = 1) • lower relative abundance of Enterobacteriaceae (p = 0.03, FDR = 1) |
Fix26 Nicaragua 2020 |
| 107 |
Single centre cohort nested in RCT (different OPV vaccination schedules) Level 4 |
OPV 2, 3, 4 m IPV-bOPV-bOPV or IPV-IPV-bOPV or IPV-IPV-tOPV |
3, 3.5, 4 m | 16S rRNA gene sequencing, V4, MiSeq (Illumina), SILVA | 3, 3.5, 4 m |
Responders (faecal IgA≥20 IU/ml or 4x increase of titre) at 0 d: • lower alpha (Shannon index, p = 0.0478) and beta diversity (UniFrac distance, p = 0.024) • lower relative abundance of Bacillota (p < 0.001) and higher relative abundance of Actynomycetota (p < 0.01) • lower relative abundance of Clostridia (p < 0.001) and higher relative abundance of unidentified Actynomycetota (p < 0.05) • lower relative abundance of Clostridiales (p < 0.001) and higher relative abundance of unidentified Bifidobacteriales (p < 0.05) • lower relative abundance of Clostridium sensu stricto (p < 0.01) |
Zhao41 China 2020 |
| 291 |
Single centre cohort nested in RCT (vitamin A supplement) Level 4 |
OPV: 0, 6, 10, 14 w TT-HBV: 6, 10, 14 w BCG: 0 w |
6, 11, 15 w |
Bifidobacterium-specific PCR and T-RFLP 16S rRNA gene sequencing, V4, MiSeq (Ilumina), Greengenes May 2013 |
6, 15 w, 2 y |
Higher BCG-specific T-cell responses at 15 w and 2 y: • higher relative abundance of Bifidobacterium at 6,11,15 w (p < 0.05) • higher relative abundance of B. longum and B. longum subspecies infantum at 6w (p < 0.05) (only associated with response at 15 w) Higher tetanus-specific T cell responses at 15 w and 2 y: • higher relative abundance of Bifidobacterium at 6,11,15 w (p < 0.05) • higher relative abundance of B. longum and B. longum subspecies infantum at 6w (p < 0.05) (only associated with response at 15 w) Higher tetanus-specific IgG levels in serum at 15 w and 2 y: • higher relative abundance of Bifidobacterium at 6,11,15 w (p < 0.05) (only associated with response at 2 y) • higher relative abundance of B. longum at 6w (p < 0.05) (only associated with response at 15 w) Higher HepB-specific T cell responses at 15 w: • higher relative abundance of Bifidobacterium at 6,11,15 w (p < 0.05) • higher relative abundance of B. longum at 6w (p < 0.05) Higher HepB-specific IgG levels in serum at 15 w or 2 y: • lower relative abundance of B. breve at 6w (p < 0.05) (only associated with response at 15 w) • lower relative abundance of B. longum subspecies longum at 6w (p < 0.05) (only associated with response at 2 y) Higher polio-specific faecal IgA at 2 years (serotypes 1–3) • higher relative abundance of Bifidobacterium at 6,11,15 w (p < 0.05) Higher polio-specific plasma IgA at 2 years • higher relative abundance of Bifidobacterium and B.longum at 6w (p < 0.05) (Serotype 3) • lower relative abundance of B.breve at 6w (p < 0.05) (Serotype 1) Higher polio-specific plasma IgG at 15 w or 2 y • higher relative abundance of Bifidobacterium at 6,11,15 w and B.bifidum at 6 w (p < 0.05) (all strains, only associated with response at 2 years). • lower relative abundance of B.longum longum at 6w (p < 0.05) (all strains, only associated with response at 2 years). |
Huda31 Bangladesh 2019 |
| 120 |
Single centre cohort nested in RCT (azithromycin on immunogenicity of OPV) Level 4 |
OPV (mOPV3) Single dose at 6 to 11 m |
2w before and on vaccination day |
16S rRNA gene sequencing, V4, MiSeq (Ilumina), RDP Pathogens: real-time PCR Taqman array card |
3w after vaccination day |
Responders (serum neutralising IgG ≥1:8): • no difference in relative abundance of specific bacterial taxa Non-vaccine-shedders had higher numbers of bacterial taxa (number of OTUs, p < 0.05), higher diversiy (Shannon index, p < 0.05) and higher relative abundance of Clostridia (p = 0.04) |
Praharaj38 India 2019 |
| 30 |
Single centre matched case-control, nested in RCT (different ORV dosing) Level 3 |
ORV (movalent) 6, 10, 14 w or 6, 10 w or 10, 14 w |
Before vaccination and 7 d after last dose | Human Intestinal Tract Chip (Microarray) | Before vaccination and 28 d after last dose |
Responders (serum IgA ≥ 20 IU/mL): • higher relative abundance of Bacillota (Clostridium cluster XI) (p = 0.02, FDR = 0.36) and Pseudomonadota (p = 0.04, FDR = 0.36) • higher relative abundance of Gram negative bacteria related to Serratia (p = 0.01, FDR = 0.19) and E. coli (p < 0.01, FDR = 0.05) |
Harris27 Pakistan, Netherlands 2018 |
| 325 |
Single centre case-control, nested in RCT (zinc and probiotic supplement) Level 3 |
ORV (movalent) plus OPV 6, 10 w |
6, 10 w |
Pathogens: real-time PCR Taqman array card 16S rRNA gene sequencing, V4, MiSeq (Ilumina), RDP |
6, 14 w |
Responders (serum rotavirus IgA 20 ≥ IU/ml or >4x increase of titre): • no differences in composition or diversity (Shanon index for alpha diversity, Unifrac distances for beta diversity) • more likely to harbour ≥ 1 bacterial enteropathogen (26 vs 13%, p = 0.006) at dose 1 (not dose 2) Rotavirus shedding was associated with a higher number of bacterial taxa (greater OTU count) |
Parker37 India 2018 |
| 78 |
Single centre matched case-control nested in RCT (different ORV dosing) Level 3 |
ORV (monovalent) 6, 10, 14 w or 6, 10 w |
6 w | Human Intestinal Tract Chip (Microarray) | 12 w |
Responders (serum IgA ≥ 20 IU/mL): • no difference in alpha diversity (Shannon index) (p = 0.87) • higher Enterobacteria-Bacteroides ratio (p = 0.04) • lower relative abundance of Bacteroidota (FDR = 0.003), and several Bacteroides and Prevotella species (FDR = 0.03-0.1) • higher relative abundance of Bacillota (FDR = 0.027) and Streptococcus bovis (FDR = 0.008) |
Harris28 Ghana 2016 |
| 48 |
Single centre cohort nested in RCT (vitamin A supplement) Level 4 |
OPV: 0, 6, 10, 14 w TT-HBV: 6, 10, 14 w BCG: 0 w |
6, 11, 15 w |
Bifidobacterium-specific PCR and T-RFLP 16S rRNA gene sequencing, V4, MiSeq (Ilumina), ns |
15 w |
Higher BCG-, polio-, TT- and HBV-specific T cell responses: • higher relative abundance of Actynomycetota (B. longum) (except HBV), Bifidobacteriales and Bifidobacteriaceae (except HBV, OPV), and Bifidobacterium and Corynebacterium (only TT), and Coriobacteriales and Coriobacteriaceae (only OPV) (p < 0.05). • higher relative abundance of Porphyromonadaceae (OPV), Enterococcaceae (OPV and BCG) and Enterococcus (BCG) (p < 0.05). • higher relative abundance of β-Proteobacteria and Burkholderiales (BCG); and Pseudomonaceae (TT) (p < 0.05) • lower relative abundance Bacillota, Clostridia, Clostridiales and Lactobacillaceae (TT); Veillonella (OPV) and Lactococcus (OPV and BCG) (p < 0.05). • lower relative abundance of Pseudomonadales, Moraxellaceae (except OPV) and Acinetobacter (except HBV and OPV); γProteobacteria, Enterobacteriales, Enterobacteriaceae, Escherichia and Shigella (TT), Pseudomonaceae (OPV) (p < 0.05) Higher HBV-, polio-, and TT-specific serum IgG levels and DTH skin-test response for tuberculosis: • higher relative abundance of Actinomycetales (BCG and TT) and Actinomyces (TT); Micrococcaceae and Rothia (BCG and HBV); Bidifobacteriales, Bifidobacteriaceae (BCG) and Bifidobacterium (BCG and OPV) (p < 0.05). • lower relative abundance of Prevotellaceae and Prevotella (BCG) (p < 0.05). • lower relative abundance of Staphylococcaceae (OPV) and Staphylococcus (TT); Carnobacteriaceae and Lachnospiraceae (HBV); Clostridium XI, Finegoldia, Peptoniphilus, Megasphaera (BCG) (p < 0.05). • lower relative abundance of Pseudomonadota, βProteobacteria and γProteobacteria, Enterobacteriales and Enterobacteriaceae (BCG); Pseudomonales, Moraxellaceae and Acinetobacter (OPV). |
Huda32 Bangladesh 2014 (subgroup of31) |
| 20 |
Single centre cohort nested in RCT (fermented vs standard infant formula) Level 3 |
DTaP-IPV/Hib: 8, 12, 16 w 9 fed bifidogenic formula |
4, 8, 12, 16 w |
Bacterial culture Multiplex PCR for Bifidobacterium |
16 w |
Higher polio-specific faecal IgA levels: • detectable levels of B. longum subspecies B. infantis at 16 w (p < 0.002) |
Mullie34 France 2004 |
| Adults | |||||||
| 36 |
Multicentre cohort Level 4 |
Intramuscular SARS-CoV-2 vaccine (inactivated) 0*, 28 d, 7 m Mean 53 years (IQR: 48-56) |
Before 0* d | Shotgun metagenomic sequencing, NovaSeq 6000 (Illumina), MetaPhlAn3 | 12* m |
Serum neutralising antibodies high responders (virus microneutralization assay titre ≥1:10). • lower relative abundance of Eubacterium rectale (p = 0.002), Collinsella aerofaciens (p = 0.038), and Streptococcus salivarius (p = 0.021). |
Zhang47 Hong Kong 2024 |
| 14 |
Single centre cohort Level 4 |
Intramuscular SARS-CoV-2 vaccine (mRNA BNT162b2 and mRNA-1273) 0*, 21-28 d Mean 30 years (range 18-48) |
Before 0* d | 16S rRNA gene sequencing, MiSeq (Illumina), SILVA | 0*, 14, 28-35 d |
Higher anti-SARS-CoV-2 spike IgG levels (28-35 d, as continuous) • higher alpha (Shannon index, p = 0.048) and beta diversity (Jaccard distances, p = 0.019) • higher relative abundance of Desulfobacterota, Bilophila and Oscillospiraceae and Alistipes (FDR < 0.05) • lower relative abundance of Colidextribacter, Clostridium innocuum, Lachnoclostridium, genus UCG 004 (Lachnospiraceae) and Bacteroides (FDR < 0.05) |
Daddi48 USA 2023 |
| 75 | Single centre cohort nested in RCT for SARS-CoV-2 BNT162b2 vaccine Level 4 |
Intramuscular SARS-CoV-2 vaccine (mRNA BNT162b2) 0*, 21 d Median 54 years, (IQR: 38-64) |
0* d | 16S rRNA gene sequencing, MiSeq (Illumina), SILVA v132 | 0*, 35 d |
High anti-SARS-CoV-2 spike IgG responders day 35 • lower alpha (Shannon index, FDRadjusted = 0.009) and beta diversity (NMDS, p < 0.01) • higher relative abundance of Bacteroidota (p < 0.01), Bacteroides, Sutterella, and Lachnospiraceae FCS020 group (all p < 0.05) • lower relative abundance of Bacillota (p < 0.01), several Ruminococcaceae Alloprevotella, Anaerofilum, Succinivibrio, Moryella, and Negativibacillus (all p < 0.05) High spike CD4 + T-cell response • lower alpha (Shannon index, FDRadjusted = 0.003) and beta diversity (NMDS, p < 0.01) • higher relative abundance of Lactobacillaceae (p = 0.01) and Lactobacillus (p = 0.014) • lower relative abundance of Akkermansiaceae, Akkermansia, Ruminiclostridium, Hydrogenoanaerobacterium and Marvinbryantia (all p < 0.05) |
Ray50 Sweden 2023 |
| 127 |
Multicentre cohort Level 4 |
Intramuscular SARS-CoV-2 vaccine (mRNA BNT162b2 or inactivated) Schedule ns, 2 doses mRNA BNT162b2: median 42 years (IQR: 29-54) Inactivated: median 55 years (IQR: 40-57) |
0* to 3d, 28 d after last dose | Shotgun metagenomic sequencing, NovaSeq 6000 (Illumina), MetaPhlAn4 | 0* to 3d, 28 d, 6 m after last dose |
Serological tests: plasma surrogate virus neutralisation test and spike receptor-binding domain IgG at 6 m after 2nd dose High responders to mRNA BNT162b2 • higher relative abundance of several Lachnospiraceae and other Clostridia, Bifidobacterium adolescentis, B. bifidum, Parasutterella excrementihominis and Fusobacterium ulcerans (p < 0.05) • lower relative abundance of Clostridiaceae, Roseburia, Latilactobacillus sakei and Alistipes dispar (p < 0.05) High responders to inactivated vaccine • higher relative abundance of several Lachnospiraceae, Oscillospiraceae and Clostridiaceae, Solobacterium,Phocaeicola dorei, Actynomyces, and Rikenellaceae bacterium (p < 0.05) • lower relative abundance of several Bacteroidales, Lachnospiraceae, Oscillospiraceae and Clostridiaceae, Intestinimonas butyticiproducens, Emergencia timonensis and Citrobacter freundii (p < 0.05) |
Peng49 Hong Kong 2023 |
| 86 |
Single centre cohort Level 4 |
Intramuscular SARS-CoV-2 vaccine (mRNA BNT162b2) 0*, 22 d Mean 52 years (range: 20-81) |
0* d | 16S rRNA gene sequencing, V3, V4, MiSeq (Illumina), SILVA v119 | 24*, 30, 63 d |
High anti-SARS-CoV-2 spike IgG responders (value for definition not specified) • No association with alpha diversity (Shannon index) • No association with specific bacterial taxa when adjusted for age, sex and timing of stool sampling High T-cell responders (value for definition not specified) • No association with alpha diversity (Shannon index) • No association with specific bacterial taxa when adjusted for age, sex and timing of stool sampling |
Hirota30 Japan 2023 |
| 138 |
Multicentre cohort Level 4 |
Intramuscular SARS-CoV-2 vaccine (mRNA BNT162b2 or inactivated) Schedule ns, 2 doses Median 47 years (range: 18-67) |
0* to 3d, 28 d after last dose | Shotgun metagenomic sequencing, NovaSeq 6000 (Illumina), MetaPhlAn3 | 0* to 3d, 28 d after last dose |
Serological tests: plasma surrogate virus neutralisation test and spike receptor-binding domain IgG at 28 d after 2nd dose High responders to mRNA BNT162b2 • higher relative abundance of Eubacterium rectale, Roseburia faecis, Bacteroides thetaiotaomicron and Bacteroides OM05-12 (p < 0.05) High responders to inactivated vaccine • higher relative abundance of Bifidobacterium adolescentis (p < 0.05) • lower relative abundance of Bacteroides vulgatus, Bacteroides thetaiotaomicron and Ruminococcus gnavus (p < 0.05) |
Ng35 Hong Kong 2022 (same as49) |
| 207 |
Single centre cohort Level 4 |
Intramuscular SARS-CoV-2 vaccine (inactivated BBIBP-CorV) 0*, 28 d Median 34 years (IQR: 29-46) |
0*, 14, 42 d | Shotgun metagenomic sequencing, NovaSeq 6000 (Illumina) | 0*, 14, 42 d |
High ACE2-RBD inhibiting antibody at day 42 • No association with alpha diversity (Shannon index) • higher relative abundance of Collinsella aerofaciens, Fusicatenibacter saccharivorans, Eubacterium ramulus, and Veillonella dispar (p < 0.05) • lower relative abundance of Lawsonibacter asaccharolyticus (p < 0.05) |
Tang45 China 2022 |
| 27 | Single centre cohort nested in RCT (new OCV vaccine Phase I) Level 4 |
OCV (Oral MucoRice CTB) 1 dose Mean 29 years (range 20-40) |
0*, 2, 4, 6, 8, 16 w | Shotgun metagenomic sequencing, HiSeq 2500 (Illumina) | 0*, 2, 4, 6, 8, 16 w |
Responders (≥4-fold increase of titre of serum CTB IgA and IgG) • higher beta diversity (Unifrac distances, p = 0.044), no difference in alpha diversity • higher relative abundance of Shigella dysentariae (p = 0.018), S. flexneri (p = 0.032), S. sonnei (p = 0.037), Anaerobaculum mobile (p = 0.001) and Bacillus licheniformis (p = 0.002) • lower relative abundance of Bacteroides |
Yuki46 Japan 2021 |
| 69 |
Single centre cohort nested in RCT (OCV stored at different temperatures) Level 4 |
OCV 0* d (sd) or 0, 14 d (td-14) or 0, 30 d (td-30) Mean 29 years (range 18-44) |
0* d | 16S rRNA gene sequencing, V4, MiSeq (Illumina), SILVA v138 |
All: 0*, 3, 7, 90 d Additionally: 30d (sd); 17, 42, 180 d (td-14); 60, 180 d (td-30) |
V. cholerae O polysaccharide-specific response (plasma IgA and IgG ≥4-fold change): • no association with gut microbiota diversity (phylum level abundance or alpha diversity by inverse Simpson, and beta diversity by Bray-Curtis distance) • higher relative abundance of Clostridiales (Sarcina and Clostridiales sensu stricto species) (p < 0.001) • lower relative abundance of Enterobacterales (p < 0.01) |
Chac22 Bangladesh 2021 |
| 122 |
Single centre cohort Level 4 |
IM inactivated trivalent influenza vaccine 1 dose Mean 35 years (range 18-64) |
0*, 28 d | 16S rRNA gene sequencing, V1-V3, MiSeq (Illumina), RDP | 0*, 28, 180 d |
High responder (≥4-fold increase in serum IgG titre against H1 and H3): • no differences in diversity (Shanon index for alpha diversity, Bray-Curtis index for beta diversity) • higher relative abundance of Bacillota (several OTUs), Alphaproteobacteria, Sutterella, Parabacteroides (H1N1) (p < 0.05) • higher relative abundance of Bacillota (several OTUs), Bifidobacterium bifidum, Prevotella and cyanobacteria (H3N2) (p < 0.05) • lower relative abundance of Bacillota (several OTUs) and Butyricimonas (H1N1) (p < 0.05) • lower relative abundance of Bacillota (several OTUs) and H. parainfluenzae (H3N2) (p < 0.05) In naive influenza vaccination: high response to H1N1 and H3N2 associated with higher relative abundance of Bacillota (several OTUs) (p < 0.05) |
Cait21 New Zealand 2021 |
| 15 |
Single centre cohort Level 4 |
HBV 0*, 28, 180 d ns (range: 44-73 years) |
-14, 0*, 14 d | 16S rRNA gene sequencing, V4, MiSeq (Illumina), | -14*, 28, 180, 208 d |
Higher Anti-HBsAg plasma IgG at 208 d • higher relative abundance of Butyricicoccus, Clostridium, Phascolarctobacterium and Lachnospiraceae (p < 0.05) • lower relative abundance of Bacteroidota, Desulfovibrionales, Bacteroides, Atopobium, Gardenerella, Clostridiales, Dialister, Blautia, Desulfovibrionales and Fusobacterium (p < 0.05) |
Shannon44 Canada 2020 |
| 125 |
Single centre cohort (feasibility study) Level 3 |
IM inactivated trivalent influenza vaccine 1 dose Mean 35 years (range: 18-64) |
0*, 28 d | 16S rRNA gene sequencing, V4, MiSeq (Illumina), RDP | 0*, 28, 180 d | No association between intestinal microbiota enterotypes and serum influenza-specific IgG response (not powered for this outcome, feasibility study) |
Shortt40 N. Zealand 2018 (Same as21) |
| 66 |
Single centre cohort nested in RCT (broad vs narrow spectrum antibiotics vs control) Level 4 |
ORV (monovalent) 1 dose Mean 24 years (SD: 4-4.5) |
0* d and before antibiotic | 16S rRNA gene sequencing, V4, MiSeq (Illumina), RDP | 7*, 14, 28 d |
Rotavirus IgA boosting ( ≥ 2-fold increase in rotavirus-specific serum IgA titre) and shedders • higher relative abundance of Prevotellaceae and Porphyromonadaceae and Ruminococcaceae (p < 0.05) • lower relative abundance of Fusobacteriaceae and Erysipelotrichaceae (p < 0.05) |
Harris29 Netherlands 2018 |
| 13 |
Single centre non-randomised trial Level 3 |
Oral Salmonella typhi live-attenuated vaccine (Ty21a) Schedule ns n = 6: 4 doses n = 7: 1 dose Median 26 years (range: 19-50) |
- 7, 0*, 2, 4, 7, 10, 14, 28, 42, 56 d | 16S rRNA gene sequencing, V1, V2, Roche 454 (Roche diagnostics), Greengenes March 2011 | - 7, 0*, 2, 4, 7, 10, 14, 28, 42, 56 d |
Multiphasic cell-mediated immune response • more diverse, complex bacterial communities: higher alpha diversity (inverted Simpson index (p < 0.0001), Shannon index (p < 0.0001)) and phylogenetic diversity (p < 0.0001) (mostly within order Clostridiales, predominantly within Lachnospiraceae and Ruminococcaceae (q value < 0.05). Responders (≥4x increase of titre of serum IgA and IgG) • no difference in overall community diversity (Shannon, inverted Simpson and phylogenetic diversity) |
Eloe-Fadrosh25 USA 2013 |
BCG Bacille-Calmette-Guérin vaccine, CTB cholera toxin B, d days, DTaP-IPV/Hib diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis-H. influenzae type b vaccine, FDR false discovery rate, HBV Hepatitis B vaccine, IM intramuscular, Ig immunoglobulin (G/A), IPV intramuscular poliovirus vaccine, IQR interquartile range, m months, MenC meningococcus C polysaccharide vaccine, ns not specified, OCV oral cholera vaccine, OPV (t/b) oral poliovirus vaccine (trivalent/bivalent), ORV oral rotavirus vaccine, OTU operational taxonomic unit, (q)PCR (quantitavie) polymerase chain reaction, PCV pneumococcal conjugate vaccine, RCT randomised controlled trial, RDP ribosomal database project, RNA ribonucleic acid, SARS-CoV-2 Severe acute respiratory syndrome coronavirus 2, sd single dose, SD standard deviation, td(-14/30) two doses, second after 14 or 30 days, T-RFLP terminal restriction fragment length polymorphism, TT tetanus vaccine, UK United Kingdom, w weeks. # They are all prospective studies; * 0 = day 1st dose; ## Oxford Centre for Evidence-Based Medicine 2011 Levels of Medicine **(A/Victoria/4897/2022 (H1N1)pdm09-like strain, A/Darwin/9/2021 (H3N2)-like strain, B/Austria/1359417/2021-like strain, B/Phuket/3073/2013 like strain).
All included publications had an overall risk of bias score (JBI standardised critical appraisal checklist, yes%) over 60% (acceptable quality), and 80% (24/30) of publications had an overall score ≥80% (good quality) (Supplementary Table 2). The most frequent risk of bias was a lack of identification of confounding factors and strategies to address these (present in 57% (17/30) studies). Some studies did not describe clearly how many children completed follow-up or the reasons for lost to follow-up. In some of the adult studies, ≥4-fold increase in antibody levels were used to overcome the difficulty of including seronegative or vaccinated naive subjects.
In total, 87% (26/30) of the publications reported an association between the composition of the intestinal microbiota and vaccine responses, though 3 of them found only an association with alpha or beta diversity and not with relative abundance of specific bacteria. We did not observe important differences between the studies assessing oral vaccines and those assessing parenteral vaccine responses. The findings are summarised in Tables 1 and 2, and in Supplementary Table 3.
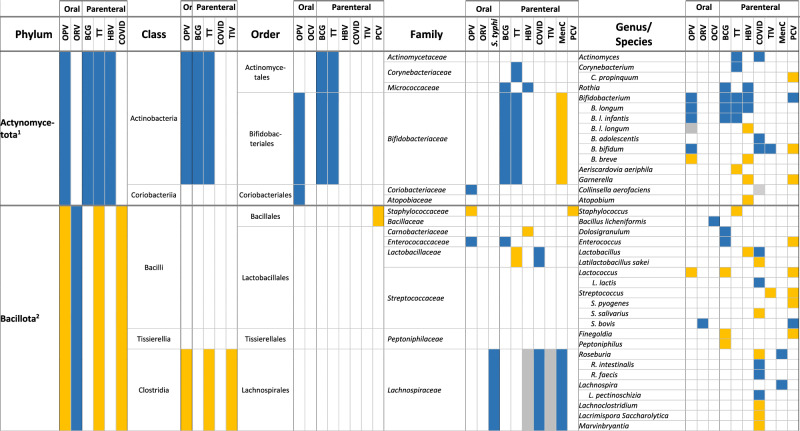
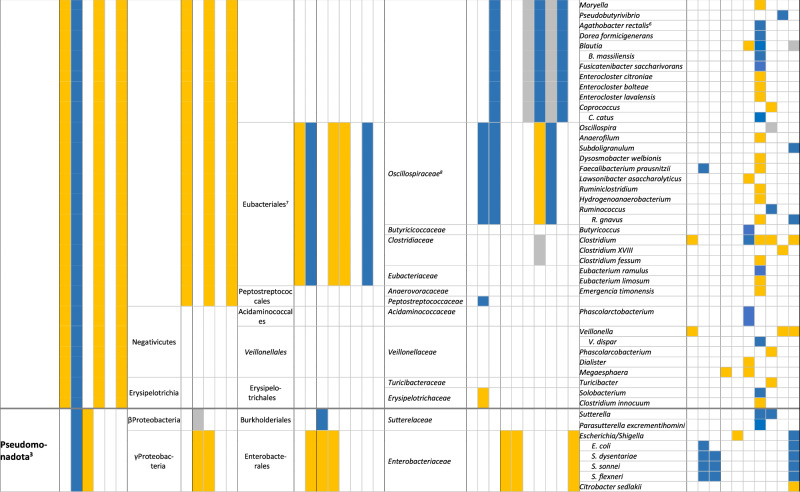
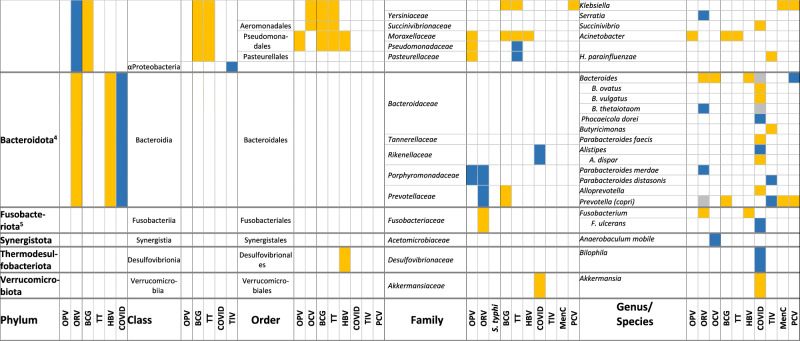
Table 2.
Associations between the composition of the intestinal microbiota at different taxonomic levels and vaccine responses (colour coding: higher relative abundance associated with higher vaccine response (blue); lower vaccine response (yellow), inconsistent findings (grey); all p < 0.05)
BCG Bacille-Calmette-Guérin vaccine, COVID SARS-CoV-2 vaccine, HBV HepB vaccine, MenC meningococcus C polysaccharide vaccine, OCV oral cholera vaccine, OPV oral poliovirus vaccine, ORV oral rotavirus vaccine, PCV pneumococcal conjugate vaccine, S. typhi: oral Salmonella typhi vaccine, TIV trivalent influenza vaccine, TT tetanus vaccine
1: Previous Actinobacteria; 2: Previous Firmicutes; 3: Previous Proteobacteria; 4: Previous Bacteroidetes; 5: Previous Fusobacteria; 6: Previous Eubacterium rectale; 7: Previous/other: Clostridiales; 8: Previous Ruminocaccaceae.
Oral vaccines
Six publications in infants26–28,36,37,39,42 and one in adults29 reported on the association between the composition of the intestinal microbiota and serum IgA response to ORV, defining high responders as an IgA titre of ≥20 IU/mL or a ≥4-fold increase in titre (Tables 1 and 2). Five studies used the monovalent ORV vaccine27–29,37,39 and three the pentavalent26,36,42. Two publications reporting findings from the same study found a lower alpha and beta diversity of the intestinal microbiota among responders, but only in India and Malawi and not in the UK36,42. In the other studies, a higher relative abundance of Bacillota, Ruminococcaceae and Peptostreptococcaceae, Streptococcus bovis and Faecalibacterium prausnitzii and a lower relative abundance of Erysipelotrichaceae and Clostridium XVII were associated with ORV vaccine responders (all p < 0.05)27–29. Furthermore, a higher relative abundance of Pseudomonadota (Escherichia coli and Serratia), and Bacteroidota (Porphyromonadaceae and Prevotellaceae, and Bacteroides thetaiotaomicron) were associated with responders (p < 0.05), while for Bacteroides and Prevotella contradicting findings were reported27–29,39. A lower relative abundance of Fusobacteriaceae was also associated with higher vaccine responses (p < 0.05)29. One study found no association between the diversity or composition of the intestinal microbiota, but responders more often had more than one bacterial enteropathogen (26 vs 13%, p = 0.006)37.
Responses to OPV were assessed in 5 infant studies23,31,32,38,41, one of them using a subgroup of participants from a larger study31,32. Vaccine response assessment included polio-specific intestinal IgA, serum and plasma IgA and IgG titres, as well as T-cell responses23,31,32,38,41. Only one study found an association between a lower alpha and beta diversity and higher vaccine responses (intestinal polio-specific IgA, p < 0.05)41. Other studies reported that a higher relative abundance of Actynomycetota (from phylum to family level) and a lower relative abundance of Bacillota (from phylum to species level) and Pseudomonadota (from order to species level) were associated with higher OPV vaccine responses (p < 0.05)31,32,41. Exceptions to this were an association between a higher relative abundance of Enterococcaceae (Bacillota)32,41 and lower relative abundance of Bifidobacterium breve (Actynomycetota)31 with higher OPV vaccine responses (p < 0.05). A higher relative abundance of Prophyromonadaceae (Bacteroides) was also associated with higher OPV vaccine response (p < 0.05)32. One study found no association between the relative abundance of specific bacterial taxa in the intestinal microbiota and polio-specific serum IgG responses38.
Two studies investigated the association between the composition of the intestinal microbiota and OCV responses (plasma IgA and IgG ≥4-fold change in titre) in adults22,46. One study reported a higher beta diversity (UniFrac distances, p = 0.044) and vaccine response among high OCV responders46. These studies found an association between a higher relative abundance of Clostridiales22, Shigella, Anaerobaculum mobile and Bacillus licheniformis46, a lower relative abundance of Enterobacteriales22 and Bacteroides46, and higher memory B cell responses (p < 0.05).
Another study in adults found a higher alpha diversity and phylogenetic diversity with a higher relative abundance of Clostridiales (Lachnospiraceae and Ruminococcaceae) was associated with multiphasic cell-mediated immune response after an oral Salmonella typhi vaccine (p < 0.05). No association between the composition of the intestinal microbiota and serum IgA or IgG response was found25.
Parenteral vaccines
Three infant studies analysed the effect of the composition of the intestinal microbiota on the response to PCV24,33,43. The first study found no association between PCV vaccine response and intestinal microbiota diversity or composition43. The second study found that a higher relative abundance of Escherichia/Shigella, Bifidobacterium, Bacteroides, Ruminococcaceae, and Streptococcus bovis (adjusted p < 0.05) and a lower relative abundance of Bacillota, Enterobacteriaceae, Prevotella, Bifidobacterium bifidum and Garnerella was associated with higher pneumococcal-specific saliva IgG levels (adjusted p < 0.05)24. The third study found no association between the beta diversity of intestinal microbiota or the abundance of specific taxa and serum pneumococcal IgG titre33. The second study also investigated the association of the intestinal microbiota composition at 2 and 12 months and meningococcal-C saliva IgG titres in response to meningococcus C polysaccharide vaccine given at 18 months. This study found that a higher relative abundance of Lachnospiraceae at 2 months and Pseudobutyrivibrio, Lachnospira and Roseburia at 12 months and lower relative abundance of Bifidobacteriaceae, Veillonella and Klebsiella at 2 months were associated with higher responses to meningococcus C polysaccharide vaccine (adjusted p < 0.05)24.
Humoral and T-cell response to SARS-COV-2 vaccine were investigated in seven adult studies30,35,45,47–50. Three studies assessed the humoral response to inactivated SARS-CoV-2 vaccines35,45,47, and five studies to mRNA vaccines (BNT162b2, one also for mRNA-1273)30,35,48–50. Two of the mRNA vaccine studies also assessed T-cell responses30,50. A higher relative abundance of several Actynomycetota (Actynomyces, Bifidobacterium adolescentis and B. bifidum)35,49 was associated with a higher vaccine response, while findings for Collinsella aerofaciens were contradictory45,47. The phylum Bacillota was negatively associated with SARS-CoV-2 vaccine responses50, with mixed findings for its classes and orders (higher relative abundance of Clostridia, and lower relative abundance of Eubacteriales among higher responders)45,49,50. There were also inconsistent findings for most of the remaining families and genera: a higher abundance of Lactobacillaceae was associated with higher vaccine responses, but findings for Lachnospiraceae, Clostridiaceae, certain Lactobacillales and Oscillospiraceae were contradictory45,48–50. For the Pseudomonadota phylum, a higher relative abundance of Sutterella and Parasutterella excrementihomini, and a lower relative abundance of Succinivibrio and Citrobacter were associated with higher vaccine responses49,50. While a higher relative abundance of the phylum Bacteroidota and the family Rikenellaceae were associated with higher vaccine response, there were contradictory findings for several genus and species within this phylum, including Bacteroides, Parabacteroides, Alistipes and other48–50. Finally, a higher relative abundance of Bilophila (a Thermodesulfobacteriota) and a lower abundance of Akkermansiaceae and Akkermansia (Verrucomicrobiotas) were associated with higher SARS-CoV-2 vaccine responses48,50. One study found no association between the alpha diversity of the intestinal microbiota or relative abundance of specific taxa and plasma-specific IgG responses to the mRNA BNT162b2 vaccine30.
One study in adults analysed the association between intestinal microbiota and serum influenza-specific IgG after intramuscular IIV, with results reported in two manuscripts21,40. The first report classified intestinal microbiota findings into enterotypes and found no association with these enterotypes and influenza-specific IgG responses40, stating that the study was underpowered. The second report did an ad hoc analysis on the effect of fibre intake on humoral response to IIV and found that a higher relative abundance of Alphaproteobacteria, certain Bacillota (Clostridiales, Ruminococcaceae, Ruminococcus), and Bacteroides (Parabacteroides and Prevotella), B. bifidum and Sutterella, and a lower relative abundance of several Bacillota OTUs (including Clostridia), H. parainfluenzae and Butyricimonas were associated with higher serum antibody responses to H1N1 or H3N2 (all adjusted p < 0.05), with contradicting findings for Lachnospiraceae and Oscillospora. In this study, no association was found between the alpha or beta diversity of the intestinal microbiota and vaccine responses21.
Vaccine response to tetanus toxoid vaccine in relation to intestinal microbiota was assessed in three infant studies (serum IgG and T-cell responses)23,31–33. A multicentre prospective birth study including 472 infants found no association between the relative abundance of Bifidobacterium infantis and B. longum and tetanus serum IgG levels23. A birth cohort study found that a lower relative abundance of Aeriscardovia aeriphila was associated with higher serum IgG responses to tetanus toxoid vaccine33. Results from 291 infants from Bangladesh were published in two publications. Stool samples were analysed at three different time points (6, 11, 15 weeks), and serum tetanus-specific IgG and T-cell responses were measured at 15 weeks and 2 years of age. A higher relative abundance of Bifidobacterium and B. longum was associated with higher T-cell responses and serum IgG titres at different time-point combinations31. In a sub-sample of 48 infants, a higher relative abundance of Actynomycetota (Bifidobacteriales, Bifidobacteriaceae, Corynebacterium, Bifidobacterium) and Pseudomonadaceae and a lower relative abundance of certain Bacillota (Clostridia, Clostridiales and Lactobacillaceae), and Gammaproteobacteria (Pseudomonadales, Enterobacteriales, Moraxellaceae, Enterobacteriaceae, Escherichia, Shigella and Acinetobacter), were associated with higher T-cell responses to tetanus. A higher relative abundance of Actinomycetales and Actinomyces, and a lower relative abundance of Staphylococcus were also associated with higher serum IgG response to tetanus32.
A birth cohort in the USA, studied the humoral response to all vaccine components in the DTaP/Hib vaccine43, and reported a negative correlation between alpha diversity (evenness of genera, p = 0.04) and median IgG DTaP/Hib titres, but no association with relative abundance of specific intestinal bacterial taxa43.
Two studies investigated responses to HBV vaccine, one study in infants31,32 and one in adults44. Among infants, a higher relative abundance of Bifidobacterium and B. longum and lower relative abundance of Pseudomonales and Moraxellaceae were associated with higher T-cell responses and a higher relative abundance of Micrococcaceae and Rothia, and lower relative abundance of Carnobacteriaceae, Lachnospiraceae, B. breve and B. longum were associated with higher serum IgG titres31,32. In adults, a higher relative abundance of Butyricicoccus and Phascolarctobacterium, and a lower relative abundance of Clostridiales and Bacteroides (all p < 0.05) were associated with higher anti-HBsAg plasma IgG in a multiomics model44.
This same study in infants also investigated the response to BCG vaccine31,32, and reported that a higher relative abundance of Actynomycetota (Bifidobacteriales, Bifidobacteriaceae), certain Bacillota (Enterococcaceae, Enterococcus) and Betaproteobacteria (Burkholderiales), and a lower abundance of certain Gammaproteobacteria (Pseudomonales, Moraxellaceae and Acinetobacter) were associated with higher T-cell responses. Furthermore, a higher relative abundance of certain Actynomycetota (Actinomycetales, Micrococcaceae, Rothia, Bidifobacteriales, Bifidobacteriaceae and Bifidobacterium) and a lower abundance of several Bacteroidota (Prevotellaceae and Prevotella) and Bacillota OTUs were associated with positive delayed-type hypersensitivity skin-test responses (p < 0.05)31,32.
One cohort study in infants found an association between detectable levels of B. longum subspecies infantis and higher polio-specific intestinal IgA titres as a response to IPV (p < 0.002)34. Two of the studies mentioned above which assessed response to OPV, also used IPV as a combination with OPV in different schedules, or according to country schedule (see above)23,41.
We assessed differences between the associations between the gut microbiota relative abundance of specific bacterial taxa and vaccine responses among infants (Supplementary Table 4) and adults (Supplementary Table 5). For the Actynomycetota phylum, there was more evidence of an increased relative abundance among higher vaccine responders in infant studies, with some exceptions such as Gardnerella and some Bifidobacterium species. In adults, Bifidobacterium showed also a positive association with vaccine response. Among the Bacillota phylum, findings were variable for both infants and adults, though more infant studies reported a lower relative abundance of Bacillota among higher vaccine responders compared to adults. A lower relative abundance of Pseudomonota (from phylum to family) was associated with vaccine response in infants, while adult studies reported positive associations more frequently. However, Escherichia and Shigella genus and species were also positively associated with vaccine response among infants. Bacteroidota phylum bacteria findings were very variable, with a general negative association with vaccine response for Bacteroides genus and species among adults.
Discussion
Understanding the underlying causes for the variability in vaccine effectiveness among individuals is crucial to improving vaccine performance. Our systematic review shows that the composition of the intestinal microbiota can impact responses to oral and parental vaccines, affecting both humoral and cellular responses. Despite some variability in results among studies, in general, there was a beneficial effect of Actynomycetota (particularly Bifidobacterium), and a detrimental effect of Pseudomonadota (especially Gammaproteobacteria) on vaccine responses. The alpha and beta diversity of the intestinal microbiota was not strongly associated with differences in vaccine responses.
Three possible immunological mechanisms have been proposed to explain how the intestinal microbiota modulates vaccine responses: provision of natural adjuvants, modulation of antigen-presenting cells by microbial metabolites, and presence of cross-reactive antigens encoded in the microbiota14. Certain microbiota components, such as lipopolysaccharide or flagellin, have been shown to act as vaccine adjuvants by activating antigen-presenting cells through pattern-recognition receptors (PRRs)51,52. The microbiota has also been shown to enhance different antigen-presenting cells, such as dendritic cells53,54, macrophages52 or intestinal epithelial cells55. Memory CD4 + T cells encoded for specific pathogen antigens have been found in individuals not previously exposed to these pathogens56, and CD4 + T cells reactive to intestinal microbiota can also be found in humans57. Some researchers have therefore hypothesised that some of these CD4 + T cells may cross-react to certain epitopes shared between the intestinal microbiota and pathogen antigens present in vaccines14. The evidence supporting these potential immunological mechanisms is sometimes contradictory and based mainly on mouse models. A potential issue to consider is that variations in the microbiome may represent a marker of poor vaccine response rather than the cause: individuals with a weaker immune response to vaccines may have specific microbiome profiles, reflecting an association rather than a causal relationship.
Nonetheless, animal studies provide strong evidence for the impact of the composition of the intestinal microbiota on vaccine responses. Antibiotic-treated infant mice mount reduced humoral responses to common licensed live and adjuvanted vaccines51. Similarly, pups born to mothers treated with antibiotics show impaired humoral responses to the model antigen ovalbumin58. In relation to the role of the activation of PRRs, mice deficient in Toll-like receptor 5 (TLR5) or in nucleotide-binding oligomerization domain-containing (Nod) 2 (both PRRs), and germ-free mice show impaired antibody responses to influenza, IPV and cholera toxin vaccines, with a restored response after bacterial reconstitution52,59. However, this reduced humoral response in TLR5-deficient mice depends on the type of vaccine and is not observed after adjuvanted and live vaccines52. Some of these studies reported a rescued vaccine response after the intestinal microbiota was restored, especially when restored with flagellated Escherichia coli51,52.
Studies in mice have also reported differences between adult and infant mice, with no impaired vaccine response in antibiotic-treated adult mice51,52. In our systematic review, an effect of the intestinal microbiota on vaccine responses was reported in both adults and infants, though none included both age groups in the same study. The only vaccine investigated across both age groups, albeit in separate studies, was the ORV. Variations in the specific bacterial taxa associated with differences in vaccine response were observed between adults and infants, as well as between different studies in infants26–29,36,37,39, precluding definitive conclusions. Further studies including different age groups using the same methods and vaccine are needed to establish the impact of age on the intestinal microbiota on vaccine responses in humans.
A key characteristic of intestinal microbiota is that it can be modified through interventions. Studies have shown how the use of probiotics, prebiotics, and antibiotics modify the diversity and composition of intestinal microbiota60. A systematic review on the effect of probiotics on vaccine response, reported that half of the 26 included randomised placebo-controlled trials (RCTs), showed a beneficial effect, especially for oral vaccines and parenteral influenza vaccine61. Again, study designs, study vaccine, dosing and timing of the probiotic varied between studies, making it difficult to draw firm conclusions61. Also, some of the RCTs did not report previous exposure to antibiotics or excluded participants exposed to antibiotics. Studies assessing the effect of probiotics in infants and adults with disrupted intestinal microbiotas would be highly relevant but currently lacking. Further RCTs in children published since the review have continued to report both positive (for Haemophilus influenzae type B vaccine)62 or no associations (pneumococcus, diphtheria, tetanus and pertussis vaccines)62,63 between probiotic administration and vaccine responses. In adults, a meta-analysis on the effect of pre- and probiotics on immune response to influenza vaccines that included 20 RCTs concluded that their use was associated with an improved response to H1N1, H2N3 and B influenza strains64. As discussed above, antibiotic-treated mice have a reduced humoral response to influenza vaccine52. However, few human clinical studies have assessed the effect of antibiotics on vaccine responses through disruption of the intestinal microbiota. A retrospective study in children, showed an inverse dose-dependent effect of antibiotic exposure on antibody responses to infant vaccines (DTaP, Hib, IPV and PCV), though intestinal microbiota changes were not assessed65. Nonetheless, a trial on the effect of azithromycin on OPV vaccine response in Indian infants, did not improve vaccine immunogenicity despite reducing pathogenic intestinal bacteria prevalence, environmental enteropathy and reducing the relative abundance of Pseudomonadota and Verrucomicrobia66,67. In adults, a trial showed that antibiotic-driven intestinal microbiota alterations led to a reduced H1N1-specific IgG1 response only among those with low pre-existing titres, with no effect on participants with high baseline titres or on H3N2 influenza response68. Finally, vaccines may themselves generate changes in the intestinal microbiota composition, as was shown in one the studies included in this review, which reported an association between SARS-CoV-2 intramuscular vaccine and a decrease in relative abundance of Bacteroides caccae and Clostridiales35. However, other studies showed no effect of human immunodeficiency virus (HIV)-1 (intranodal and intramuscular), oral typhoid, or BCG vaccines on intestinal microbiota composition25,69–71. Overall, there is good evidence that vaccine responses can be modulated by changes in the intestinal microbiota.
The strengths of the study include the comprehensive search strategy with no age, region, language, or time restriction to avoid study selection bias.
Our review is inevitably affected by limitations inherent in the reported studies. While all studies were prospective, predominantly cohort studies, many had small sample sizes (only 3 studies included more than 200 participants), potentially resulting in underpowered analyses. Some studies might be subject to bias from multiple significance testing when they included multiple analyses with several vaccines, multiple humoral and cellular responses, and several time points to measure vaccine response. Thirdly, the heterogeneity in study designs (different vaccines and schedules, timing of stool collection and vaccine response outcome measures, as well as stool analysis techniques), make it difficult to find common patterns. Similarly, results of intestinal microbiota composition were reported at different taxonomic levels. Finally, like all reviews, our analysis depends on published data, which could be affected by reporting and publication biases, potentially leading to the omission of studies with negative findings.
Despite the increasing evidence to support a key role of the composition of the intestinal microbiota on modulating vaccine responses, it is not yet sufficient for translation to clinical practice. Larger multicentre studies applying the same methods and studying the same vaccine responses in different locations and age groups are needed to assess the impact of variations in the intestinal microbiota impact on vaccine response, and their relative importance in relation to other potential vaccine response modulators, such as environmental, perinatal, nutritional and intrinsic host factors3. In the present study, we reported both similarities and differences between studies in infants and adults, but this was limited by the use of different vaccines and settings. It would be of interest to do studies in different age groups for vaccines that are used all along the life span, such as SARS-CoV-2 or influenza vaccines. Future studies should investigate other components of the intestinal microbiota such as fungi, archaea and viruses, as there is some evidence for an effect of the intestinal virome on vaccine responses72. Further, they should apply more detailed stool analysis techniques, such as whole metagenomic next-generation sequencing, to identify key intestinal microbiota bacteria at the species and strain level. This may lead to adequately powered clinical trials on interventions (probiotics or antibiotics) directed at optimising the balance of key intestinal microbiota bacteria associated with modulating vaccine responses.
Findings from this systematic review support the concept that the composition of the intestinal microbiota impacts vaccine responses. Although further confirmation is needed in more robust studies, these findings have exciting implications for potential microbiota-targeted interventions to optimise vaccine responses.
Methods
Studies were identified, selected, appraised and synthesised following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews73.
Eligibility criteria
Studies investigating the association between the composition of intestinal microbiota composition and any type of vaccine response (humoral and cellular) in humans were included. For intervention studies in which antibiotics, probiotics and supplements were administered, inclusion was contingent upon the presence of a control group receiving no intervention or that the association between the intestinal microbiota and vaccine responses was observed across all groups. Studies that included participants already infected by the targeted pathogen of the vaccine were excluded.
Information sources and search strategy
In June 2024, MEDLINE (1946 to present) and Embase (1947 to present) were searched without any language restrictions with the following search terms: microbiota AND stool AND vaccine response. The detailed search terms can be found in Supplementary Table 1. Duplicates were removed. References of retrieved articles were hand-searched for additional publications.
Study selection, data extraction and quality assessment
Titles and abstracts were screened for eligibility, followed by screening of full texts. Relevant data was extracted into detailed tables, including study characteristics (design, location, and year), population (size and age), exposure (type of vaccine and schedule, timing and technique used for stool sample analysis), outcome measures (timing and technique used for vaccine response analysis), and results (Table 1).
Quality and risk of bias assessment
The level of evidence of each study was classified according to the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence74. Risk of bias was assessed using the 2017 Joanna Briggs Institution (JBI) standardised critical appraisal checklist for case-control and cohort studies75.
Synthesis of results
The findings were summarised into tables, grouped by specific intestinal bacteria (from phylum to species) and vaccine investigated.
Supplementary information
Acknowledgements
We thank Ms Poh Chua, Librarian, Royal Children’s Hospital Melbourne for her assistance with the literature search. C.A.G. was funded by a Postdoctoral Mobility Fellowship from the Swiss National Science Foundation (grant number P500PM_217605). The funder played no role in the study design, data collection, analysis and interpretation of data, or the writing of this manuscript. P.Z. is supported by the Swiss National Science Foundation (Grant 10000835 and PZPGP3_193140).
Author contributions
C.A.G. and P.Z. designed the study. C.A.G. did the literature search and study selection and extracted the relevant information. C.A.G. and P.Z. synthesised the data. C.A.G. wrote the first draft of the manuscript. P.Z. and N.C. supervised the overall work. All authors critically revised successive drafts of the paper and approved the final manuscript.
Data availability
The data used in this systematic review was extracted from published studies, and we do not possess specific datasets to share. All data generated during this study are included in this published article and its supplementary information files.
Competing interests
Authors C.A.G. and N.C. declare no financial or non-financial competing interests. Author P.Z. serves as an Associate Editor of this journal and had no role in the peer-review or decision to publish this manuscript. Author P.Z. declares no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-024-01000-0.
References
- 1.Andre, F. E. et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ.86, 140–146 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Praharaj, I., John, S. M., Bandyopadhyay, R. & Kang, G. Probiotics, antibiotics and the immune responses to vaccines. Philos. Trans. R. Soc. B: Biol. Sci.370, 20140144 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann, P. & Curtis, N. Factors influencing the intestinal microbiome during the first year of life. Pediatr. Infect. Dis. J.37, e315–e335 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Ritz, N., Mui, M., Balloch, A. & Curtis, N. Non-specific effect of Bacille Calmette-Guerin vaccine on the immune response to routine immunisations. Vaccine31, 3098–3103 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Nakaya, H. I. et al. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity43, 1186–1198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Junqueira, A. L. et al. Safety and immunogenicity of hepatitis B vaccine administered into ventrogluteal vs. anterolateral thigh sites in infants: a randomised controlled trial. Int. J. Nurs. Stud.47, 1074–1079 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Ciabattini, A. et al. Vaccination in the elderly: the challenge of immune changes with aging. Semin. Immunol.40, 83–94 (2018). [DOI] [PubMed]
- 8.PrabhuDas, M. et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat. Immunol.12, 189–194 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann, P. & Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev.32, e00084–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe, Y. J., Blatt, D. B., Lee, H. J. & Choi, E. H. Associations between geographic region and immune response variations to pneumococcal conjugate vaccines in clinical trials: a systematic review and meta-analysis. Int. J. Infect. Dis.92, 261–268 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Clark, A. et al. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect. Dis.19, 717–727 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallander, H. et al. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine21, 138–145 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Lalor, M. K. et al. Population differences in immune responses to Bacille Calmette-Guerin vaccination in infancy. J. Infect. Dis.199, 795–800 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynn, D. J., Benson, S. C., Lynn, M. A. & Pulendran, B. Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. Nat. Rev. Immunol.22, 33–46 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann, P. & Curtis, N. The influence of the intestinal microbiome on vaccine responses. Vaccine36, 4433–4439 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Bäckhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe17, 690–703 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Brushett, S., Sinha, T., Reijneveld, S. A., De Kroon, M. L. & Zhernakova, A. The effects of urbanization on the infant gut microbiota and health outcomes. Front. Pediatr.8, 408 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci.107, 14691–14696 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasolli, E. et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell176, 649–62.e20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segata, N. Gut microbiome: westernization and the disappearance of intestinal diversity. Curr. Biol.25, R611–R613 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Cait, A. et al. Potential association between dietary fibre and humoral response to the seasonal influenza vaccine. Front. Immunol.12, 765528 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chac, D. et al. Gut microbiota and development of vibrio cholerae-specific long-term memory B cells in adults after whole-cell killed oral cholera vaccine. Infect. Immun.89, e0021721 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colston, J. M. et al. Intestinal colonization with bifidobacterium longum subspecies is associated with length at birth, exclusive breastfeeding, and decreased risk of enteric virus infections, but not with histo-blood group antigens, oral vaccine response or later growth in three birth cohorts. Front. Pediatr.10, 804798 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Koff, E. M. et al. Mode of delivery modulates the intestinal microbiota and impacts the response to vaccination. Nat. Commun.13, 6638 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eloe-Fadrosh, E. A. et al. Impact of oral typhoid vaccination on the human gut microbiota and correlations with s. Typhi-specific immunological responses. PLoS One8, e62026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fix, J. et al. Association between gut microbiome composition and rotavirus vaccine response among Nicaraguan infants. Am. J. Tro. Med. Hyg.102, 213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris, V. et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes9, 93–101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris, V. C. et al. The infant gut microbiome correlates significantly with rotavirus vaccine response in rural Ghana. J. Infect. Dis.215, 34–41 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris, V. C. et al. Effect of antibiotic-mediated microbiome modulation on rotavirus vaccine immunogenicity: a human, randomized-control proof-of-concept trial. Cell Host-Microbe24, 197–207.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirota, M. et al. Human immune and gut microbial parameters associated with inter-individual variations in COVID-19 mRNA vaccine-induced immunity. Commun. Biol.6, 368 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huda, M. N. et al. Bifidobacterium abundance in early infancy and vaccine response at 2 years of age. Pediatrics143, e20181489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huda, M. N. et al. Stool microbiota and vaccine responses of infants. Pediatrics134, e362–e372 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moroishi, Y. et al. A prospective study of the infant gut microbiome in relation to vaccine response. Pediatr. Res.93, 725–731 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullié, C. et al. Increased poliovirus-specific intestinal antibody response coincides with promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in infants: a randomized, double-blind, placebo-controlled trial. Pediatr. Res.56, 791–795 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Ng, S. C. et al. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut71, 1106–1116 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker, E. P. K. et al. Impact of maternal antibodies and microbiota development on the immunogenicity of oral rotavirus vaccine in African, Indian, and European infants. Nat. Commun.12, 7288 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker, E. P. K. et al. Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south India. Vaccine36, 264–272 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Praharaj, I. et al. Influence of nonpolio enteroviruses and the bacterial gut microbiota on oral poliovirus vaccine response: a study from South India. J. Infect. Dis.219, 1178–1186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson, R. C. et al. The fecal microbiome and rotavirus vaccine immunogenicity in rural Zimbabwean infants. Vaccine39, 5391–5400 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shortt, N. et al. A feasibility study: association between gut microbiota enterotype and antibody response to seasonal trivalent influenza vaccine in adults. Clin. Transl. Immunol.7, e1013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, T. et al. Influence of gut microbiota on mucosal IgA antibody response to the polio vaccine. NPJ Vaccines5, 47 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham-Oakes, E. et al. Increased bacterial taxonomic and functional diversity is associated with impaired rotavirus vaccine immunogenicity in infants from India and Malawi. BMC Microbiol.23, 354 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaffer, M. et al. Very early life microbiome and metabolome correlates with primary vaccination variability in children. Msystems8, e00661–23 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shannon, C. P. et al. Multi-omic data integration allows baseline immune signatures to predict hepatitis B vaccine response in a small cohort. Front. Immunol.11, 578801 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang, B. et al. Correlation of gut microbiota and metabolic functions with the antibody response to the BBIBP-CorV vaccine. Cell Rep. Med.3, 100752 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuki, Y. et al. Oral MucoRice-CTB vaccine for safety and microbiota-dependent immunogenicity in humans: a phase 1 randomised trial. Lancet Microbe2, e429–e440 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Zhang, L.-N. et al. Association between gut microbiota composition and long-term vaccine immunogenicity following three doses of CoronaVac. Vaccines12, 365 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daddi, L. et al. Baseline gut microbiome signatures correlate with immunogenicity of SARS-CoV-2 mRNA vaccines. Int. J. Mol. Sci.24, 11703 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng, Y. et al. Baseline gut microbiota and metabolome predict durable immunogenicity to SARS-CoV-2 vaccines. Signal Transduct. Target. Ther.8, 373 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray, S. et al. Impact of the gut microbiome on immunological responses to COVID-19 vaccination in healthy controls and people living with HIV. npj Biofilms Microbiomes9, 104 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lynn, M. A. et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe23, 653–60.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Oh, J. Z. et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity41, 478–492 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruane, D. et al. Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses. J. Exp. Med.213, 53–73 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaupp, L. et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells. Cell181, 1080–96.e19 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Koyama, M. et al. MHC class II antigen presentation by the intestinal epithelium initiates graft-versus-host disease and is influenced by the microbiota. Immunity51, 885–98.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su, L. F., Kidd, B. A., Han, A., Kotzin, J. J. & Davis, M. M. Virus-specific CD4+ memory-phenotype T cells are abundant in unexposed adults. Immunity38, 373–383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hegazy, A. N. et al. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology153, 1320–1337.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamousé-Smith, E. S., Tzeng, A. & Starnbach, M. N. The intestinal flora is required to support antibody responses to systemic immunization in infant and germ free mice. PloS One6, e27662 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim, D. et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat. Med.22, 524–530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zimmermann, P. & Curtis, N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J. Infect.79, 471–489 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Zimmermann, P. & Curtis, N. The influence of probiotics on vaccine responses–a systematic review. Vaccine36, 207–213 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Kukkonen, K., Nieminen, T., Poussa, T., Savilahti, E. & Kuitunen, M. Effect of probiotics on vaccine antibody responses in infancy–a randomized placebo‐controlled double‐blind trial. Pediatr. Allergy Immunol.17, 416–421 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Sørensen, C. A. et al. Probiotics and the immunological response to infant vaccinations; a double-blind randomized controlled trial. Clin. Microbiol. Infect.25, 511 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Lei, W.-T., Shih, P.-C., Liu, S.-J., Lin, C.-Y. & Yeh, T.-L. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients9, 1175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chapman, T. J., Pham, M., Bajorski, P. & Pichichero, M. E. Antibiotic use and vaccine antibody levels. Pediatrics149, e2021052061 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grassly, N. C. et al. The effect of azithromycin on the immunogenicity of oral poliovirus vaccine: a double-blind randomised placebo-controlled trial in seronegative Indian infants. Lancet Infect. Dis.16, 905–914 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Parker, E. P. K. et al. Changes in the intestinal microbiota following the administration of azithromycin in a randomised placebo-controlled trial among infants in south India. Sci. Rep.7, 9168 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hagan, T. et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell178, 1313–28.e13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pastor-Ibáñez, R. et al. Impact of transcriptome and gut microbiome on the response of hiv-1 infected individuals to a dendritic cell-based hiv therapeutic vaccine. Vaccines9, 694 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmermann, P. et al. The effect of bacille Calmette-Guérin vaccination on the composition of the intestinal microbiome in neonates from the MIS BAIR trial. Pediatr. Infect. Dis. J.43, 378–389 (2024). [DOI] [PubMed] [Google Scholar]
- 71.Borgognone, A. et al. Gut microbiome signatures linked to HIV-1 reservoir size and viremia control. Microbiome10, 59 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Praharaj, I. et al. Enterovirus and gut virome profile in Indian infants and immune response to oral rotavirus vaccine. Int. J. Infect. Dis.101, 492–493 (2020). [Google Scholar]
- 73.Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med.151, 264–269 (2009). [DOI] [PubMed] [Google Scholar]
- 74.Howick J. et al. Oxford centre for evidence-based medicine 2011 levels of evidence. Centre for Evidence-Based Medicine Retrieved July from https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-21pdf. (2011).
- 75.Moola S. M. Z. et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E MZ, editor. JBI Manual for Evidence Synthesis (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this systematic review was extracted from published studies, and we do not possess specific datasets to share. All data generated during this study are included in this published article and its supplementary information files.