Abstract
1. Rat thymus cells were incubated in homologous serum (10%) and medium 199. The effects of various quantities of thymidine or deoxycytidine (0–30μm) on the radioactive labelling of cells with the corresponding radioactive deoxynucleoside were examined. From plots of the reciprocal of the radioactivity incorporated against the total deoxynucleoside concentration (`isotope-dilution plots'), values were obtained for (a) the Vmax. of the rate-limiting step governing incorporation of the deoxynucleoside, and (b) the concentration of the pool of compounds competing with the radioactive deoxynucleoside at that rate-limiting step. From changes in these values under different experimental conditions inferences were drawn on the position and control of the rate-limiting step within intact cells. 2. Isotope-dilution plots for deoxycytidine were linear, whereas plots for thymidine were bimodal, indicating an abrupt increase in both the Vmax. and pool concentration at a critical thymidine concentration (approx. 5μm). The bimodality was removed by amethopterin. The Vmax. determined with deoxy[U-14C]cytidine was approximately equal to the sum of the Vmax. determined with deoxy[5-3H]cytidine and the Vmax. determined with [Me-3H]thymidine at thymidine concentrations above 5μm. 3. The thymidine competitor pool at thymidine concentrations above 5μm was approximately equal to the sum of the deoxycytidine competitor pool and the thymidine competitor pool at thymidine concentrations below 5μm. The pools were independent of cell concentration and dependent on serum concentration. 4. These results were explained on the following basis. Deoxycytidine in serum (16μm) is the major source of both cytosine and, by way of thymidylate synthetase, thymine, in the DNA of thymus cells. Serum deoxycytidine normally maintains a sufficient intracellular concentration of dTTP to inhibit partially the activity of thymidine kinase. When the dTTP concentration is lowered, either by decreasing the concentration of deoxycytidine or by inhibiting thymidylate synthetase, the activity of thymidine kinase increases. The activity of thymidine kinase may also be increased by concentrations of thymidine greater than 5μm, which overcome the inhibition of the enzyme by dTTP. At concentrations of thymidine below 5μm, thymidine kinase limits the rate of labelling with [Me-3H]thymidine and the radioactivity is diluted by a pool of unlabelled thymidine in serum (4μm). At thymidine concentrations greater than 5μm, the activity of DNA polymerase limits the rate of labelling and the radioactivity is diluted both by serum thymidine and, indirectly, by serum deoxycytidine.
Full text
PDF

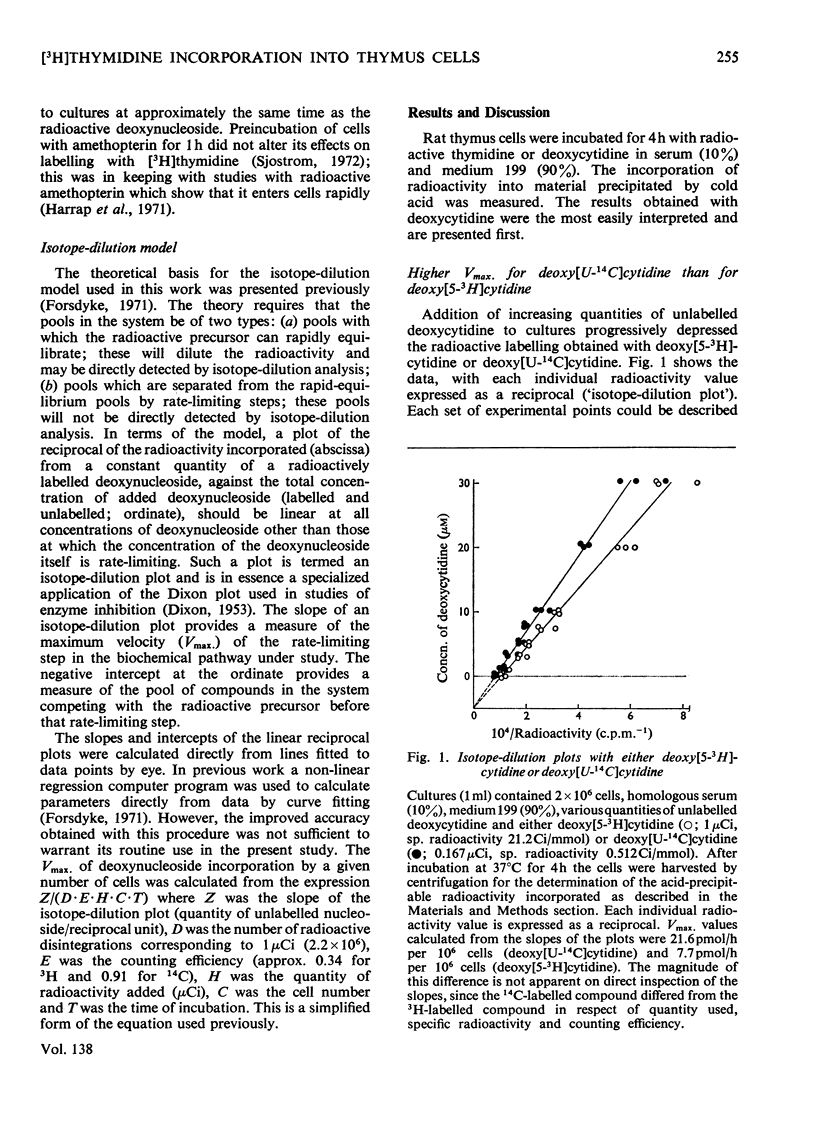
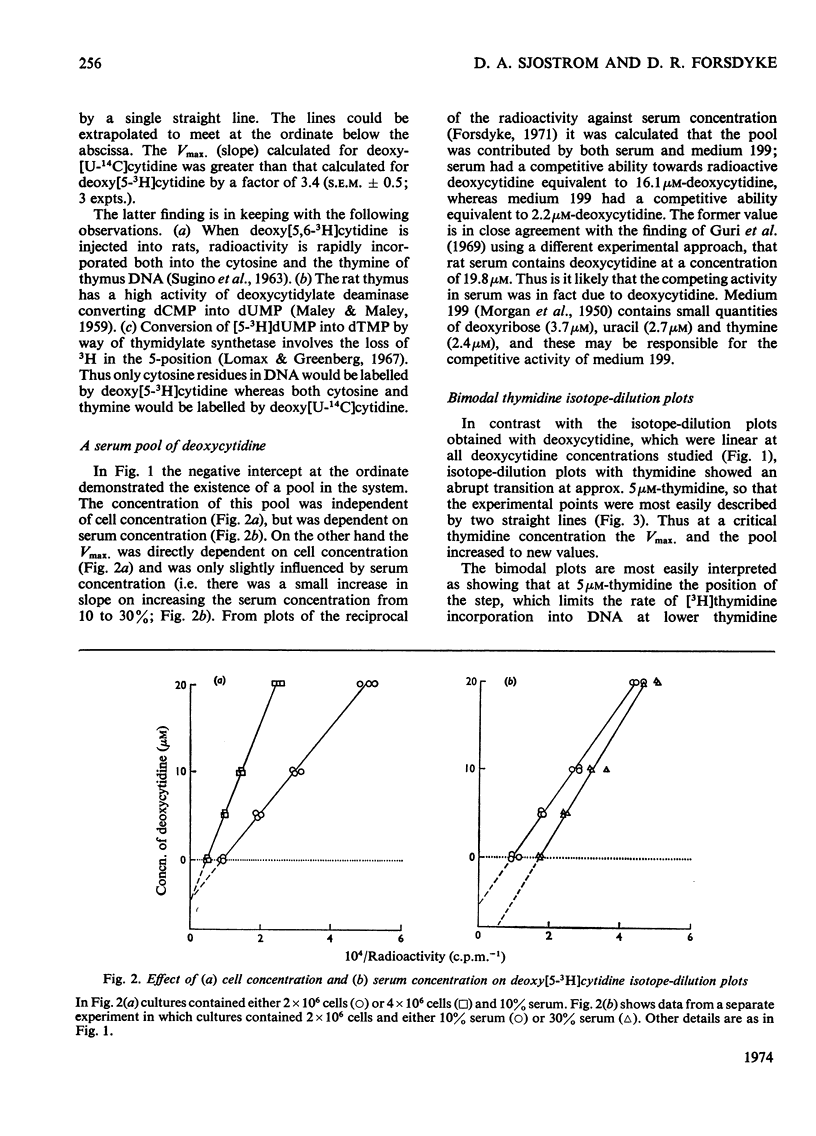
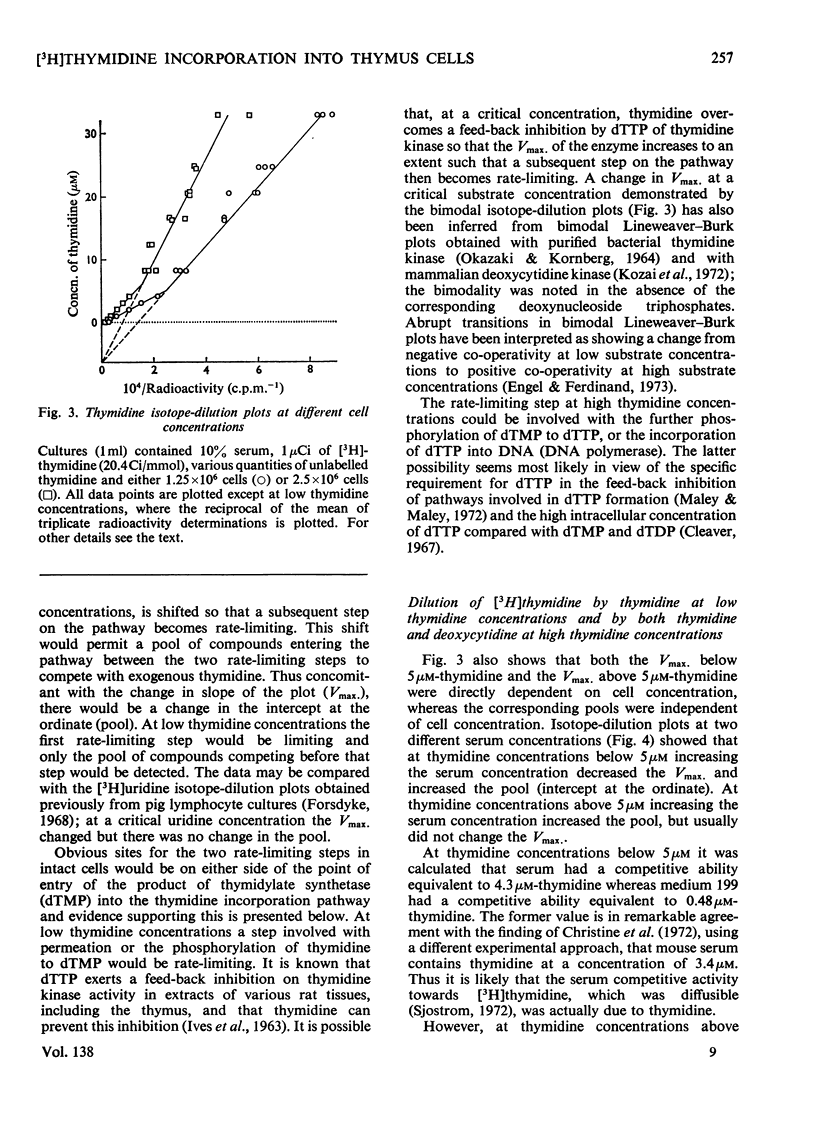
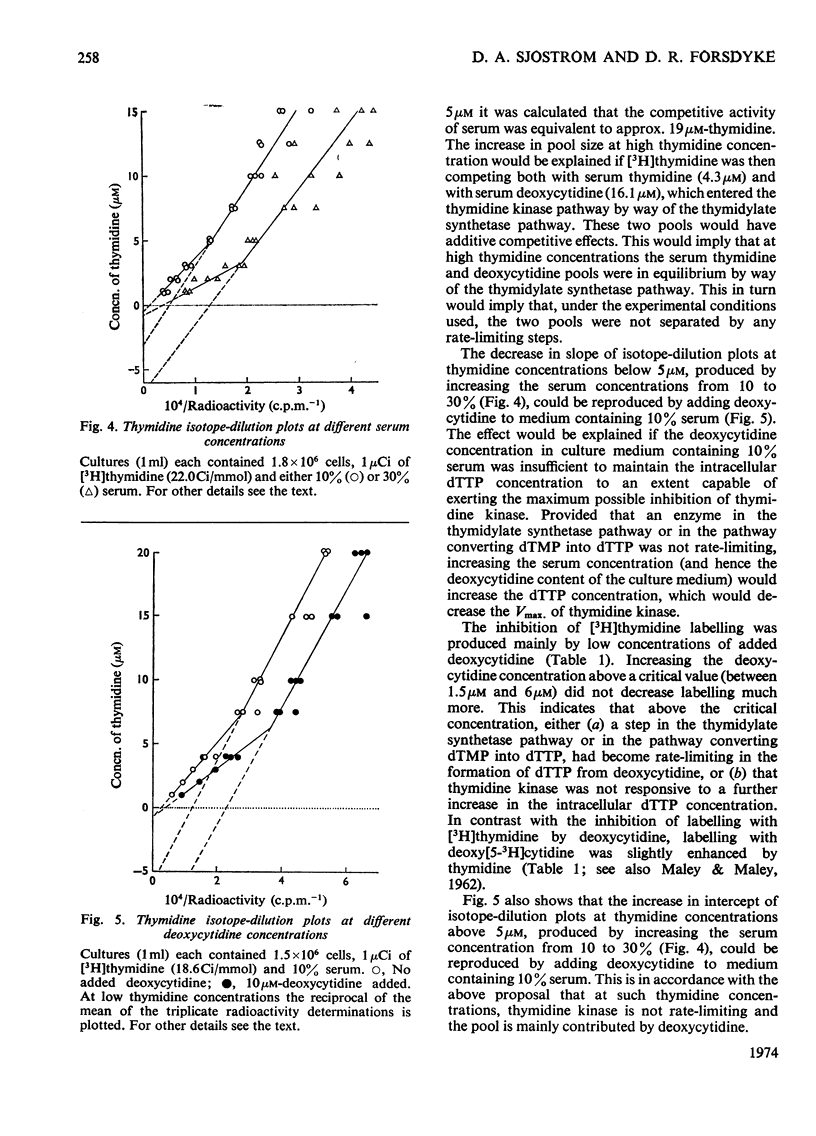

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Berryman S., Thomson A. Deoxyribonucleoside triphosphate pools in synchronized and drug-inhibited L 929 cells. Biochim Biophys Acta. 1971 Jul 29;240(4):455–462. doi: 10.1016/0005-2787(71)90702-7. [DOI] [PubMed] [Google Scholar]
- Beltz R. E., Smith T. R., Waters R. N. Kinetics of the clearing of 14 C-labeled deoxyribonucleosides from rat blood in vivo and in vitro. Biochim Biophys Acta. 1973 Feb 28;297(2):258–267. doi: 10.1016/0304-4165(73)90072-x. [DOI] [PubMed] [Google Scholar]
- Bryant B. J. Renewal and fate in the mammalian thymus: mechanisms and inferences of thymocytokinetics. Eur J Immunol. 1972 Feb;2(1):38–45. doi: 10.1002/eji.1830020109. [DOI] [PubMed] [Google Scholar]
- COOPER E. H., MILTON J. D. THE INCORPORATION AND DEGRADATION OF PYRIMIDINE DNA PRECURSORS BY HUMAN LEUCOCYTES. Br J Cancer. 1964 Dec;18:701–713. doi: 10.1038/bjc.1964.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P. C., Ferdinand W. The significance of abrupt transitions in Lineweaver-Burk plots with particular reference to glutamate dehydrogenase. Negative and positive co-operativity in catalytic rate constants. Biochem J. 1973 Jan;131(1):97–105. doi: 10.1042/bj1310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDKIN M., WOOD H. I. V. Utilization of thymidine-C14 by bone marrow cells and isolated thymus nuclei. J Biol Chem. 1956 Jun;220(2):639–651. [PubMed] [Google Scholar]
- Forsdyke D. R. Application of the isotope-dilution principle to the analysis of factors affecting the incorporation of (3H) uridine and (3H) cytidine into cultured lymphocytes. Evaluation of pools in serum and culture media. Biochem J. 1971 Dec;125(3):721–732. doi: 10.1042/bj1250721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdyke D. R. Quantitiative nucleic acid changes during phytohaemagglutinin-induced lymphocyte transformation in vitro. Dependence of the response on phytohaemagglutinin-serum rati. Biochem J. 1967 Nov;105(2):679–684. doi: 10.1042/bj1050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdyke D. R. Studies on the incorporation of [5-3H] uridine during activation and transformation of lymphocytes induced by phytohaemagglutinin. Dependence on the incorporation rate on uridine concentration at certain critical concentrations. Biochem J. 1968 Mar;107(2):197–205. doi: 10.1042/bj1070197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F., Goldberg A. L. Problems in the use of (Me- 3H) thymidine for the measurement of DNA synthesis. Biochim Biophys Acta. 1973 Apr 11;299(4):521–532. doi: 10.1016/0005-2787(73)90224-4. [DOI] [PubMed] [Google Scholar]
- Guri C. D., Minot H., Swingle K. F., Cole L. J. Early increase in the miscible deoxycytidine pool in rats after x-irradiation. Radiat Res. 1969 Jul;39(1):155–163. [PubMed] [Google Scholar]
- Harrap K. R., Hill B. T., Furness M. E., Hart L. I. Sites of action of amethopterin: intrinsic and acquired drug resistance. Ann N Y Acad Sci. 1971 Nov 30;186:312–324. doi: 10.1111/j.1749-6632.1971.tb46986.x. [DOI] [PubMed] [Google Scholar]
- Heidelberger C. Fluorinated pyrimidines. Prog Nucleic Acid Res Mol Biol. 1965;4:1–50. doi: 10.1016/s0079-6603(08)60783-7. [DOI] [PubMed] [Google Scholar]
- Hitchings G. H., Burchall J. J. Inhibition of folate biosynthesis and function as a basis for chemotherapy. Adv Enzymol Relat Areas Mol Biol. 1965;27:417–468. doi: 10.1002/9780470122723.ch9. [DOI] [PubMed] [Google Scholar]
- IVES D. H., MORSE P. A., Jr, POTTER V. R. Feedback inhibition of thymodine kinase by thymodine triphosphate. J Biol Chem. 1963 Apr;238:1467–1474. [PubMed] [Google Scholar]
- Lomax M. I., Greenberg G. R. A new assay of thymidylate synthetase activity based on the release of tritium from deoxyuridylate-5-3-H. J Biol Chem. 1967 Jan 10;242(1):109–113. [PubMed] [Google Scholar]
- MALEY F., MALEY G. F. On the nature of a sparing effect by thymidine on the utilization of deoxycytidine. Biochemistry. 1962 Sep;1:847–851. doi: 10.1021/bi00911a017. [DOI] [PubMed] [Google Scholar]
- MALEY G. F., MALEY F. Nucleotide interconversions in embryonic and neoplastic tissues. I. The conversion of deoxycytidylic acid to deoxyuridylic acid and thymidylic acid. J Biol Chem. 1959 Nov;234:2975–2980. [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- Merey D., Cox R. P. Stability of tritiated thymidine during prolonged labelling of human blood leukocyte cultures. Experientia. 1972 Jun 15;28(6):711–712. doi: 10.1007/BF01944996. [DOI] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- Reichard P. Control of deoxyribonucleotide synthesis in vitro and in vivo. Adv Enzyme Regul. 1972;10:3–16. doi: 10.1016/0065-2571(72)90003-9. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., FRENKEL E. P., POTTER R. L. EFFECT OF X-RADIATION ON DNA METABOLISM IN VARIOUS TISSUES OF THE RAT. V. DNA METABOLISM IN REGENERATING THYMUS. Radiat Res. 1963 Aug;19:682–700. [PubMed] [Google Scholar]
- Skoog L., Nordenskjöld B. Effects of hydroxyurea and 1-beta-D-arabinofuranosyl-cytosine on deoxyribonucleotide pools in mouse embryo cells. Eur J Biochem. 1971 Mar 1;19(1):81–89. doi: 10.1111/j.1432-1033.1971.tb01290.x. [DOI] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittes R. E., Kidwell W. R. A kinetic approach to the determination of the S phase pool size of thymidine triphosphate in exponentially growing mouse L cells. J Mol Biol. 1973 Aug 15;78(3):473–486. doi: 10.1016/0022-2836(73)90469-5. [DOI] [PubMed] [Google Scholar]


