Abstract
Invasive alien plants pose a significant threat to biodiversity and the agricultural economy. The invasive weed (Ammannia coccinea) competes with rice in paddy fields, potentially threatening rice production. Despite the crucial need to estimate the global geographical distribution and ecological niche dynamics of A. coccinea for effective early warning, control strategies, and global rice security, relevant research remains scarce. This study utilized the Biomod2 platform, which integrates multiple single models into ensemble model, incorporating environmental and species data to analyze the distribution range shifts of A. coccinea under current and future climate scenarios. It also quantified and analyzed shifts in the species’ ecological niche across these climate scenarios. The results indicated that the potential suitable areas for A. coccinea were mainly in Southern North America, northern and south-eastern South America, south-western Europe, the Middle East, central Africa, western Asia, south-eastern Asia, with a gradual increase in mid-high suitability habitat over time and radiation levels. While the overall ecological niche of A. coccinea remains stable, minor shifts are expected under future conditions. Temperature, precipitation, and the human impact index were the key factors influencing the future distribution of A. coccinea. Climate change contributes to the expansion of A. coccinea's highly suitable areas and shifts its ecological niche. Organizations efforts should focus on preventing the spread of A. coccinea in regions where its potential distribution overlaps with key rice production areas. The findings of this study provide critical insights into the global distribution and ecological niche dynamics of A. coccinea, aiding in the development of early warning and control strategies to mitigate its impact on biodiversity, agriculture, and particularly rice production under future climate scenarios.
Keywords: Ammannia coccinea, Biomod2, Climate change, Ecological niche, Habitat distribution
Subject terms: Ecology, Plant sciences, Climate sciences, Ecology, Environmental sciences
Introduction
In recent years, with the acceleration of international exchanges and transnational trade, an increasing number of organisms have crossed spatial boundaries to reach other regions, where they have proliferated and caused adverse impacts on the local ecological ecosystems. As a result, the invasion of exotic organisms has emerged as a significant environmental concern for humanity in the twenty-first century1. Among these invasive species, plants play a major role, accounting for approximately 20% of the world’s flora2. Invasive alien plants pose a serious threat to native species, biodiversity, ecological balance, agricultural production, and even economic stability3,4. For example, species like Mikania micrantha Kunth and Eupatorium odoratum L. have caused significant ecological and economic damages in regions such as China and Australia, contributing to billions in financial losses5–7. These losses include reduced crop yields, increased costs of management and eradication, and disruption of local ecosystems.
In the context of global warming, future climate change will alter the land surface temperature and precipitation pattern, significantly affecting invasive species distribution8. Higher temperatures typically accelerate their metabolism, development, and reproductive cycles, promoting their spread. For example, the red imported fire ant (Solenopsis invicta) has expanded into higher latitude regions as warmer climate allow it to thrive in new habitats9. Similarly, species are likely to shift to higher altitudes and latitudes under global climate change, accelerating the invasion process of exotic species10. In addition to temperature changes, alterations in precipitation patterns can also facilitate the invasion of alien plants. Some studies have shown that genetic alterations and accelerated evolution, triggered by changes in precipitation, contribute to the spread of invasive species. For example, two annual grass species, Avena barbata and Bromus madritensis, have shown reduced growth and reproduction in areas with decreased precipitation in the United States11. Human activities and land use further exacerbate these shifts by modifying habitats and ecosystems12,13. Once invasive species successfully colonize new areas, eradication becomes highly challenging. Therefore, it is crucial to investigate the global distribution of invasion plants under climate change conditions. Such research will provide the foundation for developing effective early warning and control systems to mitigate the threats posed by invasive species14.
To enhance the efficiency of invasive species control, predicting the potential distribution habitats of invasive plants using species distribution models (SDMs) has become a key focus in biological invasion research15. SDMs predict the actual and potential distribution of a species by using algorithms to determine its ecological requirements, projecting these results in a specific spatiotemporal context. This process considers both the known distribution points and the associated environmental variables16. Numerous SDMs are available, each differing in principles, algorithms, and predictive performance17. We applied 10 modelling algorithms from the Biomod2 platform, including Generalized Linear Model (GLM), Generalized Boosted Model (GBM), Generalized Additive Model (GAM), Classification Tree Analysis (CTA), Artificial Neural Network (ANN), one Rectilinear Envelope Similar to BIOCLIM (SRE), Flexible Discriminant Analysis (FDA), Multivariate Adaptive Regression Splines (MARS), Random Forest (RF), Maximum Entropy Models (MaxEnt)18. Biomod2 integrates results from multiple single models to improve the accuracy of species distribution predictions19. Ensemble model refer to the modeling approach where multiple individual models (often referred to as “single models”) are combined to make a final prediction. The greater reliability of ensemble model compared to single models has made them widely used for studying the potential suitable areas for invasive alien plants9, such as Centaurea solstitialis L20, Heracleum mantegazzianum21, Aegilops tauschii and Ambrosia artemisiifolia (common ragweed)22–24.
Ammannia coccinea is a noxious weed that competes with rice and is frequently found in rice paddies. It also thrives in various wet environments including wet meadows, rivers, riverbanks, floodplains, ponds, lakes, and marshes25,26. Native to North and Central America, A. coccinea has been introduced to many countries, including France, Spain, Portugal, Italy, Bulgaria, Greece, Turkey, Australia, Africa, Morocco, and others25,27,28. It was introduced to China, Japan, Korea, Malaysia, and other Asian countries in the 1950s28,29. It is now one of the most widely distributed nuisance weeds in the region30,31. The seeds of A. coccinea are small and highly prolific, averaging 270 seeds per capsule and producing over 500,000 seeds per plant32. In shaded conditions, A. coccinea adapts by increasing the ratio and stem node length while reducing stem diameter, branch number, and stem node count33. A. coccinea contains flavonoids, such as quercetin, that are converted to protective compounds when exposed to UV-B radiation34. These flavonoids scavenge free radicals and improve the plant’s resistance to fungal pathogens35. A. coccinea thrives in freshwater at a depth of up to 0.5 meters25,30. These biological characteristics traits enhance the competitiveness and invasiveness of A. coccinea32. In California rice fields, A. coccinea is highly competitive, outcompeting rice 45 days after sowing, with densities of 110 plants/m2 causing a 39% reduction in rice yields33,36.
Given A. coccinea's high competitiveness and its significant impact on paddy fields, predicting its global distribution patterns under climate change and understanding its niche dynamics are of critical importance. However, comprehensive research on this subject remains limited. This study addresses this gap by employing advanced species distribution models and niche analysis methods to examine A. coccinea's distribution and niche shifts in globally suitable areas. This research reveals A. coccinea's invasion dynamics, offering critical insights for developing effective biosecurity measures and management strategies to mitigate its impact on global agriculture.
Materials and methods
Acquisition and screening of occurrence records
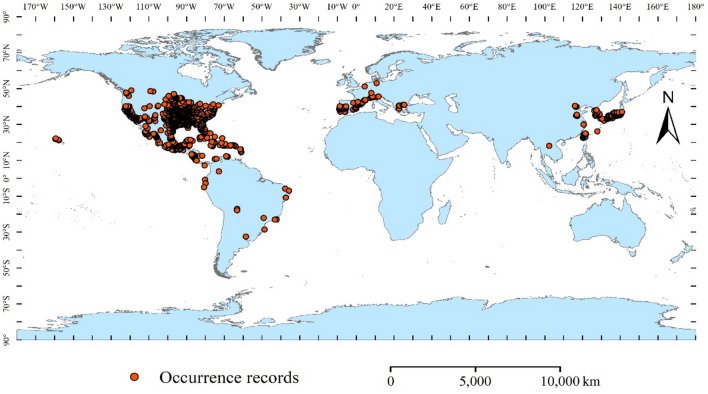
Occurrence records for A. coccinea were gathered from literature resource, field surveys, the Global Biodiversity Information Network (http://www.GBIF.org; https://doi.org/10.15468/dl.d9vykx)37, China Plant Image Bank (https://ppbc.iplant.cn), and China Digital Herbarium (https://www.cvh.ac.cn/index.php). Once the occurrence locations were identified from China Plant Image Bank and China Digital Herbarium, the coordinate picker tool (https://lbs.amap.com/tools/picker) was used to retrieve latitude and longitude data. 2862 occurrences were collected after removing some duplicates. To ensure data accuracy and precision, occurrence records that were not on land or had incorrect coordinates were excluded. Concurrently, to ensure compatibility with the resolution of our environmental variables, we refined the occurrence data using ENMTools v1.4.438. This process filtered the data to retain only one occurrence record per 10 × 10 km grid, effectively minimizing spatial autocorrelation. If unaddressed, spatial autocorrelation can skew results due to the clustering of data points39. Finally, 1138 occurrence records of A. coccinea were obtained for modeling its potential global distribution using Biomod2 (Fig. 1).
Fig. 1.
Screened global distribution occurrence records of Ammannia coccinea.
Obtaining and filtering environment variables
Current climate data (1970–2000) and future climate data (2041–2060, 2061–2080, 2081–2100) were downloaded from WorldClim (www.worldclim.org)40. The current climate data come from WorldClim 2.1 and include 19 variables related to temperature and precipitation, along with digital elevation data (elev), all at a resolution of 5 arc-minutes. To account for the influence of human activities and land use on invasive plant distribution34,35, we included Human Impact Index (HII;2.5arc-minutes) and global land cover data (GlobCover 2009; http://due.esrin.esa.int/page_globcover.php; 2.5arc-minutes). Both variables were resampled to 5 arc-minute resolution in ArcGIS. Future climate data for the 2050s, 2070s, and 2090s were derived from three shared socioeconomic pathways (SSPs) under CMIP6: SSP126, SSP245, and SSP585. These scenarios reflect different levels of radiative forcing in 2100: 2.6 W/m2 in a sustainability-focused world (SSP126), 4.5 W/m2 in a middle-of-the-road scenario (SSP245), and 8.5 W/m2 in a high-growth, high-energy world (SSP585)41,42. Data from the BCCCSM2-MR model were used, which combines SSPs with Representative Concentration Pathways (RCPs) to project future climate trends43.
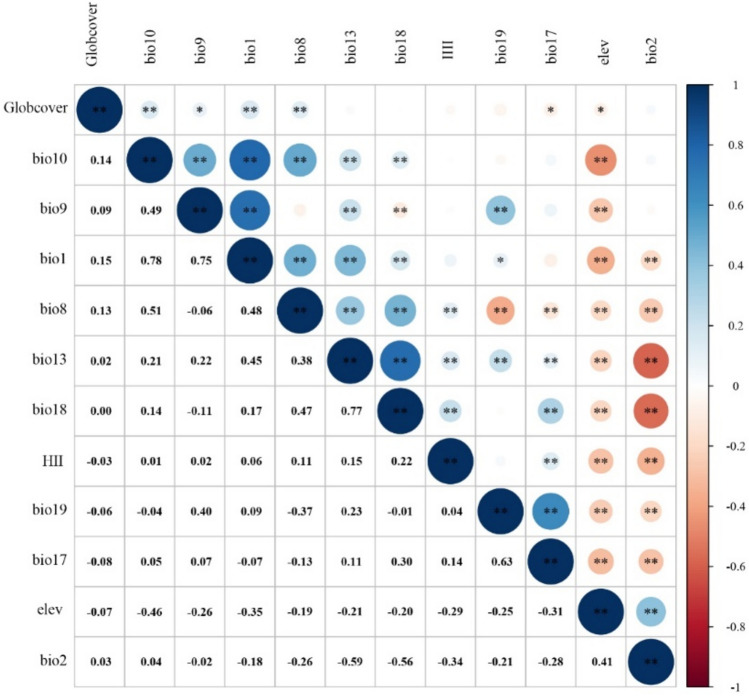
To prevent model misinterpretation due to multicollinearity between environmental variables44, we conducted a correlation analysis on the 22 bioclimatic variables using R. The variables were first extracted to the sample points using ArcGIS’s multi-value extraction module and then analyzed for correlation using R. Variables with a correlation coefficient |r|> 0.8 were removed, keeping the one with the higher contribution to the models based on jackknife importance values. This process reduced the 22 variables to 12 predictors, minimizing redundancy and enhancing model robustness (Table 1, Fig. 2). Correlation analysis is essential for identifying multicollinearity, preventing highly correlated variables from distorting model outcomes. Jackknife importance values assess each variable’s independent contribution to the models. Combining these two approaches ensured the models remained both robust and interpretable.
Table 1.
Selected climatic variables influencing the distribution of Ammannia coccinea.
| Code | Description | Unit | Weather to use A. coccinea for modeling | Importance (Jackknife) |
|---|---|---|---|---|
| bio1 | Annual mean temperature | °C | Yes | 6 |
| bio2 | Mean diurnal range | °C | Yes | 2.5 |
| bio3 | Isothermality | °C | No | 2 |
| bio4 | Temperature seasonality | – | No | 5.1 |
| bio5 | Maximum temperature of the warmest month | – | No | 1.5 |
| bio6 | Minimum temperature of the warmest month | °C | No | 0.4 |
| bio7 | Annual mean temperature range | °C | No | 1.1 |
| bio8 | Mean temperature of the wettest quarter | °C | Yes | 0.1 |
| bio9 | Mean temperature of the driest quarter | °C | Yes | 0.1 |
| bio10 | Mean temperature of the warmest quarter | °C | Yes | 10.9 |
| bio11 | Mean temperature of the coldest quarter | °C | No | 0.9 |
| bio12 | Annual precipitation | mm | No | 0.4 |
| bio13 | Precipitation of the wettest month | mm | Yes | 1.3 |
| bio14 | Precipitation of the driest month | mm | No | 0.6 |
| bio15 | Precipitation seasonality ( CV) | – | No | 0.8 |
| bio16 | Precipitation of the wettest quarter | mm | No | 0.2 |
| bio17 | Precipitation of the driest quarter | mm | Yes | 3.6 |
| bio18 | Precipitation of the warmest quarter | mm | Yes | 0.3 |
| bio19 | Precipitation of the coldest quarter | mm | Yes | 7.8 |
| HII | Human Influence Index | – | Yes | 44.4 |
| Globcover | Global land cover | Km2 | Yes | 8.6 |
| elev | above sea level | M | Yes | 1.3 |
Fig. 2.
Correlation analysis of 12 selected environment variables.
Construction and evaluation of ensemble models
The Biomod2 includes 10 single models, enabling a wide range of configurations to meet diverse research needs45. By integrating these single models, we leverage their respective sensitivities and explanatory powers to enhance the diversity, robustness, and comprehensiveness of predictions. This ensemble approach reduces individual model bias, minimizes errors, and improves overall prediction accuracy by combining outputs, thereby better assessing uncertainty19. Studies have shown that combining multiple single models generally results in higher predictive accuracy than using a single model, thereby improving the reliability and practicality of scientific research17.
In this study, we used 10 single models (GAM, GBM, GLM, CTA, MARS, ANN, SRE, FDA, RF, MaxEnt) from the R package Biomod2 to predict potentially suitable areas for A. coccinea based on species occurrence records and environmental variables. We employed the disk method in Biomod2 to generate pseudo-absence samples equal in number to the presence points. This approach enhances the accuracy of classification algorithms like classification trees and random forests46. In each species distribution models setup, 75% of the data was used for training and the remaining 25% for testing to evaluate model accuracy and reliability47. The division of training and test data was repeated randomly 5 times and the models were repeated 10 times. Additionally, 500 pseudo-negative sample points were randomly selected 46,48,49 and repeated three times, resulting in a total of 150 modeled species distributions.
The true skill statistic (TSS), area under the receiver operating characteristic curve (AUC), and Cohen’s kappa (Kappa) were used to evaluate model accuracy, with values closer to 1 indicating more reliable predictions50–52. The evaluation criteria for AUC, TSS and Kappa are presented in Table 2. Of the 150 constructed models, those with TSS and AUC values greater than 0.75 were selected to build the ensemble species distribution models. Model weighting in the ensembles was based on the evaluation score. Models with higher ratings received greater weighting in the combined models50. The importance of each environmental factor was assessed using Biomod2. Finally, the ensemble model was used to predict the potential global distribution of A. coccinea under climate change scenarios.
Table 2.
Evaluation standard for AUC, TSS and Kappa.
| Evaluation index | Fail | Bad | Medium | Good | Excellence |
|---|---|---|---|---|---|
| AUC | 0.50–0.60 | 0.60–0.70 | 0.70–0.80 | 0.80–0.90 | 0.90–1.00 |
| TSS | 0.00–0.40 | 0.40–0.55 | 0.55–0.70 | 0.70–0.85 | 0.85–1.00 |
| Kappa | 0.00–0.40 | 0.40–0.55 | 0.55–0.70 | 0.70–0.85 | 0.85–1.00 |
We employed multiple integrated methods to construct ensemble models for the potential distribution areas of A. coccinea, including EMmean (ensemble mean), and EMca (consensus average) etc. This multifaceted approach enabled a comprehensive evaluation of the models, ensuring the selection of the optimal ensemble model for accurate prediction.
Division of the suitable areas
The critical value of ensemble model were used as the threshold to differentiate between suitable and unsuitable zones. The ASCII raster layer, ranging from 0 to 1,000 in the ensemble model, indicated the occurrence probability (p) of A. coccinea. A larger P-value indicates a higher probability of occurrence of A. coccinea9,20. We divided the potential global distribution of A. coccinea into four categories using ArcGIS: high-suitability habitat (600 ≤ P ≤ 1000), moderate-suitability habitat (400 ≤ P < 600), low-suitability habitat (200 ≤ P < 400), and unsuitable habitat (0 ≤ P < 200). We used the raster map of suitable zones for A. coccinea to create binary maps through the reclassification function in ArcGIS 10.2, and applied the SDM Toolbox v2.0 plugin to calculate the spatial patterns of these suitable zones. When binarizing the prediction results, we used 0.2 as the boundary between suitable and non-suitable zones. We compared current and future projections of total suitable area and assessed changes by calculating areas of expansion, contraction, and rates of gain and loss.
Ecological Niche comparison measures
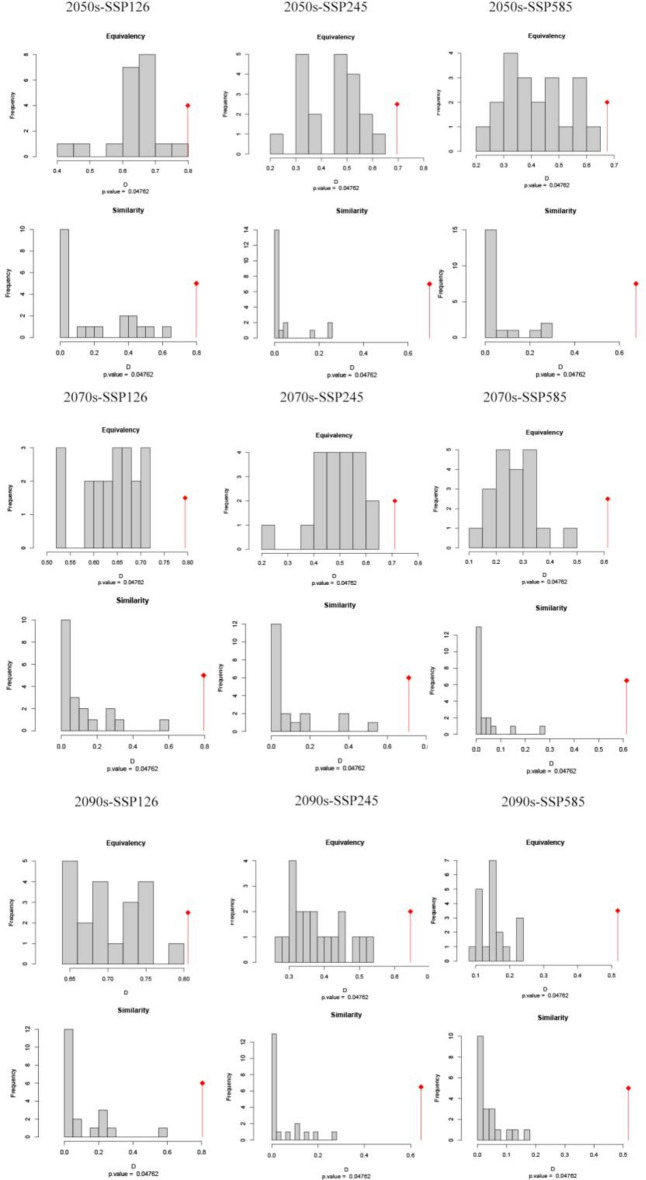
We utilized the Principal Component Analysis (PCA) method from the ecospat software package53 to investigate ecological niche shifts of A. coccinea under current and future climatic scenarios. This approach is widely used to analyze the ecological niche dynamics of invasive alien species54,55. We employed the Schoener’s D metric was used to quantify ecological niche overlap, which ranges from 0 to 1, with larger values indicating greater overlap between the two areas56. This index is crucial for understanding how the ecological niche of A. coccinea changes or remains stable under current and future climate scenarios. Niche equivalency tests and similarity were conducted to evaluate the significance of niche overlap across geographic areas56,57. The niche equivalency test assessed whether the niches of the two entities were equal (full overlap), moderately similar (partial overlap), or distinctly different (no overlap). The niche similarity test assessed whether the niches of the two entities being compared were more similar (or different) than expected by chance, also considering the surrounding environmental conditions throughout the geographic area56,58. The test was repeated randomly 100 times, and the null hypothesis of ecological niche equivalence or similarity could be rejected if the observed niche values (D) were significantly lower than the overlap value from the null distribution (P ≤ 0.05)57.
To assess niche dynamics, an environments was considered to indicate niche expansion if it is available in both current and future ranges but was only occupied in the future range56. Similarly, an environment indicated niche stability if occupied in both current and future ranges, while niche unfilling if it was used in the current range but available yet unexploited in the future range58. Values for expansion, stability, and unfilling ranging from 0 to 100% were deemed significant if greater than 10%. Niche expansion is regarded as the sole measure that accurately reflects shifts in a realized niche54,55.
Results
Evaluation of model accuracy
We evaluated the performance of 10 individual models (GAM, GBM, GLM, CTA, SRE, MARS, FDA, ANN, RF, and MaxEnt) using TSS, Kappa, and AUC as evaluation metrics (Table 3). Since the TSS values of SRE and ANN did not meet the threshold of greater than 0.75, they were not included in the construction of the ensemble models. Ultimately, we selected GAM, GBM, GLM, CTA, MARS, FDA, RF, and MaxEnt to construct the ensemble models. We found that the EMca version had the highest accuracy, with TSS and AUC values greater than those of EMmean (Table 3). Therefore, we selected the EMca version for further visualization and analysis.
Table 3.
Evaluation metric values for individual models and ensemble models.
| Evaluation index | MARS | RF | MAXENT | CTA | FDA | GAM | GBM | GLM | EMca | EMmean |
|---|---|---|---|---|---|---|---|---|---|---|
| TSS | 0.844 | 0.992 | 0.857 | 0.882 | 0.828 | 0.836 | 0.883 | 0.807 | 0.891 | 0.876 |
| ROC | 0.967 | 0.997 | 0.944 | 0.972 | 0.961 | 0.974 | 0.986 | 0.958 | 0.983 | 0.982 |
| KAPPA | 0.847 | 0.991 | 0.832 | 0.884 | 0.831 | 0.844 | 0.892 | 0.816 | – | – |
Significance of environmental variables
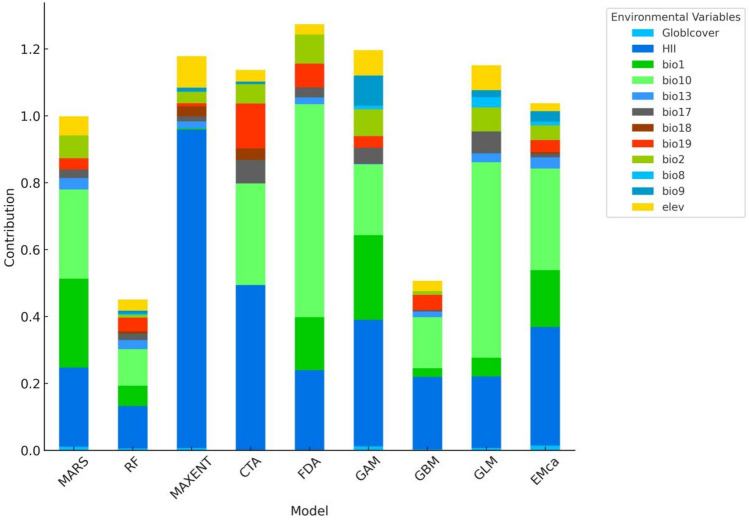
Eight single models was used to assess the contribution of environmental variables affecting the distribution of A. coccinea, and the results were shown in Fig. 4. The top contributing variables across different models varied slightly, but the most influential variables included the Human Impact Index (HII), mean annual temperature (bio1), mean temperature of the warmest quarter (bio10), and precipitation of the coldest quarter (bio19). Specifically, the ensemble model results (Fig. 3) confirmed that these four environmental variables were consistently the most influential across all models, indicating that two temperature variables, one precipitation variable, and one anthropogenic factor primarily drive the distribution of suitable habitats for A. coccinea. This highlights the central role of temperature and human influence in determining the species’ suitable habitats.
Fig. 4.
Changes in the ecological niche of A. coccinea under different future climate scenarios compared to the current.
Fig. 3.
Contribution values of environmental variables in single models and the ensemble model.
Ecological Niche analysis
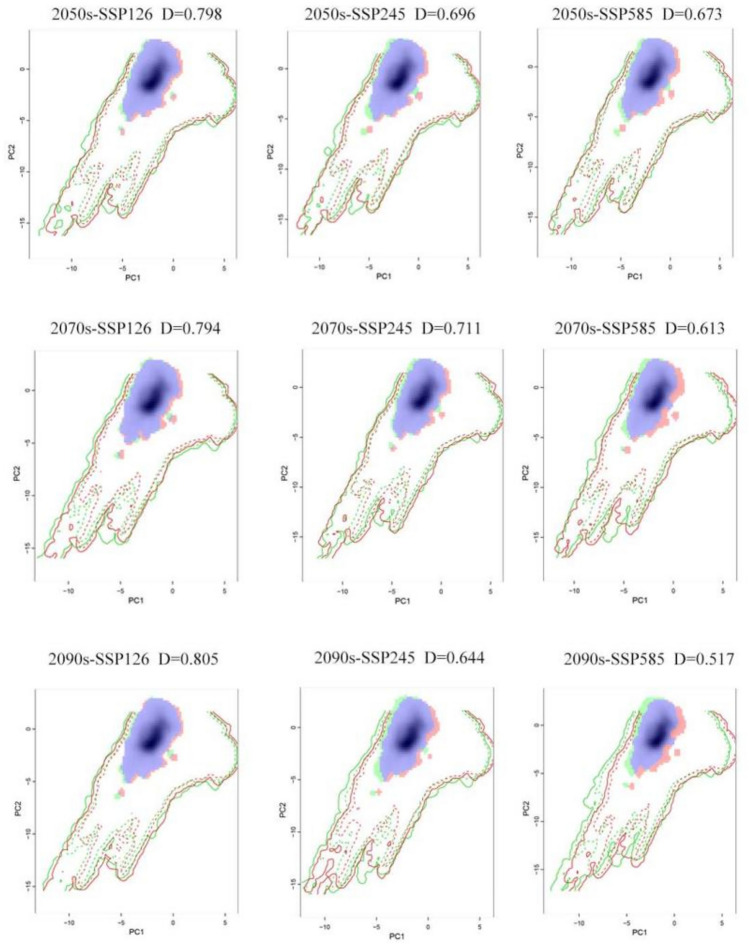
Niche analyses contrasting current and future climatic conditions revealed a moderate degree of similarity across all scenario comparisons, with Schoener’s D values ranging from 0.518 for current vs SSP585-2090s to 0.805 for current vs SSP126-2050s (Fig. 4; Table 4). These Schoener’s D values indicate moderate to high similarity between suitability distributions in different climatic scenarios. The pattern suggests a prospective shift in climatic suitability, with a potential reduction of A. coccinea's current niche under future climate conditions. The results showed that the Schoener’s D values for the SSP126 scenarios are all greater than the SSP245 scenarios, while the Schoener’s D for the SSP245 scenarios are all greater than the SSP585 scenarios. In short, there was a decreasing trend in the Schoener’s D values with an increasing radiation intensity over time. The results of the climatic background PCA have been presented in Table 4 and Figure S3. Across all PCA analyses, PC1 consistently captures the largest source of climatic variation, typically associated with temperature-related variables (e.g., bio1, bio10). PC2, on the other hand, reflects the second-largest variation in the climatic dataset, generally driven by precipitation variables (e.g., bio19). These principal components together provide a comprehensive summary of the key climatic factors influencing the environmental variation across different time periods and SSP scenarios. This result is consistent with the importance of environment variables in Biomod2.
Table 4.
Niche comparisons and variation between the current and future projected distribution ranges of A. coccinea.
| Niche comparison pairs | PC1 (%) | PC2 (%) | Niche overlap (D) | Niche expansion (%) | Niche stability (%) | Niche unfilling (%) |
|---|---|---|---|---|---|---|
| Current vs SSP126-2050s | 38.08 | 23.21 | 0.799 | 0.008 | 0.992 | 0.004 |
| Current vs SSP126-2070s | 38.10 | 23.18 | 0.794 | 0.007 | 0.993 | 0.003 |
| Current vs SSP126-2090s | 38.01 | 23.32 | 0.805 | 0.006 | 0.994 | 0.003 |
| Current vs SSP245-2050s | 38.05 | 23.22 | 0.696 | 0.006 | 0.994 | 0.007 |
| Current vs SSP245-2070s | 37.91 | 23.18 | 0.711 | 0.009 | 0.991 | 0.007 |
| Current vs SSP245-2090s | 37.75 | 23.15 | 0.645 | 0.012 | 0.987 | 0.012 |
| Current vs SSP585-2050s | 37.78 | 23.20 | 0.673 | 0.01 | 0.99 | 0.008 |
| Current vs SSP585-2070s | 37.40 | 23.21 | 0.613 | 0.026 | 0.974 | 0.01 |
| Current vs SSP585-2090s | 37.11 | 23.01 | 0.518 | 0.037 | 0.963 | 0.022 |
Furthermore, we extended the exploration of niche equivalency and similarity. In pairwise analyses of climate ecological niches of species under current and future climate scenarios, the p-values for equivalence were less than 0.05, leading to the rejection of the null hypothesis of ecological niche equivalency and similarity in all pairwise comparisons (Fig. 4; Table 4). This suggested that A. coccinea may experience significant differences in its ecological niche characteristics under future climate scenarios (Fig. 5).
Fig. 5.
The ecological niche equivalency and similarity of A. coccinea under different future climate scenarios compared to the current.
Red areas indicate expansion, green areas represent unfilling, and blue areas show overlap (Table 4). The values of ecological niche expansion and stability suggested that A. coccinea will experience minimal niche expansion (< 10%) in the future. However, as radiation intensity increases over time, the extent of ecological niche expansion and unfilling is expected to increase, while niche stability is likely to decrease.
Current potential global distribution of A. coccinea
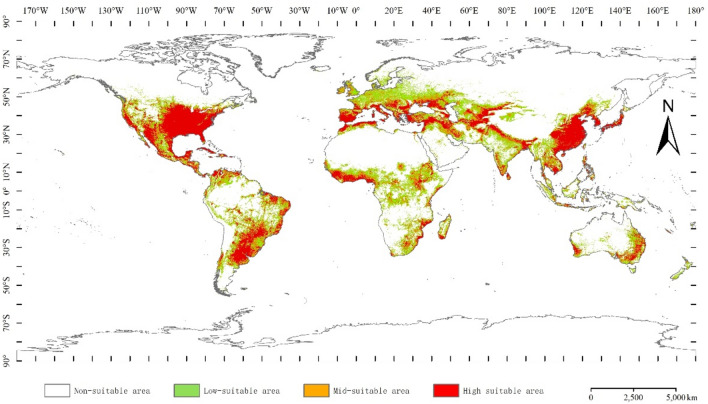
We employed the ensemble model in Biomod2 to predict the potential global distribution of A. coccinea under current climate conditions (Fig. 6). Under current climatic conditions, the potential global geographic distribution of A. coccinea is concentrated in southern North America, northern and southeastern South America, southwestern Europe, the Middle East, central Africa, western Asia, Southeast Asia, etc. (150 °E–120 °W, 40 °S–60 °N). Specifically, the high suitable habitat area was 1,759.12 × 104 km2, accounting for 11.81% of the global land area, covering regions such as the United States, Mexico, Cuba, Puerto Rico, Jamaica, northern Colombia, northern Venezuela, Portugal, the eastern coast and south of Brazil, northeastern Argentina, Paraguay, Spain, Italy, Greece, Turkey, the southwestern corner of Russia, Iran, India, northern China, Cambodia, Vietnam, the Philippines, Korea, Japan, southeastern Australia, Guinea, Liberia, the southern coast of Nigeria, and other areas. North, southeastern China, Cambodia, Vietnam, Philippines, Korea, Japan, southeastern Australia, Guinea, Liberia, southern coast of Nigeria and other regions. The moderately suitable habitat area was 101.76 × 104 km2, accounting for 8.1% of the global land area, mainly surrounding the highly suitable area. The low-suitable habitat area was 1920.86 × 104 km2, accounting for 12.89% of the global land area, covering regions such as the northwestern United States, central Mexico, Peru, Bolivia, central Colombia, Venezuela, Fatima, Germany, Poland, Hungary, Rome, Ukraine, Turkey, Iran, Uzbekistan, Pakistan, central India, Myanmar, Indonesia, Cameroon, Central Africa, the Congo, the Sudan, South Africa, Zimbabwe, Mozambique, Madagascar and other regions.
Fig. 6.
Potential global distribution of A. coccinea under current climate conditions.
Future potential global distribution of A. coccinea
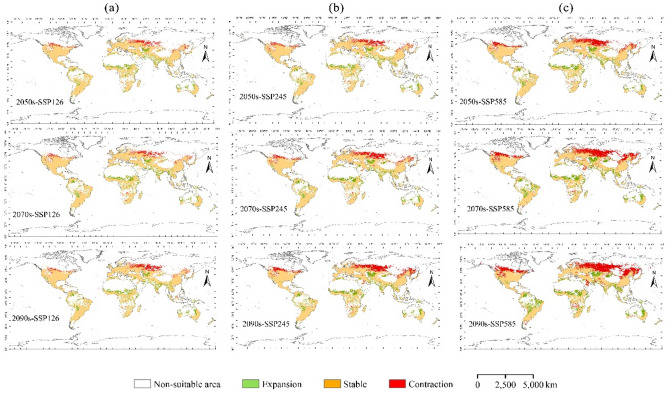
The potential geographic distribution and suitable area of A. coccinea under future climate conditions are presented in the supplemental materials (Figures S1 and S2, Table S1). The changes in the area of A. coccinea distribution under future climate change conditions compared to the current distribution are shown in Fig. 7.
Fig. 7.
Projected changes in potential global suitable habitats for A. coccinea under future climate scenarios compared to the current climate: (a) SSP126 for the 2050s, 2070s, and 2090s; (b) SSP245 for the 2050s, 2070s, and 2090s; (c) SSP585 for the 2050s, 2070s, and 2090s.
Compared with the current conditions, mid-high suitable areas were expected to expand under future climate scenarios, with the most significant expansion observed under the SSP585 scenario in the 2090s (Figures S1, S2). While the distribution of suitable areas remains relatively stable in some regions, overall, significant geographical shifts are anticipated under future climate conditions. Except for SSP245 in 2090 and SSP585 in 2070 and 2090, habitat contraction exceeds expansion, and total suitable habitat area decreases under all other climate scenarios. Notable expansion were expected in regions such as northeastern Bolivia, eastern Colombia, central Brazil, Nigeria, South Sudan, Uzbekistan, central India, Myanmar, Thailand, northwestern China, and parts of Australia. The contractions areas of the suitable areas were mainly located in the southwestern Russia, northern Kazakhstan, southern Africa, northern China, and southern Cana.
Discussion
Accuracy of model predictions
Variations in the prediction process and parameter algorithms across individual models may lead to uncertainty in their prediction results9. Ensemble model constructed using the Biomod2 platform were applied to predict the potential habitat distribution of the invasive plant A. coccinea, effectively reducing the uncertainty and simulation bias with single models. To mitigate sampling bias, the occurrence records of A. coccinea were carefully screened. To avoid multicollinearity among environmental variables, Pearson correlation analysis and jacknife method were used to remove highly correlated and less importance factors. Nine climate factors, one terrain factor, one human disturbance factor, and one land type coverage factor were selected for the distribution models. The predicted values of AUC, TSS, and Kappa for the single models met the “excellent” standard, while the of AUC and TSS values for the ensemble model reached the standard of "excellent." This indicated that the model predictions were accurate and reliable, and closely aligned with the species, actual distribution. Thus, these models can be utilized to analyze the global potential distribution of A. coccinea.
Key environmental and anthropogenic factors influencing the global distribution of A. coccinea
The key environmental variables influencing the potential global distribution of A. coccinea included annual mean temperature (bio1), mean temperature of the warmest quarter (bio10), precipitation of the coldest quarter (bio19), and human impact index (HII). In summary, the potential global geographic distribution of A. coccinea was determined by the synergistic effects of three environmental variables: temperature, precipitation and anthropogenic impacts. In areas where A. coccinea has successfully invaded, favorable temperature and precipitation have facilitated its colonization and expansion. This has led to faster reproduction and greater adaptability in A. coccinea25,33,34. Research has shown that A. coccinea is photoblastic, requiring temperature above 15 °C and a soil burial depth of less than 3 cm for successful germination and seedling emergence29. Dormant A. coccinea seeds require at least 100 days of cold stratification, diurnal temperature fluctuations, and light for optimal germination59. Generally, A. coccinea thrives in warm environments, with temperatures above 28 °C favoring its growth32. Climate warming has increased A. coccinea,s environmental tolerance, expanding its invasive range. These studies support and validate our findings.
The coldest season precipitation (bio19) significantly influences the potential geographic distribution of A. coccinea. Precipitation plays a crucial role in determining the establishment and spread of invasive species, as it directly affects soil moisture levels and plant physiology51. For A. coccinea, adequate precipitation during colder months may promote germination and seedling survival, particularly in regions with moderate or seasonal droughts. Insufficient precipitation can limit the species’ growth by reducing soil water availability, leading to lower reproductive success and slower population expansion60. Studies have shown that altered precipitation patterns, driven by climate change, have already begun reshaping the geographic distribution of invasive species globally, facilitating their spread into previously unsuitable areas11,61,62. Therefore, changes in precipitation patterns due to climate change could enhance the invasiveness of A. coccinea in regions with favorable conditions.
Anthropogenic influences have also partially shaped the potential geographic distribution of A. coccinea. Studies have shown that A. coccinea frequently occurs in rice fields, which are heavily impacted by human activities. Human influences have also facilitated multiple dispersal pathways of A. coccinea. The seeds of A. coccinea can be dispersed through various pathways, including agricultural trade, water flow, and attachment to mud or machinery tires used in cultivation22. The spread of A. coccinea is accelerated by human activities. Therefore, under suitable temperature, precipitation, and anthropogenic activity, A. coccinea will flourish.
Changes in potential geographic distribution of A. coccinea
Our results showed that, under current climatic, the potential geographical distribution of A. coccinea is mainly in Southern North America, northern and southeastern South America, southwestern Europe, West Asia, and Southeast Asia. This was consistent with previous research32, indicating a strong agreement between the simulated potential geographic distribution of A. coccinea and its actual range under the current climatic scenarios. Research indicates that under future climate conditions, the potential areas of moderate to high suitability for A. coccinea are expected to expand. Furthermore, with ongoing climate change, particularly the rise in radiation intensity, the global invasion range of A. coccinea is expected to expand rapidly in moderate to high suitability zones. This suggests that different socioeconomic development pathways significantly impact the global spread of A. coccinea, with high fossil fuel consumption accelerating its spread63. Additionally, the Schoener’s D values and niche analysis results indicate that as radiation intensity increases (e.g., in the SSP585 scenario), the climatic suitability of A. coccinea’s current niche gradually decreases. This reduction in suitability may drive the species to seek new habitats at higher latitudes where temperature and precipitation conditions are more favorable. Specifically, the declining trend in Schoener’s D values suggests that A. coccinea's current niche will become less suitable in the future, promoting a shift in its range to higher latitudes to find climates that match its tolerance thresholds.
Ecological niche dynamics and adaptation of A. coccinea
Ecological niches are crucial for prediction invasive species, distribution. Invasive alien species adapt to new habitats in a variety of ways and expand their ecological niche space after colonisation, ultimately leading to differences between native and invasive ecological niches, such as thermal niche shifts64. For A. coccinea, the maximum ecological niche overlap (SSP126-2050s) was 0.805, indicating substantial similarity, while the minimal ecological niche overlap (SSP585-20900 s) decreased to 0.518, indicating greater differentiation in extreme future climate scenarios. This suggests that A. coccinea has broad ecological adaptability to various environments.
Despite overall niche stability for A. coccinea under future conditions, we observe increased niche expansion and unfilling as radiation intensity rose (e.g., SSP585), suggesting A. coccinea will exploit more diverse habitats and expand its range. As its current niche becomes less suitable, it is likely to migrate to higher latitudes in search of favorable conditions. Additionally, the PCA analysis showed significant shifts in the species’ niche under future scenarios, with niche equivalency being rejected (p < 0.05). This supports the potential for A. coccinea to expand its distribution as temperatures rise and precipitation patterns change. As the suitable areas expand, A. coccinea is likely to face competition with local species for resources and space.This competition could negatively impact local biodiversity, as invasive species often suppress or replace native species by occupying resources and altering habitats33,35. Therefore, as climate change intensifies, managing the expansion of A. coccinea will become a critical issue for ecological management.
Targeted prevention and pontrol strategies for A.coccinea
A. coccinea is a noxious weed that competes with rice in paddy fields and is spreading globally. Globalization and climate warming have promoted the invasion of alien species65,66. Managing A. coccinea is challenging due to climate change, with its global distribution influenced by temperature, precipitation, and human activities. The seeds can spread through water currents or agricultural machinery, such as rice harvesters34. Therefore, government should focus prevention and control efforts on regions where A. coccinea overlaps with key rice production areas, rather than only targeting highly suitable habitats for the weed. Tailored prevention strategies are crucial, especially in areas where rice production and A. coccinea overlap, as these regions face significant threats to food security.
Control measures should combine mechanical, chemical and biological measures. Mechanical techniques like early removal and tilling can eliminate the weed, while herbicides provide chemical control. Biological control through natural enemies like insects or pathogens can offer long-term suppression. Early and intensive control usually yields better outcomes62. Thus, agencies should conduct surveys in high-risk areas to assess the weed’s impact and provide a scientific basis for implementing effective control measures. These efforts will improve global knowledge of invasive species management and reduce the impact on rice production.
Existing deficiencies and future development directions
Our analysis of A. coccinea's potential distribution is more comprehensive and accurate compared to previous studies. The key advantages are: (1) Using the latest species distribution data, which improves accuracy and geographic coverage; (2) Implementing a stricter environmental variable selection, considering factors like the human impact index (HII) and Globcover; (3) Using the Biomod2 platform to integrate multiple models, enhancing prediction reliability; (4) Expanding the study to a global scale, predicting future distributions across different climate scenarios; (5) Quantifying niche dynamics like expansion, unfilled areas, and stability, offering deeper insights into the species’ ecological adaptability.
Despite efforts to improve model accuracy, species distribution is a complex result of biotic and abiotic interactions. Our study focused on climate, HII, and Globcover, but factors like species interactions, infrastructure development, and trade activities also influence invasive species spread, highlighting limitations in our model43. Future research should include a wider range of variables, especially species interactions, native biota, and socio-economic factors, to better understand distribution patterns of A. coccinea and other invasive species under changing environmental conditions. Although our research offers valuable insights into the species’ distribution, future studies should incorporate more comprehensive factors for deeper understanding and more effective management strategies.
Conclusions
The habitats of A. coccinea are highly susceptible to climate change, resulting in shifts in suitable areas and alterations the species, ecological niches. Using the ensemble model constructed from the best-performing single models, we achieved high accuracy in predicting the potential global distributions of A. coccinea. Under current climatic conditions, the species is predominantly found in Southern North America, northern and south-eastern South America, south-western Europe, the Middle East, central Africa, western Asia, and south-eastern Asia. Future climate scenarios predict an expansion of mid-to-high habitability areas for A. coccinea.
While the overall ecological niche of A. coccinea remains stable, minor shifts are expected under future conditions. Key environmental drivers—temperature, precipitation and human impact index (HII) -played a key role in facilitating its invasion and expansion globally. Given these findings, organizations efforts should focus on preventing the spread of A. coccinea in regions where its potential distribution overlaps with key rice production areas, rather than solely targeting the species’ highly suitable habitats. Strengthening prevention and control measures in such regions is crucial, as A. coccinea poses a threat to food security. Taking proactive measures will not only safeguard agricultural production but also contribute to achieving the United Nations Sustainable Development Goals, particularly those related to food security and environmental sustainability.
Supplementary Information
Author contributions
YanJing Zhang wrote the main manuscript text; Jie Hu and ChenBin Wang provided Formal analysis, investigation and Writing–review & editing; YaQiong Wan &Mulan Ji prepared Writing – review & editing; FangZhou Ma and YiQing Lu provided Funding acquisition, Methodology and Writing–review & editing. All authors reviewed the manuscript.
Funding
National Key Research and Development Program of China, 2021YFC2600400, Project Strengthening coordinated approaches to reduce invasive alien species (IAS) threats to globally significant agrobiodiversity and agroecosystems in China.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due (our experimental team’s policy) but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82164-6.
References
- 1.Pyšek, P. & Richardson, D. M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour.35, 25–55 (2010). [Google Scholar]
- 2.Ziwen, W. Combinatorial modeling based on the study of the habitat of invasive alien plants Manduca sexta and Prunus yellowsii in Liaoning Province. (Master’s thesis, Liaoning University) (2023). (in Chinese)
- 3.Anderson, L. G., Rocliffe, S., Haddaway, N. R. & Dunn, A. M. The role of tourism and recreation in the spread of non-native species: A systematic review and meta-analysis. PLoS One10, e0140833 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellard, C., Cassey, P. & Blackburn, T. M. Alien species as a driver of recent extinctions. Biol. Lett.10.1098/rsbl.2015.0623 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee, A. K., Mukherjee, A., Guo, W., Liu, Y. & Huang, Y. Spatio-temporal patterns of climatic niche dynamics of an invasive plant mikania micrantha kunth and its potential distribution under projected climate change. Front. Ecol. Evol.7, 291 (2019). [Google Scholar]
- 6.Bradshaw, C. J. et al. Detailed assessment of the reported economic costs of invasive species in Australia. NeoBiota67, 511–550 (2021). [Google Scholar]
- 7.Fang, Y. et al. Predicting the invasive trend of exotic plants in China based on the ensemble model under climate change: A case for three invasive plants of Asteraceae. Sci. Total Environ.756, 143841 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Chen, I.-C., Hill, J. K., Ohlemüller, R., Roy, D. B. & Thomas, C. D. Rapid range shifts of species associated with high levels of climate warming. Science333, 1024–1026 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Li, M. et al. Geographical distribution pattern and ecological Niche of Solenopsis invicta Buren in China under climate change. Diversity15, 607 (2023). [Google Scholar]
- 10.Shrestha, U. B., Sharma, K. P., Devkota, A., Siwakoti, M. & Shrestha, B. B. Potential impact of climate change on the distribution of six invasive alien plants in Nepal. Ecol. Indicat.95, 99–107 (2018). [Google Scholar]
- 11.Puritty, C. E., Mayfield, M. M., Azcárate, F. M. & Cleland, E. E. Different traits predict competitive effect versus response by Bromus madritensis in its native and invaded ranges. Biol. Invas.20, 2553–2565 (2018). [Google Scholar]
- 12.Vilà, M. & Pujadas, J. Land-use and socio-economic correlates of plant invasions in European and North African countries. Biol. Conserv.100, 397–401 (2001). [Google Scholar]
- 13.Zhao, H. et al. Constructing an ensemble model and niche comparison for the management planning of eucalyptus longhorned borer Phoracantha semipunctata under climate change. Insects14, 84 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natale, E. S., Zalba, S. M., Reinoso, H. E. & Damilano, G. Assessing invasion process through pathway and vector analysis: Case of Saltcedar (Tamarix spp.). (2012).
- 15.Elith*, J. et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography29, 129–151 (2006). [Google Scholar]
- 16.Franklin, J. Species distribution models in conservation biogeography: Developments and challenges. Divers. Distribut.19, 1217–1223 (2013). [Google Scholar]
- 17.Luo, M., Wang, H. & Lyu, Z. Evaluating the performance of species distribution models Biomod2 and MaxEnt using the giant panda distribution data. Ying yong sheng tai xue bao = The J. Appl. Ecol.28, 4001–4006 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Lantschner, M. V., de la Vega, G. & Corley, J. C. Predicting the distribution of harmful species and their natural enemies in agricultural, livestock and forestry systems: an overview. Int. J. Pest Manag.65, 190–206 (2019). [Google Scholar]
- 19.Hao, T., Elith, J., Guillera-Arroita, G. & Lahoz-Monfort, J. J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distribut.25, 839–852 (2019). [Google Scholar]
- 20.Jia, T. et al. Estimation of climate-induced increased risk of Centaurea solstitialis L. invasion in China: An integrated study based on biomod2. Front. Ecol. Evolut.11, 1113474 (2023). [Google Scholar]
- 21.Mędrzycki, P. et al. Simple yet effective: Historical proximity variables improve the species distribution models for invasive giant hogweed (Heracleum mantegazzianum sl) in Poland. PloS one12, e0184677 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung, H. I. et al. Integrated spatial model based evaluation methodology for optimal invasive species management: common ragweed in the Republic of Korea. Environ. Res. Lett.17, 034047 (2022). [Google Scholar]
- 23.Xian, X. et al. Climate change has increased the global threats posed by three ragweeds (Ambrosia L.) in the Anthropocene. Sci Total Environ859, 160252 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Qianhong, T., Donglin, Z., Jing, Z., Xiaokang, H. & Wang, T. Potential threats and their driving factors of the farmland weeds knapweed and ragweed under global climate change. J. Ecol.04, 1130–1140 (2024) ((in Chinese)). [Google Scholar]
- 25.Graham, S. A. & Gandhi, K. Nomenclatural changes resulting from the transfer of Nesaea and Hionanthera to Ammannia (Lythraceae). Harvard Papers Botany18, 71–90 (2013). [Google Scholar]
- 26.Vladimirov, V., Delcheva, M., Georgiev, V., Tsoneva, S. & Gussev, C. Ammannia coccinea Rottb. (Lythraceae): The first report for the Bulgarian alien flora. Acta Zool. Bulgarica9, 39–42 (2017). [Google Scholar]
- 27.Zheng G. Competitive effects of the exotic invasive longleaf water amaranth on rice and its drug resistance. (Zhejiang University. MA thesis)( 2017).(in Chinese)
- 28.Park, S. H. New illustrations and photographs of naturalized plants of Korea 559 (Ilchokak Pulishing Co, 2009). [Google Scholar]
- 29.Shen, X., Pyon, J.-Y. & Kim, D.-S. Germination and seedling emergence of Ammannia coccinea as influenced by environmental factors. Korean J. Weed Sci.30, 84–93 (2010). [Google Scholar]
- 30.Naqinezhad, A. & Larijani, N. N. Ammannia coccinea (Lythraceae), a new record for the Flora Iranica area. Phytol. Balcan.23, 35–38 (2017). [Google Scholar]
- 31.Hwang, S., Kil, J., Kim, Y. & Kim, S. Spreading and distribution of exotic weed Ammannia coccinea in Korea. Weed Turfgrass Sci.3, 292–298 (2014). [Google Scholar]
- 32.Zhu, J. et al. New invasive plant - Long-leaved water amaranth. Plant Quarant.04, 64–66 (2015) ((in Chinese)). [Google Scholar]
- 33.Caton, B. P., Foin, T. C. & Hill, J. E. Phenotypic plasticity of Ammannia spp. in competition with rice. Weed Res.37, 33–38 (1997). [Google Scholar]
- 34.Graham, S. A., Timmermann, B. N. & Mabry, T. J. Flavonoid glycosides in Ammannia coccinea (Lythraceae). J. Nat. Prod.43, 644–645 (1980). [Google Scholar]
- 35.Harborne, J. B. & Williams, C. A. Advances in flavonoid research since 1992. Phytochemistry55, 481–504 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Shen, X.-R., Kim, D.-S. & Pyon, J.-Y. Competitive effect of Ammannia coccinea Rottb on growth and yield of rice in paddy Field. Korean J. Weed Sci.28, 25–31 (2008). [Google Scholar]
- 37.GBIF.org (2023). GBIF occurrence Download. Global biodiversity information facility. 10.15468/dl.d9vykx (Accessed: 13 November 2024).
- 38.Warren, D. L., Glor, R. E. & Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography33, 607–611 (2010). [Google Scholar]
- 39.Wang, R. L., Li, Q., Feng, C. H. & Shi, Z. P. Predicting potential ecological distribution of Locusta migratoria tibetensis in China using MaxEnt ecological niche modeling. Acta Ecol. Sinica37, 8556–8566 (2017). [Google Scholar]
- 40.Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol.: A J. Royal Meteorol. Soci.25, 1965–1978 (2005). [Google Scholar]
- 41.Jiang, T. et al. New scenarios of CMIP6 model (SSP-RCP) and its application in the Huaihe river basin. Adv. Meteorol. Sci. Technol10, 102–109 (2020). [Google Scholar]
- 42.Zhang, L., Chen, X. & Xin, X. Overview and review of CMIP6 scenario model comparison program (ScenarioMIP). Res. Progr. Climate Change15, 519–525 (2019). [Google Scholar]
- 43.Zhang, X. et al. Potential distribution prediction of Amaranthus palmeri S. Watson in China under current and future climate scenarios. Ecol. Evol.12, e9505 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruz-Cárdenas, G., López-Mata, L., Villaseñor, J. L. & Ortiz, E. Potential species distribution modeling and the use of principal component analysis as predictor variables. Revista mexicana de biodiversidad85, 189–199 (2014). [Google Scholar]
- 45.Gao, C., Zhifeng, F., Changle, M., Jianxin, Y. & Shuai-Long, G. Prediction of suitable areas for Dianthus camelliae under climate change based on the Biomod2 combinatorial model. J Ecol10.21203/rs.3.rs-4652177/v1 (2024). [Google Scholar]
- 46.Barbet-Massin, M., Jiguet, F., Albert, C. H. & Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many?. Meth. Ecol. Evolut.3, 327–338 (2012). [Google Scholar]
- 47.Araujo, M. B., Pearson, R. G., Thuiller, W. & Erhard, M. Validation of species–climate impact models under climate change. Global Change Biol.11, 1504–1513 (2005). [Google Scholar]
- 48.Lobo, J. M., Jiménez-Valverde, A. & Real, R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecol. Biogeogr.17, 145–151 (2008). [Google Scholar]
- 49.Senay, S. D., Worner, S. P. & Ikeda, T. Novel three-step pseudo-absence selection technique for improved species distribution modelling. PloS one8, e71218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allouche, O., Tsoar, A. & Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol.43, 1223–1232 (2006). [Google Scholar]
- 51.Ben Rais Lasram, F. et al. The Mediterranean Sea as a ‘cul-de-sac’for endemic fishes facing climate change. Global Change Biol.16, 3233–3245 (2010). [Google Scholar]
- 52.Eskildsen, A. et al. Testing species distribution models across space and time: High latitude butterflies and recent warming. Global Ecol. Biogeogr.22, 1293–1303 (2013). [Google Scholar]
- 53.Di Cola, V. et al. Ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography40, 774–787 (2017). [Google Scholar]
- 54.Li, Y., Liu, X., Li, X., Petitpierre, B. & Guisan, A. Residence time, expansion toward the equator in the invaded range and native range size matter to climatic niche shifts in non-native species. Global Ecol. Biogeogr.23, 1094–1104 (2014). [Google Scholar]
- 55.Liu, C., Wolter, C., Xian, W. & Jeschke, J. M. Most invasive species largely conserve their climatic niche. Proceed. Nat. Acad. Sci.117, 23643–23651 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warren, D. L., Glor, R. E. & Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution62, 2868–2883 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Broennimann, O. et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecol. Biogeogra.21, 481–497 (2012). [Google Scholar]
- 58.Guisan, A., Petitpierre, B., Broennimann, O., Daehler, C. & Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trend Ecol. Evol.29, 260–269 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Les, D. H. Aquatic Dicotyledons of North America: Ecology, life history, and systematics (CRC Press, 2017). [Google Scholar]
- 60.Parmesan, C. & Hanley, M. E. Plants and climate change: Complexities and surprises. Ann. Botany116, 849–864 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pendergrass, A. G., Knutti, R., Lehner, F., Deser, C. & Sanderson, B. M. Precipitation variability increases in a warmer climate. Sci. Reports7, 17966 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guan, B. et al. Shifting ranges of eleven invasive alien plants in China in the face of climate change. Ecol. Inform.55, 101024 (2020). [Google Scholar]
- 63.Shunting, Y., Huichun, W., Weikun, J., Qi-Gang, W., Hui-Jun, Y., Xian-Qin, Q., & Hong-Ying, J 2024 Modeling the impact of climate change on the global distribution of wild rose. J. Appl. Ecol. 1–11
- 64.Bujan, J. et al. Increased acclimation ability accompanies a thermal niche shift of a recent invasion. J. Animal Ecol.90, 483–491 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Tu, W., Xiong, Q., Qiu, X. & Zhang, Y. Dynamics of invasive alien plant species in China under climate change scenarios. Ecol. Indicat.129, 107919 (2021). [Google Scholar]
- 66.Zenni, R. D., Bailey, J. K. & Simberloff, D. Rapid evolution and range expansion of an invasive plant are driven by provenance–environment interactions. Ecol. Lett.17, 727–735 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due (our experimental team’s policy) but are available from the corresponding author on reasonable request.