Abstract
Vagus nerve stimulation (VNS) is a therapeutic intervention previously shown to enhance fear extinction in rats. VNS is approved for use in humans for the treatment of epilepsy, depression, and stroke, and it is currently under investigation as an adjuvant to exposure therapy in the treatment of PTSD. However, the mechanisms by which VNS enhances extinction of conditioned fear remain unresolved. VNS increases norepinephrine levels in extinction-related pathways, but recent studies indicate that norepinephrine release from the locus coeruleus interferes with extinction learning. The purpose of this study is to elucidate the role of the locus coeruleus (LC) in VNS-enhanced fear extinction. Adult male and female tyrosine hydroxylase (Th)-Cre rats were implanted with a stimulating cuff electrode around the left cervical vagus nerve, and a Cre-dependent viral vector expressing the inhibitory opsin ArchT3.0 was infused bilaterally into the LC. Rats then underwent auditory fear conditioning followed by extinction training. During extinction training, rats were divided into four treatment groups: Sham stimulation, Sham with LC inhibition, VNS, and VNS with LC inhibition. Consistent with previous findings, VNS treatment during extinction training significantly reduced freezing 24 h and 2 weeks later. This effect was blocked by optogenetic LC inhibition, suggesting that VNS enhances extinction by engaging the LC.
Exposure therapy is the gold-standard approach to treating anxiety and trauma-related disorders. Success in exposure therapy depends on extinguishing conditioned fear through many repeated unreinforced exposures. Impairments in the ability to extinguish conditioned fear are a hallmark of anxiety and trauma-related disorders and can interfere with progress in therapy. Therapeutic strategies that enhance the effectiveness of exposure-based therapy could dramatically improve patient outcomes. Our preclinical studies in rats suggest that vagus nerve stimulation (VNS) can accelerate the acquisition of extinction memories (Peña et al. 2013), reverse extinction impairments (Noble et al. 2017; Souza et al. 2019; Souza et al. 2021a,b), prevent the return of fear (Noble et al. 2017; Souza et al. 2019), and increase the generalization of extinction memories (Noble et al. 2019; Souza et al. 2021a,b). VNS is approved for use in humans to treat neurological disorders such as medication-refractory epilepsy and depression. Because VNS facilitates motor cortex plasticity when paired with motor training (Engineer et al. 2019), it was recently approved as an adjuvant for rehabilitation during recovery from stroke, and it is currently under investigation as an adjuvant to exposure therapy in the treatment of PTSD (National Library of Medicine [NLM], NCT04064762 and NCT02992899). However, the mechanisms by which VNS enhances fear extinction remain unclear.
Several preclinical studies have demonstrated improvements in learning and memory performance (Clark et al. 1999; Altidor et al. 2021; Driskill et al. 2022) and enhanced neuroplasticity (Peña et al. 2014; Meyers et al. 2018; Buell et al. 2019; Morrison et al. 2019) following task-paired stimulation of the left cervical vagus nerve. One explanation for the robust nature of VNS-paired extinction memories is that VNS engages emotional arousal systems to facilitate plasticity during extinction learning. Acute stress and the release of stress hormones, which activate the vagus nerve, have been shown to enhance the consolidation of long-term memories. For example, posttraining administration of epinephrine enhances memory consolidation in rats and humans (Gold and van Buskirk 1975; Cahill et al. 2003) and vagal fibers respond to elevations in circulating epinephrine (Chen and Williams 2012). Memory-enhancing VNS increases norepinephrine (NE) levels in the amygdala similarly to that seen with peripheral administration of epinephrine or footshock avoidance training (McIntyre et al. 2002; Hassert et al. 2004; Chen and Williams 2012). Intra-amygdala infusions of NE enhance memory consolidation (Hatfield and McGaugh 1999), whereas intra-amygdala infusions of β-adrenergic antagonists block the memory-enhancing effect of systemic epinephrine (Liang et al. 1986), suggesting that the mechanism by which epinephrine enhances memory involves amygdala responses to vagal signaling. Consistent with the hypothesis that VNS engages this mechanism to enhance extinction memory, NE infusions into the basolateral amygdala (BLA) enhance consolidation of extinction of inhibitory avoidance (Berlau and McGaugh 2006). VNS also increases NE concentrations in the mPFC (Follesa et al. 2007) and blocking β-adrenergic receptors in the infralimbic (IL) region of the mPFC impairs the consolidation of fear extinction (Mueller et al. 2008; Do-Monte et al. 2015). Taken together, these data suggest that, like stress, VNS may enhance consolidation of extinction memory by increasing NE release in the amygdala and/or mPFC, but VNS does so without engaging the peripheral sympathetic response.
In contrast to the evidence that NE signaling in the mPFC and BLA plays a necessary and sufficient role in the consolidation of extinction memory, some findings indicate that stress-induced activation of the locus coeruleus (LC), the primary source of NE in the brain, can interfere with extinction memory formation (Giustino et al. 2019,2020). These findings suggest that activation of circuitry involved in fear learning, including the LC, biases fear memory over extinction memory (Giustino and Maren 2018; Maren 2022). Based on these findings, VNS may enhance the extinction of conditioned fear despite its effects on the LC rather than because of its effects on the LC. Here, we used an optogenetic approach to inhibit the LC during VNS delivery to test the hypothesis that LC activity is necessary for VNS-driven enhancement of extinction of conditioned fear.
Results
Inhibition of the LC blocks VNS enhancement of extinction of conditioned fear
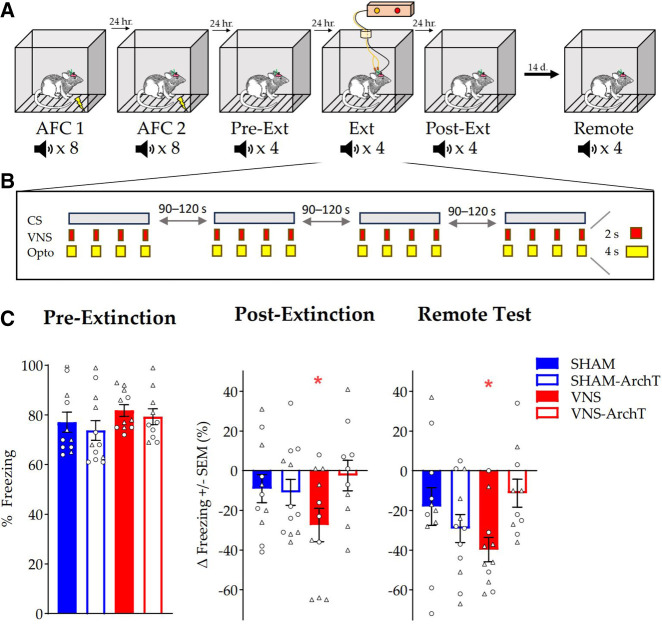
A Cre-dependent virus expressing the inhibitory opsin ArchT3.0 was infused into the LC of adult male and female Th-Cre+ rats. Four weeks later, VNS cuff electrodes were implanted on the left cervical vagus nerve. One week was allowed for recovery from surgery, then all rats underwent 2 days of auditory fear conditioning (AFC) followed by a retention test (Pre-Ext) 24 h later (Fig. 1A). During the retention test, the CS was presented four times with no shock, and mean percent time spent freezing during each presentation was recorded. During the subsequent fear extinction session (Ext), the CS was presented four times in the absence of footshock, and each 30-sec tone was paired with four VNS trains (0.8 mA, 2 sec) or no stimulation (Sham), and simultaneous delivery of yellow laser light (593 nm, 10 mW, 4 sec, overlapping each VNS train) to the LC. The four groups were: Sham-eYFP: n = 11; Sham-ArchT: n = 12; VNS-eYFP: n = 11, VNS-ArchT: n = 10 (Fig. 1B). A second retention test (Post-Ext) was given 24 h after the extinction session, and a third retention test was given 2 weeks later (Remote Test). The mean change in percent time spent freezing from Pre-Ext to Post-Ext and Pre-Ext to Remote Test was calculated for all groups (Fig. 1C). We hypothesized that inhibition of the LC with ArchT would block the VNS enhancement of extinction. Therefore, there should be less freezing in the VNS group treated with control virus (eYFP) from Pre-Ext to Post-Ext than in the VNS group expressing the inhibitory opsin (VNS-ArchT) and sham controls. To test our prediction, we used an a priori contrast analysis (sham-eYFP, −1; sham-ArchT, −1; VNS-eYFP, + 3; VNS-ArchT, −1). Contrast analysis does not make comparisons between different treatment groups. Rather, it directly answers the question of interest by testing expectations against the data using a (fixed effect) linear regression approach where the contrast coefficients are treated as a priori predictors of the experimental means (see, e.g., Rosenthal and Rosnow 1985, 2000; Abdi et al. 2009, pp. 328–333). This contrast explained 88% of the experimental variance and 11% of the total variance, and was significant: F(1,40) = 5.28, MSe = 604.4, P = 0.027. The two degrees of freedom residual experimental sum of squares (which corresponded to 12% of the experimental variance) was nonsignificant: F(2,40) = 0.34, MSe = 604.4, P = 0.44. These results are consistent with the prediction that VNS enhanced extinction of conditioned fear, but not when neuronal activity in the LC was inhibited, and they support the hypothesis that the LC is a necessary player in mediating VNS enhancement of extinction memory.
Figure 1.
VNS enhancement of extinction depends on LC activation. (A) Behavioral timeline for AFC, conditioned fear response testing, and extinction training. (B) Stimulation parameters for extinction training of VNS groups. (C) Pre-Ext freezing (%) is shown with males as circles and females as triangles. Post-Ext and Remote Testing are reported as a change in percent freezing compared to Pre-Ext freezing +/− SEM (Pre-Ext normalized to 0%). Solid bars represent animals with eYFP control virus in the LC, while empty bars are rats expressing the ArchT inhibitory virus. Only VNS-treated animals infused with control virus showed a significant reduction in freezing 24 h after extinction training. Sham animals and VNS-treated animals expressing the inhibitory opsin in the LC did not show a significant reduction in freezing. These effects were observed again 14 days later during Remote Testing. Data were analyzed using a priori contrasts. (*) P < 0.05, (**) P < 0.001, (***) P < 0.0001.
Previous findings indicate that VNS-enhanced extinction memory is maintained for 2 weeks (Souza et al. 2020). Therefore, we hypothesized that the decrease in freezing from Pre-Ext to the Remote Test would be significant in the VNS group treated with control virus, but not in the other three groups. An a priori contrast analysis explained 65% of the experimental variance and 11% of the total variance and was significant: F(1,40) = 5.17, MSe = 621.5, P = 0.028. The two degrees of freedom residual experimental sum of squares (which corresponded to 35% of the experimental variance) was nonsignificant: F(2,40) = 1.41, MSe = 621.5, P = 0.41 ns. These findings suggest that, like the 24 h extinction memory, the long-term extinction memory was enhanced with VNS administration, and concurrent LC inhibition prevented the enhancement.
Anxiety and locomotion
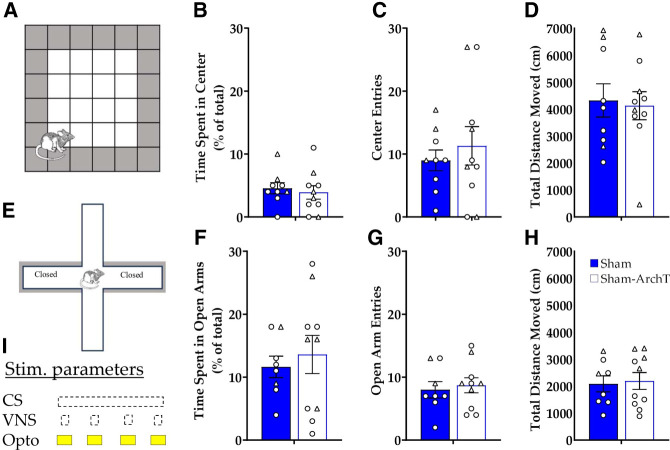
This study was designed to test the hypothesis that activation of the LC is critical for VNS enhancement of consolidation of extinction memory. Therefore, measurement of freezing during VNS or ArchT inhibition was not planned. However, a reduction in freezing was observed in the Sham-ArchT group versus Sham-eYFP. An unpaired t-test indicated a nonsignificant trend [t(21) = 1.79, P = 0.087], suggesting that the effects of LC inhibition on learned associations may be attributed to alterations in anxiety or activity during extinction training, rather than blocking a critical pathway for VNS effects. To test whether optogenetic inhibition of the LC had effects on anxiety or locomotion, the elevated plus maze and open field test were used. ArchT-expressing virus or control virus was infused bilaterally in the LC of naïve Th-Cre+ rats. Chronic optic fibers were placed dorsal to the LC. Laser light was administered using the same parameters as in the extinction experiment (593 nm, 10 mW, 4 sec, four times during 30 sec window) during the open field test and elevated plus maze. Unpaired t-tests revealed no significant difference between Sham-eYFP and Sham-ArchT groups for time spent in the center [t(17) = 0.47, P = 0.64] of the open field (Fig. 2A,B), number of center entries [t(17) = 0.64, P = 0.53] (Fig. 2C), or total distance (cm) traveled in the open field [t(17) = 0.24, P = 0.81] (Fig. 2D). In the elevated plus maze, there were no significant differences between Sham-eYFP and Sham-ArchT groups for percent of time spent in the open arms [t(16) = 0.53, P = 0.60] (Fig. 2E,F), number of open arm entries [t(16) = 0.53, P = 0.70], (Fig. 2G), or total distance traveled [t(16) = 0.24, P = 0.82] (Fig. 2H). These results suggest that LC inhibition did not reduce conditioned fear by influencing anxiety or locomotion during extinction sessions. Therefore, VNS enhancement of extinction of conditioned fear is likely driven by LC effects on consolidation of the extinction memory.
Figure 2.
Optogenetic inhibition of the LC does not affect anxiety-like behavior or locomotion. (A) Illustration of the open field arena. The unshaded region was considered the “center” of the open field. (B) Mean percentage of time spent in the center (+/− SEM) during the open field test. (C) Mean number of entries into the center of the open field (+/− SEM). (D) Mean total distance moved in the open field (+/− SEM). (E) Illustration of the elevated plus maze. The unshaded arms were “open” because they did not have walls along the sides. (F) Mean percentage of total time spent in the open arms of the elevated plus maze (+/− SEM). (G) Mean number of entries into the open arms of the elevated plus maze (+/− SEM). (H) Mean total distance traveled in the elevated plus maze (+/− SEM). (I) Stimulation parameters for optogenetic inhibition during testing. Circles indicate males, while triangles indicate females. No significant differences were seen between sham-eYFP and sham-ArchT in any of the tests.
Histological and electrophysiological verification
Fiber placements and virus expression were histologically validated in all rats after the conclusion of behavioral testing (Fig. 3A,B,D). In a separate rat that received LC-targeted ArchT infusions, we observed significant laser-driven suppression of firing in all putative noradrenergic LC neurons recorded (Fig. 3C; 5/5), functionally validating the optogenetic approach.
Figure 3.
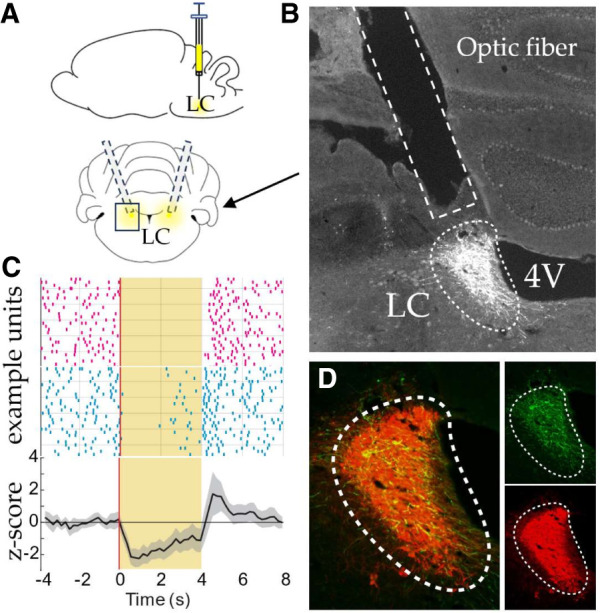
Verification of viral efficacy. (A) Depiction of optic fibers placed bilaterally dorsal to the LC and a viral infusion. (B) The optic fiber tract is seen directly dorsal to the LC (hashed white outline, virus shown in white). (C) Noradrenergic LC neurons are inhibited during laser stimulation. Peri-stimulation raster plots from five LC neurons were recorded in one rat (example units). All exhibited a reduction in firing during laser stimulation (yellow background). Average z-score normalized firing rates across the population of cells. (D) Fluorescent tag eYFP (green) bound to the ArchT opsin, and dopamine-beta hydroxylase (red) can be seen in separate panels on the right. On the left, these images are merged to see the colocalization of the virus and DBH (yellow).
Discussion
For effective treatment of symptoms of trauma-related disorders such as PTSD, it is necessary to create strong and durable fear extinction memories. According to previous results, VNS accelerates extinction and makes it resistant to reinstatement (Peña et al. 2013; Noble et al. 2017; Souza et al. 2019). Here, we tested the hypothesis that VNS enhances the extinction of conditioned fear by driving NE release from the LC to modulate ongoing synaptic plasticity during extinction training. LC inhibition during VNS blocked the extinction-enhancing effect at both the 24 h and Remote time points. These findings support the hypothesis that the fear extinction-enhancing effects of VNS are mediated by the LC. We propose that direct stimulation of the vagus nerve during fear extinction taps into brain mechanisms relevant to strengthening emotionally arousing memories. This is likely accomplished by engaging the LC to increase NE release in downstream targets that play a role in fear memory and extinction, like the BLA and IL region of the prefrontal cortex. The present results are consistent with previous studies demonstrating an increase in LC firing rate in response to 30 Hz VNS (Hulsey et al. 2017), and VNS-evoked NE elevations in both the amygdala and medial prefrontal cortex (Hassert et al. 2004; Follesa et al. 2007).
VNS is currently used to treat a variety of human disorders, including epilepsy, depression, and stroke. Eighty percent of vagus nerve afferents terminate in the nucleus of the solitary tract (NTS), which projects to the LC, raphe nucleus, and parabrachial nucleus, among other areas (Holt 2022). While the mechanisms of VNS effects are not fully understood, VNS effects in epilepsy, depression, and cortical plasticity are lost when the LC is lesioned (Krahl et al. 1998; Grimonprez et al. 2015; Hulsey et al. 2019). For example, in a motor-learning task, VNS paired with training enhanced plasticity in the motor cortex. Neurotoxic lesions of LC projections to the motor cortex blocked this plasticity (Hulsey et al. 2019). Furthermore, pharmacological inhibition of noradrenergic α2 receptors in the motor cortex also interfered with this form of VNS-induced plasticity (Tseng et al. 2021) and pairing optogenetic stimulation of the LC with training on the motor-learning task-induced plasticity in the motor cortex (Tseng et al. 2024). The current findings suggest that VNS effects on extinction, like epilepsy, depression, and cortical plasticity, critically involve activation of the LC.
Heightened LC activity is often associated with hypervigilance and anxiety-like behaviors (Aston-Jones and Bloom 1981; McCall et al. 2015; Soya et al. 2017; Llorca-Torralba et al. 2019). Based on this evidence, we considered the possibility that inhibition of the LC during extinction training could reduce anxiety, leading to more neutral associations with the conditioned stimulus and, consequently, better extinction of conditioned fear. However, when the same intermittent optogenetic LC inhibition parameters were used during testing on the elevated plus maze and in the open field, we did not observe changes in anxiety-like behaviors. These findings suggest that the effects of LC inhibition during extinction training on later expression of conditioned fear were not due to differences in anxiety state during exposure to the conditioned stimulus.
Although VNS effects on the LC/NE system have been demonstrated, there is some evidence that VNS activates other neuromodulatory systems that participate in memory and plasticity, including serotonin (Ruffoli et al. 2011) and acetylcholine (Nichols et al. 2011). Therefore, to understand the mechanisms of VNS enhancement of extinction, it was necessary to determine whether VNS effects on the LC were critically involved. We found that VNS enhancement was mediated, at least in part, by LC activity. These results suggest that the VNS-induced increase in NE release in the mPFC and amygdala does not bias memory in favor of threat over extinction. Instead of determining what memories are consolidated, studies that pair VNS with sensory, motor, or extinction training indicate that VNS-driven LC activation influences when training-induced plasticity is facilitated, and memories are consolidated.
Uematsu et al. (2017) targeted specific subpopulations of LC neurons projecting to the mPFC or amygdala and demonstrated a critical role for LC projections to the IL region of the mPFC in extinction learning and recall while inhibition of LC projections to the amygdala during exposure to the CS enhanced extinction. It is difficult to reconcile these findings with evidence that extinction-enhancing VNS increases NE in the amygdala (Hassert et al. 2004; Chen and Williams 2012). Future studies will test the hypothesis that natural extinction does not benefit from NE signaling in the amygdala, but VNS-paired extinction does. Previous findings are consistent with the hypothesis that VNS-enhanced and natural extinction produce qualitatively different effects on plasticity in a neural circuit involved in extinction, even when the level of extinction of conditioned fear is matched (Peña et al. 2014). After fear conditioning and extinction training, high-frequency stimulation in the IL produced opposing effects on local field potentials in the BLA in VNS-treated rats and Sham-treated rats (Peña et al. 2014), and, in another study, VNS-treated rats showed generalization of extinction to a CS that was not presented, whereas rats given Sham stimulation did not (Noble et al. 2019). Taken together, these findings argue that VNS does more than simply accelerate the natural extinction process. They suggest that VNS engages mechanisms that contribute to the consolidation of emotional memories, which are rapidly stored for the long term, tend to be generalized, and critically involve noradrenergic signaling.
Materials and Methods
Animals
All methods and procedures were conducted according to the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas at Dallas. Single-housed, adult (3–4 months of age) male and female Long-Evans rats weighing 250–550 g at the beginning of testing were used. All rats expressed Cre recombinase under the control of the Th promoter (Long-Evans [Th-Cre] 3.1) (Witten et al. 2011). Rats were bred in the animal care facility at the University of Texas at Dallas from breeding stock provided by the Rat Resource and Research Center (RRRC; RRID:RRRC_00659). Rats were kept on a 12h:12h light–dark cycle (lights on at 6 a.m.) and had access to food and water ad libitum. Three days before testing began, rats were handled for 10 min per day to acclimate them to the experimenter and experiment room. Behavioral training and testing rooms were immediately adjacent to rat holding rooms, and rats were habituated to the behavior room for 30 min before training or testing. All training and testing occurred during the light cycle.
Genotyping
Genotyping of transgenic rats was performed from an ear punch at weaning (postnatal day 21) using the following PCR conditions: 95°C (3 min), followed by 35 repeats of 94°C (1 min), 60.8°C (1 min), 72°C (1 min), finally 72°C (10 min) 4C (held until refrigerated). Th-Cre specific primers were used: Cre 5 (5′-GCG GCA TGG TGC AAG TTG AAT-3′), Cre 3 (5′-CGT TCA CCG GCA TCA ACG TTT-3′). Transgene-positive animals had an amplified band at 232 bp, while transgene-negative animals had no band (Witten et al. 2011).
Experimental design
To test the hypothesis that VNS-enhanced extinction of conditioned fear depends upon activation of the LC, rats were assigned to one of four groups before surgical procedures: Sham-eYFP (5F, 6M); Sham-ArchT (5F, 7M); VNS-eYFP (7F, 4M); VNS-ArchT (5F, 5M). An adeno-associated virus expressing an inhibitory ArchT opsin (AAV8-EF1a-DIO-ArchT3.0-eYFP) or an eYFP control virus (AAV8-EF1a-DIO-eYFP), in a Cre-dependent manner, was bilaterally infused into the LC (AP: −9.8, ML: ±3.75–3.90, DV: 6.43−7.43 mm from Bregma). Three weeks after virus infusions, a VNS cuff electrode and LC-targeted optic fibers were chronically implanted. Behavioral testing began 1 week after electrode and fiber implantation.
Viral production and injection procedure
Adeno-associated viruses used in this study were produced using a triple-transfection helper-free method in 239FT cells (Invitrogen). Viruses were pseudotyped as AAV8 and were purified on an iodixanol step gradient and concentrated using Amicon Ultra columns. Titers of viruses were determined using a PCR-based method as previously described (Holehonnur et al. 2014). Rats were anesthetized with isoflurane (2%–3%, inhaled; Western Medical Supply). To prevent bleeding and pain, an injection of lidocaine 2% with epinephrine (0.2 mL, Novocol Pharmaceutical) was administered subcutaneously on the skull. A 5 mm incision was made along the skull, and craniotomies were made over the targeted bilateral injection sites (needle angle 20° from vertical, AP: −9.8, ML: ± 3.75–3.80, DV: 6.43–7.43, relative to Bregma). Rats were injected with a viral vector expressing the inhibitory ArchT opsin (AAV2/8-EF1a-DIO-ArchT3.0-eYFP) or a control virus (AAV2/8-EF1a-DIO-eYFP) in the LC. One microliter of virus at a titer of ∼ 1 × 1013 Genome Copies (GC/mL) was infused bilaterally using a Hamilton 7002 syringe at a rate of 0.1 µL/min. Viral plasmids were gifts from Karl Deisseroth. The virus was allowed to diffuse for 7 min before the syringe was removed and the wound was sutured (AD Surgical, nonabsorbable nylon suture).
Cuff electrode and surgical procedure
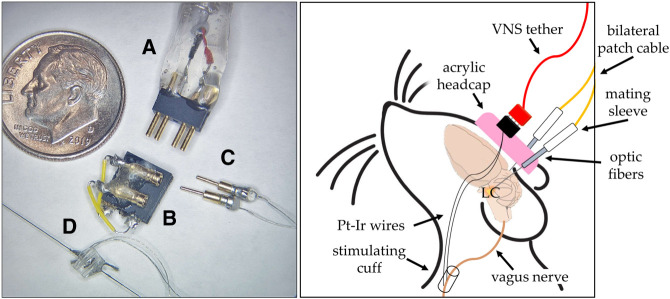
VNS stimulating cuff electrodes were prepared using previously described methods (Rios et al. 2019) (Fig. 4A–D). Three weeks after viral infusion surgery, rats were again anesthetized with isoflurane (2%–3% inhaled). The VNS cuff was implanted around the left cervical vagus nerve as previously described (Souza et al. 2021a,b). To test efficacy of the VNS cuff, a brief stimulation (0.8 mA, 20 Hz, 5 sec) was administered to induce momentary cessation of breathing, which indicated a functioning cuff. The cuff was then closed around the vagus nerve using suture wire and the wound was sutured closed. The rat was immediately transferred to a stereotaxic frame and an incision was made along the skull. Four bone screws (Technologies, 1.6 mm) were fixed to the skull to anchor the acrylic headcap. Optic fibers (400 µm, 10 mm, Thorlabs CFM14L10-10) were bilaterally implanted (needle angle 20° from vertical, AP: −9.8, ML: ± 3.75–3.80, DV: 6.43–7.43, relative to Bregma) dorsal to the LC. Acrylic was used to hold the optic fibers and VNS headcap in place (Fig. 4, right panel). Rats were given 1 week to recover before behavioral testing. During extinction training, a picoscope was used to monitor cuff impedance during each stimulation. Rats were excluded if their impedance was below 0 Ω (n = 2) or above 15 Ω (n = 3), indicating a malfunctioning cuff.
Figure 4.
(Left panel) Parts of a vagus nerve cuff are shown next to a dime for reference. The end of a VNS tether with the connector end (A), the headcap (B), gold pins that connect the cuff to the headcap (C), and the cuff itself (D). (Right panel) Depiction of a rat with bilateral LC-targeted optic fibers and left vagus nerve stimulating cuff implanted.
Behavioral apparatus
AFC, extinction, and retention all occurred in the same context. A plexiglass booth (31 × 23 × 21 cm) with electric bars for flooring was housed within a soundproof behavior box (61 × 82 × 57 cm). A speaker (Powerline) was mounted on the wall of the behavior box 11 cm from the booth. For extinction sessions, a laser (PSU-H-LED, RDW) was attached via patch cable to a commutator (Thorlabs) suspended above the behavior box. A camera (Logitech) was mounted on the far end of the box to record sessions.
Auditory fear conditioning and extinction
On days 1 and 2, rats underwent AFC. Before conditioning on day 1, two baseline tones (9 kHz, 75 dB, 30 sec) were administered to test baseline freezing behavior. Each conditioning session then consisted of eight tones (CS) paired with a footshock (Lafayette Instruments—scramble grid current generator, 0.4 mA, 0.5 sec). To increase unpredictability, the tones were presented with a pseudorandom interstimulus interval (ISI) (160–240 sec), and the footshock occurred at a pseudorandom interval during each 30 sec tone presentation. On day 3, rats underwent a preextinction conditioned fear response test (Pre-Ext CFRT). No shock was administered during the CFRT, and time spent freezing during each of four presentations of the tone CS was recorded.
On day 4 (Ext), rats received four trials in which each 30 sec CS tone presentation was paired with yellow laser light delivery to the LC that overlapped with VNS or Sham stimulation. The CS was presented four times at a pseudorandom ISI (160–240 sec). On each trial, four trains of VNS (0.8 mA, 0.5 msec biphasic pulses, 30 Hz pulse frequency, 2 sec train duration) were delivered every 7.5 sec during the 30 sec CS tone presentation. A 4 sec pulse of yellow laser light (593 nm, 10 mW) was delivered with each VNS train; laser onset began 1 sec before VNS onset and ended 1 sec after the VNS train. Sham stimulation groups were trained and tethered identically to the VNS treatment groups and received laser stimulation, but no VNS, during the fear extinction session.
A second CFRT session was given on day 5 to assess extinction learning (Post-Ext CFRT). Fourteen days following the Post-Ext CFRT, a retention test was administered to test the durability of the extinction memory. During this Remote Test session, the rats were again placed in the behavior booth, and the CS was presented four times without a footshock (Fig. 1A).
Anxiety testing
To test whether optogenetic inhibition of the LC affected anxiety-like behavior, naïve adult Long-Evans rats (3 months of age at time of injection) were bilaterally injected with ArchT (4F, 7M) or control virus (2F, 5M) in the LC. Optic fibers were implanted bilaterally dorsal to the LC 6 weeks after viral infusion. Behavior was then tested on the open field and elevated plus maze tasks using ANY-maze software.
Open field test (OFT)
Rats were placed in a corner of a large plexiglass box with a grid on the floor. The percentage of time spent near the walls was used as a measure of anxiety, while time spent in the center was considered an indication of lower anxiety. During the test, rats were tethered to a laser which administered light through the implanted optic fiber. Parameters for inhibition (593 nm, 10 mW, 4 sec, four times during 30 sec tone) were matched to those used during extinction of conditioned fear with VNS; however, no VNS or tone was administered. Video recordings were taken for later analysis of crossings into, and time spent in the center or edge during the 14-min behavior session. Videos were scored using ANY-maze software and are reported as mean percent time in the center, number of entries, and distance traveled per group.
Elevated plus maze (EPM)
Twenty-four hours after OFT, the same rats were run on an elevated plus maze as previously described (Carobrez and Bertoglio 2005; Canto-de-Souza et al. 2021). Briefly, the apparatus consisted of two opposite open arms, and two opposite enclosed arms. Rats were placed on the center of the apparatus at the intersection of these arms while tethered to a laser. Parameters for inhibition were matched to those used during the extinction of conditioned fear with VNS; however, no tone or VNS was administered. Video recordings were taken for later analysis of number of entries and time spent in open and closed arms during the 14 min behavior session (n = 1, excluded due to issues in recording the session). Sessions were scored using ANY-maze software.
Statistical analysis
Conditioned fear behavior was video recorded (Logitech camera) during each CFRT session. Two blinded experimenters scored freezing responses and results were averaged for each tone per animal. Freezing was defined as the absence of movement other than breathing or ears twitching and was transformed to a percent of total tone-exposure time score. Contrast analyses were conducted using SPSS, and an unpaired t-test was used to compare freezing in Sham-ArchT versus Sham-eYFP groups during extinction training. Graphs were made using GraphPad Prism.
Anxiety behaviors were video recorded (Logitech camera) during each test session. ANY-maze software was used to score the number of center entries (unshaded region) and percent time in the center of the open field, or number of arm entries and time spent in the open arms of the EPM. Statistical analyses and graphing were performed using GraphPad Prism. Behavioral data were analyzed using unpaired t-tests to compare: percent time in compartment, crossings into compartment, or total distance traveled in ArchT-expressing rats versus eYFP control rats. For all statistical results, significance is reported for P < 0.05.
Histological verification
Rats were perfused using 1 M phosphate buffered saline (60 mL/min, 3 min) followed by 4% paraformaldehyde in 1 M PBS (60 mL/min, 2 min) 24 h after the last day of behavioral testing. Brains were placed in 4% paraformaldehyde for 1 h followed by a 30% sucrose solution for 24–48 h. The LC of each brain was sectioned at 30 µm and stained for dopamine beta hydroxylase (DBH) and GFP. The presence of DBH was used as an indicator of NE-producing neurons while GFP antibodies were used to enhance the eYFP viral tag. A series of tris washes were used followed by incubation in mouse anti-DBH (1:10,000; Sigma-Aldrich MAB308) and rabbit anti-GFP (1:2000; Abcam 598) diluted in tris buffer. After incubating for 48 h at 4°C in the primary antibody, sections were again washed in tris solutions. Next, sections were incubated in Alexa-555 conjugated donkey anti-mouse (1:400, Invitrogen A31570) and Alexa-488 conjugated goat anti-rabbit (1:200, Invitrogen A32731) secondary antibodies in tris B for 1 h. Finally, they were rinsed in tris buffer. For imaging, sections were wet mounted onto slides with tris buffer and coverslipped using VECTASHIELD Antifade Mounting Medium with DAPI (Vector Laboratories H1200). Slices were imaged with a fluorescent microscope.
Electrophysiology
Electrophysiology recordings were performed in an untrained female TH-Cre+ rat (27 weeks old at recording) to validate ArchT-mediated suppression of LC firing. At least 4 weeks after receiving unilateral ArchT-expressing virus infusion into the LC, the rat was anesthetized with ketamine hydrochloride (70 mg/kg) and xylazine (5 mg/kg) injected intraperitoneally, and received supplementary doses of ketamine hydrochloride (70 mg/kg) as needed to maintain stable anesthesia throughout the recording session. A small craniotomy was made to target the LC (AP: −12 mm, ML: −1.25 mm from Bregma), and an optrode consisting of a 200 µm core optical fiber (Thorlabs) glued to a high-impedance (∼1 MW) bipolar tungsten matrix microelectrode (FHC Inc., SKU 30255) was slowly lowered to 5.2 mm from the surface of the brain at an angle of 20° posterior to the vertical axis. Neural activity was then recorded every 100–200 μm along the recording track from depths between 5.2 and 6.8 mm. Putative LC neural activity was identified by stereotaxic recording depth, the presence of long-duration positive–negative action potential waveforms, and a burst of spikes following toe pinch (Martins and Froemke 2015; McCall et al. 2015; Hulsey et al. 2017). During recordings, a 4 sec pulse of 593 nm laser light was delivered with a random intertrial interval between 15 and 20 sec. Neural activity was recorded using a Plexon OmniPlex Data Acquisition System (Plexon Inc.). Wide-band continuous activity was filtered from 0.1 Hz to 10 kHz and digitally sampled at 40 kHz. To capture single-unit activity, wide-band signals were digitally filtered (50 Hz–10 kHz), and spikes were sampled at 40 kHz for a duration of 2.5 msec around the time when a voltage threshold crossing was detected. Following recording, histology was performed to confirm the anatomical position of the optrode and virus expression in the LC.
Recorded spikes were manually sorted offline into clusters using Plexon Offline Sorter (Plexon Inc.). Data analysis was performed in MATLAB. Clusters exhibiting low tonic firing rates (<9 Hz) and long-duration positive–negative action potential waveshapes (peak duration > 375 μsec) were identified as putative noradrenergic LC units, and accepted for further analysis if unit activity was stably recorded during at least 20 trials. To determine whether each unit exhibited laser-evoked responses, peri-stimulation time histograms were constructed from –4 to +8 sec around laser onset, using a 200 msec bin width, and were smoothed using a 3-bin moving average. Baseline firing rate for each unit was computed from the smoothed PSTH during a 3 sec window before stimulation. Units were identified as having laser-evoked suppression of firing if the PSTH was 2 SD below the baseline firing rate for at least 1.5 sec following laser onset.
Competing interest statement
C.K.M. is an author of a patent titled “Methods for Enhancing Exposure Therapy using Vagus Nerve Stimulation.” The remaining authors declare no conflict of interest.
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under award number R01MH126516. We acknowledge Camilo Sanchez for helping pilot electrophysiology data, Peyton Demetrovich for piloting methods for viral transduction in the locus coeruleus, Joe Epperson for assistance with the Arduino and behavior box, and Philip Martin for coding the behavioral program, which was used throughout all experiments. We are thankful for a wonderful team of research assistants who helped throughout: Merlyn George, Kavya Donepudi, Erik Bellinghausen, Malavika Ramaswamy, Zara Siddiqui, Brandon Myers, and Antoinette Cotton. Finally, we thank Karl Deisseroth for sharing plasmids that were critical for this research.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053958.124.
Freely available online through the Learning & Memory Open Access option.
References
- Abdi H, Edelman B, Valentin D, Dowling WJ. 2009. Experimental design and analysis for psychology. Oxford University Press, Oxford. [Google Scholar]
- Altidor LKP, Bruner MM, Deslauriers JF, Garman TS, Ramirez S, Dirr EW, Olczak KP, Maurer AP, Lamb DG, Otto KJ, et al. 2021. Acute vagus nerve stimulation enhances reversal learning in rats. Neurobiol Learn Memory 184: 107498. 10.1016/j.nlm.2021.107498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. 1981. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1: 876–886. 10.1523/JNEUROSCI.01-08-00876.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. 2006. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Memory 86: 123–132. 10.1016/j.nlm.2005.12.008 [DOI] [PubMed] [Google Scholar]
- Buell EP, Borland MS, Loerwald KW, Chandler C, Hays SA, Engineer CT, Kilgard MP. 2019. Vagus nerve stimulation rate and duration determine whether sensory pairing produces neural plasticity. Neuroscience 406: 290–299. 10.1016/j.neuroscience.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. 2003. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn Memory 10: 270–274. 10.1101/lm.62403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto-de-Souza L, Demetrovich PG, Plas S, Souza RR, Epperson J, Wahlstrom KL, Nunes-de-Souza RL, LaLumiere RT, Planeta CS, McIntyre CK. 2021. Daily optogenetic stimulation of the left infralimbic cortex reverses extinction impairments in male rats exposed to single prolonged stress. Front Behav Neurosci 15: 780326. 10.3389/fnbeh.2021.780326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. 2005. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 29: 1193–1205. 10.1016/j.neubiorev.2005.04.017 [DOI] [PubMed] [Google Scholar]
- Chen CC, Williams CL. 2012. Interactions between epinephrine, ascending vagal fibers, and central noradrenergic systems in modulating memory for emotionally arousing events. Front Behav Neurosci 6: 35. 10.3389/fnbeh.2012.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. 1999. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 2: 94–98. 10.1038/4600 [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Quinõnes-Laracuente K, Quirk GJ. 2015. A temporal shift in the circuits mediating retrieval of fear memory. Nature 519: 460–463. 10.1038/nature14030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskill CM, Childs JE, Itmer B, Rajput JS, Kroener S. 2022. Acute vagus nerve stimulation facilitates short term memory and cognitive flexibility in rats. Brain Sci 12: 1137. 10.3390/brainsci12091137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, Hays SA. 2019. Targeted vagus nerve stimulation for rehabilitation after stroke. Front Neurosci 13: 280. 10.3389/fnins.2019.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand A, Jacquemet V, Verner R, Owens M, Beaumont E. 2023. Vagus nerve stimulation parameters evoke differential neuronal responses in the locus coeruleus. Physiol Rep 11: e15633. 10.14814/phy2.15633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Giustino TF, Seemann JR, Maren S. 2015. Noradrenergic blockade stabilizes prefrontal activity and enables fear extinction under stress. Proc Natl Acad Sci 112: E3729–E3737. 10.1073/pnas.1500682112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, Puligheddu M, Marrosu F, Biggio G. 2007. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res 1179: 28–34. 10.1016/j.brainres.2007.08.045 [DOI] [PubMed] [Google Scholar]
- Giustino TF, Maren S. 2018. Noradrenergic modulation of fear conditioning and extinction. Front Behav Neurosci 12: 43. 10.3389/fnbeh.2018.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Fitzgerald PJ, Ressler RL, Maren S. 2019. Locus coeruleus toggles reciprocal prefrontal firing to reinstate fear. Proc Natl Acad Sci 116: 8570–8575. 10.1073/pnas.1814278116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Ramanathan KR, Totty MS, Miles OW, Maren S. 2020. Locus coeruleus norepinephrine drives stress-induced increases in basolateral amygdala firing and impairs extinction learning. J Neurosci 40: 907–916. 10.1523/JNEUROSCI.1092-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, van Buskirk RB. 1975. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol 13: 145–153. 10.1016/s0091-6773(75)91784-8 [DOI] [PubMed] [Google Scholar]
- Grimonprez A, Raedt R, Portelli J, Dauwe I, Larsen LE, Bouckaert C, Delbeke J, Carrette E, Meurs A, De Herdt V, et al. 2015. The antidepressant-like effect of vagus nerve stimulation is mediated through the locus coeruleus. J Psychiatr Res 68: 1–7. 10.1016/j.jpsychires.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Hassert DL, Miyashita T, Williams CL. 2004. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci 118: 79–88. 10.1037/0735-7044.118.1.79 [DOI] [PubMed] [Google Scholar]
- Hatfield T, McGaugh JL. 1999. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol Learn Mem 71: 232–239. doi: 10.1006/nlme.1998.3875 [DOI] [PubMed] [Google Scholar]
- Holehonnur R, Luong JA, Chaturvedi D, Ho A, Lella SK, Hosek MP, Ploski JE. 2014. Adeno-associated viral serotypes produce differing titers and differentially transduce neurons within the rat basal and lateral amygdala. BMC Neurosci 15: 28. 10.1186/1471-2202-15-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt MK. 2022. The ins and outs of the caudal nucleus of the solitary tract: an overview of cellular populations and anatomical connections. J Neuroendocrinol 34: 10.1111/jne.13132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. 2017. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 289: 21–30. 10.1016/j.expneurol.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey DR, Shedd CM, Sarker SF, Kilgard MP, Hays SA. 2019. Norepinephrine and serotonin are required for vagus nerve stimulation directed cortical plasticity. Exp Neurol 320:112975. 10.1016/j.expneurol.2019.112975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahl SE, Clark KB, Smith DC, Browning RA. 1998. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 39: 709–714. 10.1111/j.1528-1157.1998.tb01155 [DOI] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. 1986. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res 368: 125–133. 10.1016/0006-8993(86)91049-8 [DOI] [PubMed] [Google Scholar]
- Llorca-Torralba M, Suárez-Pereira I, Bravo L, Camarena-Delgado C, Garcia-Partida JA, Mico JA, Berrocoso E. 2019. Chemogenetic silencing of the locus coeruleus–basolateral amygdala pathway abolishes pain-induced anxiety and enhanced aversive learning in rats. Biol Psychiatr 85: 1021–1035. 10.1016/j.biopsych.2019.02.018 [DOI] [PubMed] [Google Scholar]
- Maren S. 2022. Unrelenting fear under stress: neural circuits and mechanisms for the immediate extinction deficit. Front Syst Neurosci 16: 888461. 10.3389/fnsys.2022.888461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins ARO, Froemke RC. 2015. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat Neurosci 18: 1483–1492. 10.1038/nn.4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. 2015. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87: 605–620. 10.1016/j.neuron.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. 2002. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci 16: 1223–1226. 10.1046/j.1460-9568.2002.02188 [DOI] [PubMed] [Google Scholar]
- Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL, Kilgard MP, Hays SA. 2018. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke 49: 710–717. 10.1161/STROKEAHA.117.019202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RA, Hulsey DR, Adcock KS, Rennaker RL, Kilgard MP, Hays SA. 2019. Vagus nerve stimulation intensity influences motor cortex plasticity. Brain Stimul 12: 256–262. 10.1016/j.brs.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. 2008. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci 28: 369–375. 10.1523/JNEUROSCI.3248-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. 2011. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience 189: 207–214. 10.1016/j.neuroscience.2011.05.024 [DOI] [PubMed] [Google Scholar]
- Noble LJ, Gonzalez IJ, Meruva VB, Callahan KA, Belfort BD, Ramanathan KR, Meyers E, Kilgard MP, Rennaker RL, McIntyre CK. 2017. Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Transl Psychiatr 7: e1217. 10.1038/tp.2017.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LJ, Meruva VB, Hays SA, Rennaker RL, Kilgard MP, McIntyre CK. 2019. Vagus nerve stimulation promotes generalization of conditioned fear extinction and reduces anxiety in rats. Brain Stimul 12: 9–18. 10.1016/j.brs.2018.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña DF, Engineer ND, McIntyre CK. 2013. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol Psychiatr 73: 1071–1077. 10.1016/j.biopsych.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S. 2014. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the Amygdala. Front Behav Neurosci 18: 327. 10.3389/fnbeh.2014.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios MU, Bucksot JE, Rahebi KC, Engineer CT, Kilgard MP, Hays SA. 2019. Protocol for construction of rat nerve stimulation cuff electrodes. Methods Prot 2: 1–30. 10.3390/mps2010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow R. 1985. Contrast analysis: focused comparisons in the analysis of variance. Cambridge University Press, Oxford. [Google Scholar]
- Rosenthal R, Rosnow R. 2000. Contrasts and effect sizes in behavioral research: a correlational approach. Cambridge University Press, Oxford. [Google Scholar]
- Ruffoli R, Giorgi FS, Pizzanelli C, Murri L, Paparelli A, Fornai F. 2011. The chemical neuroanatomy of vagus nerve stimulation. J Chem Neuroanat 42: 288–296. 10.1016/j.jchemneu.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Souza RR, Robertson NM, Pruitt DT, Gonzales PA, Hays SA, Rennaker RL, Kilgard MP, McIntyre CK. 2019. Vagus nerve stimulation reverses the extinction impairments in a model of PTSD with prolonged and repeated trauma. Stress 22: 509–520. 10.1080/10253890.2019.1602604 [DOI] [PubMed] [Google Scholar]
- Souza RR, Robertson NM, Mathew E, Tabet MN, Bucksot JE, Pruitt DT, Rennaker RL, Hays SA, McIntyre CK, Kilgard MP. 2020. Efficient parameters of vagus nerve stimulation to enhance extinction learning in an extinction-resistant rat model of PTSD. Prog Neuro-Psychopharmacol Biol Psychiatr 99: 109848. 10.1016/J.PNPBP.2019.109848 [DOI] [PubMed] [Google Scholar]
- Souza RR, Oleksiak CR, Tabet MN, Rennaker RL, Hays SA, Kilgard MP, McIntyre CK. 2021a. Vagus nerve stimulation promotes extinction generalization across sensory modalities. Neurobiol Learn Mem 181: 107425. 10.1016/j.nlm.2021.107425 [DOI] [PubMed] [Google Scholar]
- Souza RR, Robertson NM, McIntyre CK, Rennaker RL, Hays SA, Kilgard MP. 2021b. Vagus nerve stimulation enhances fear extinction as an inverted-U function of stimulation intensity. Exp Neurol 341: 113718. 10.1016/j.expneurol.2021.113718 [DOI] [PubMed] [Google Scholar]
- Soya S, Takahashi TM, McHugh TJ, Maejima T, Herlitze S, Abe M, Sakimura K, Sakurai T. 2017. Orexin modulates behavioral fear expression through the locus coeruleus. Nat Commun 8. 10.1038/s41467-017-01782-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CT, Gaulding SJ, Dancel CLE, Thorn CA. 2021. Local activation of α2 adrenergic receptors is required for vagus nerve stimulation induced motor cortical plasticity. Sci Rep 11: 21645. 10.1038/s41598-021-00976-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CT, Welch HF, Gi AL, Kang EM, Mamidi T, Pydimarri S, Ramesh K, Sandoval A, Ploski JE, Thorn CA. 2024. Frequency specific optogenetic stimulation of the locus coeruleus induces task-relevant plasticity in the motor cortex. J Neurosci 44: e1528232023. 10.1523/JNEUROSCI.1528-23.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Tan BZ, Ycu EA, Cuevas JS, Koivumaa J, Junyent F, Kremer EJ, Witten IB, Deisseroth K, Johansen JP. 2017. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat Neurosci 20: 1602–1611. 10.1038/nn.4642 [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. 2011. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72: 721–733. 10.1016/j.neuron.2011.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]