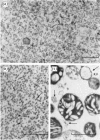
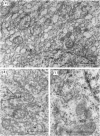
Abstract
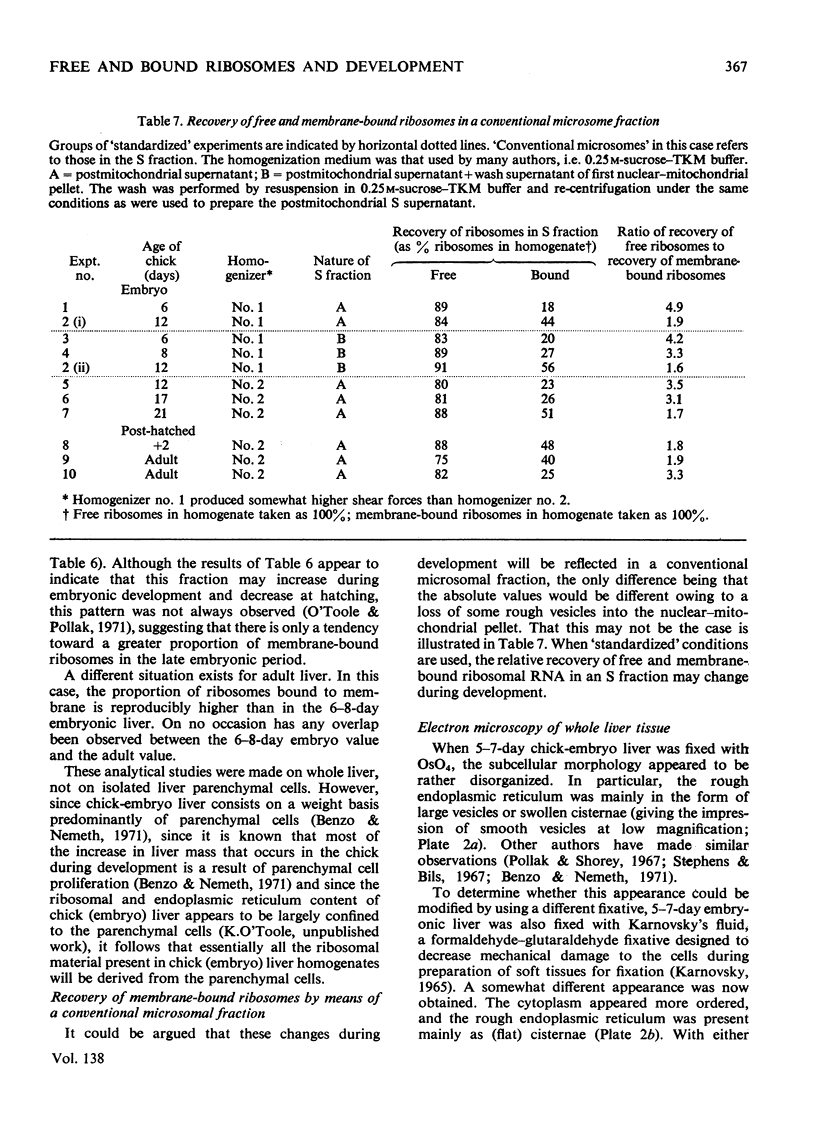
A major difficulty in studying quantitative changes in free and membrane-bound ribosomes in a tissue under different physiological conditions is that membrane-bound ribosomes are not usually recovered quantitatively in a conventional microsomal fraction. This problem was resolved for developing chick liver by determining the conditions for the isolation of a microsomal fraction containing the highest practicable yield of rough vesicles, and then separating it into free-ribosome- and rough-vesicle-containing fractions. With the aid of a marker enzyme for the microsomal membranes and the RNA content of the recovered membrane-bound ribosomes, it was possible to correct for the recovery of rough vesicles and hence to determine the concentration of membrane-bound ribosomes in the homogenate. Despite the fact that morphological studies have suggested that most of the cellular ribosomes are not bound to membrane in chick liver cells at the earliest developmental age studied (6 days of egg incubation), 49% of the total ribosomes were found to be membrane-bound by using the new fractionation technique. This fraction increased (to 66%) during development. The discrepancy between the cell-fractionation and morphological approaches could not be attributed to artifacts of the separation method but rather to difficulties inherent in the morphological approach.
Full text
PDF
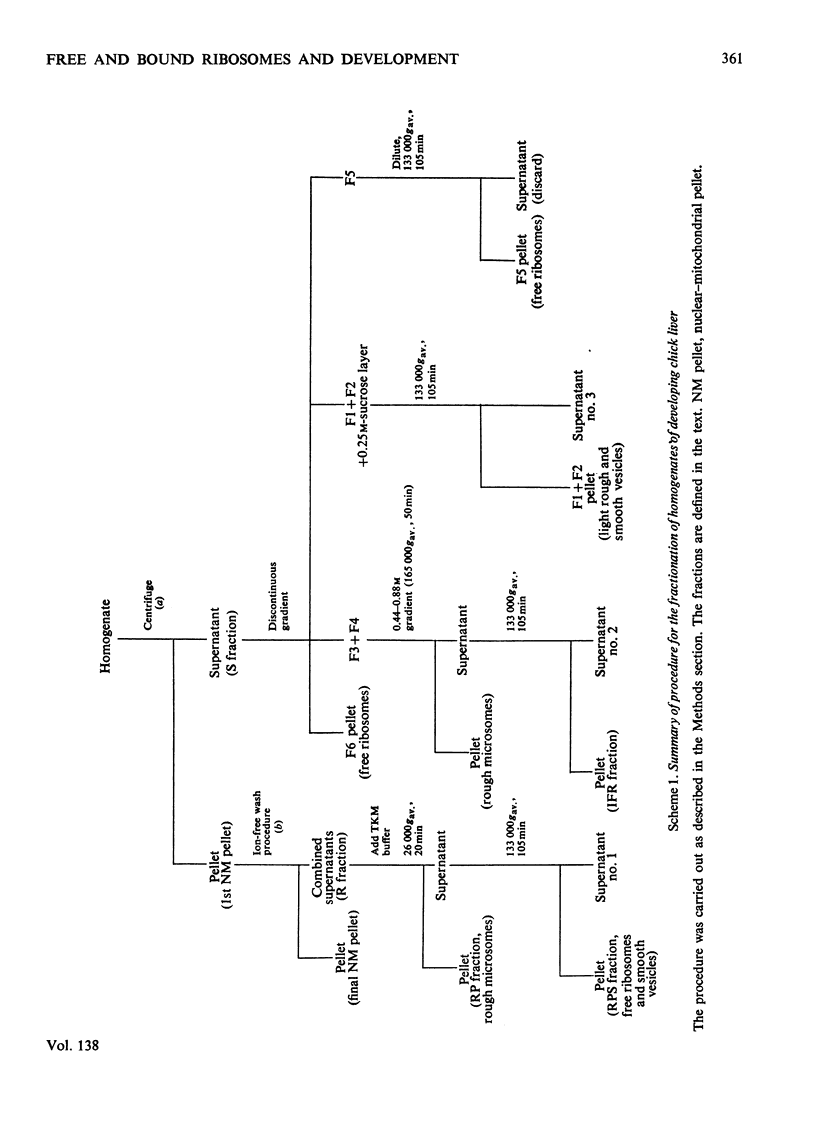






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. M., Tata J. R. Protein synthesis by membrane-bound and free ribosomes of secretory and non-secretory tissues. Biochem J. 1971 Feb;121(4):683–694. doi: 10.1042/bj1210683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi B., Cravioto B., Attardi G. Membrane-bound ribosomes in HeLa cells. I. Their proportion to total cell ribosomes and their association with messenger RNA. J Mol Biol. 1969 Aug 28;44(1):47–70. doi: 10.1016/0022-2836(69)90404-5. [DOI] [PubMed] [Google Scholar]
- BLOEMENDAL H., BONT W. S., BENEDETTI E. L. PREPARATION OF RAT-LIVER POLYSOMES WITHOUT THE UTILIZATION OF DETERGENT. Biochim Biophys Acta. 1964 May 18;87:177–180. doi: 10.1016/0926-6550(64)90064-7. [DOI] [PubMed] [Google Scholar]
- BRAUER R. W., JULIAN L. M., KREBS J. S. MATURATION OF LIVER FUNCTION IN THE CHICK EMBRYO AS EXPLORED WITH S35--SULFOBROMOPHTHALEIN--VASCULAR FACTORS, BILIARY SECRETION, AND CONJUGATION. Ann N Y Acad Sci. 1963 Dec 30;111:136–156. doi: 10.1111/j.1749-6632.1963.tb36954.x. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967 Jun 14;26(2):279–292. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Bont W. S., de Vries M., Benedetti E. L. Isolation and properties of polyribosomes and fragments of the endoplasmic reticulum from rat liver. Biochem J. 1967 Apr;103(1):177–182. doi: 10.1042/bj1030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshes R. A. Drosophila polyribosomes. The characterization of two populations by cell fractionation and isotopic labeling with nucleic acid and protein precursors. J Cell Biol. 1970 Sep;46(3):477–490. doi: 10.1083/jcb.46.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghouts J. T., Stols A. L., Bloemendal H. Free and membrane-bound polyribosomes in normal and Rauscher-virus-infected mouse spleen cells. Biochem J. 1970 Oct;119(4):749–756. doi: 10.1042/bj1190749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C., SCHNEEBELI J. Isolation of smooth vesicles and free ribosomes from rat liver microsomes. J Cell Biol. 1962 Jan;12:17–29. doi: 10.1083/jcb.12.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. N. Functions of polyribosomes attached to membranes of animal cells. FEBS Lett. 1970 Mar 16;7(1):1–7. doi: 10.1016/0014-5793(70)80603-2. [DOI] [PubMed] [Google Scholar]
- DALLNER G. STUDIES ON THE STRUCTURAL AND ENZYMIC ORGANIZATION OF THE MEMBRANOUS ELEMENTS OF LIVER MICROSOMES. Acta Pathol Microbiol Scand Suppl. 1963:SUPPL166–SUPPL16694. [PubMed] [Google Scholar]
- Daillie J., Grasset L., Prudhomme J. -C., Beck J. -P., Ebel J. -P. The properties of free and membrane bound polysomes studied in a cell free system of Bombix mori silkglands. FEBS Lett. 1971 Apr 2;13(6):321–324. doi: 10.1016/0014-5793(71)80251-x. [DOI] [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. I. Structural and chemical differentiation in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):73–96. doi: 10.1083/jcb.30.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender B. R., Wettstein F. O. Differences in the ribosomal protein of free and membrane bound polysomes of chick embryo cells. Biochem Biophys Res Commun. 1970 Apr 24;39(2):247–253. doi: 10.1016/0006-291x(70)90785-0. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Williams C. A. In vitro synthesis of different categories of specific protein by membrane-bound and free ribosomes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1370–1376. doi: 10.1073/pnas.63.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaye P., Denamur R. Acides ribonucléiques et polyribosomes de la glande mammaire de la lapine au cours de la lactogénèse induite par la prolactine. Biochim Biophys Acta. 1969 Jul 22;186(1):99–109. [PubMed] [Google Scholar]
- Gaye P., Denamur R. Preferential synthesis of beta lactoglobulin by the bound polyribosomes of the mammary gland. Biochem Biophys Res Commun. 1970 Oct 9;41(1):266–272. doi: 10.1016/0006-291x(70)90498-5. [DOI] [PubMed] [Google Scholar]
- Glaumann H. Studies on the synthesis and transport of albumin in microsomal subfractions from rat liver. Biochim Biophys Acta. 1970 Nov 12;224(1):206–218. doi: 10.1016/0005-2787(70)90634-9. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Green H. Collagen synthesis on polyribosomes of cultured mammalian fibroblasts. J Mol Biol. 1967 May 28;26(1):1–18. doi: 10.1016/0022-2836(67)90257-4. [DOI] [PubMed] [Google Scholar]
- HALLINAN T., MUNRO H. N. A RAPID METHOD FOR PREPARING GRANULAR AND AGRANULAR ENDOPLASMIC RETICULUM AND FREE RIBOSOMES FROM RAT LIVER. Q J Exp Physiol Cogn Med Sci. 1965 Jan;50:93–103. doi: 10.1113/expphysiol.1965.sp001774. [DOI] [PubMed] [Google Scholar]
- HENSHAW E. C., BOJARSKI T. B., HIATT H. H. PROTEIN SYNTHESIS BY FREE AND BOUND RAT LIVER RIBOSOMES IN VIVO AND IN VITRO. J Mol Biol. 1963 Aug;7:122–129. doi: 10.1016/s0022-2836(63)80041-8. [DOI] [PubMed] [Google Scholar]
- HERS H. G., BERTHET J., BERTHET L., DE DUVE C. Le système hexose-phosphatasique. III. Localisation intra-cellulaire des ferments par centrifugation fractionnée. Bull Soc Chim Biol (Paris) 1951;33(1-2):21–41. [PubMed] [Google Scholar]
- HOWELL R. R., LOEB J. N., TOMKINS G. M. CHARACTERIZATION OF RIBOSOMAL AGGREGATES ISOLATED FROM LIVER. Proc Natl Acad Sci U S A. 1964 Nov;52:1241–1248. doi: 10.1073/pnas.52.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks S. J., Drysdale J. W., Munro H. N. Preferential synthesis of ferritin and albumin by different populations of liver polysomes. Science. 1969 May 2;164(3879):584–585. doi: 10.1126/science.164.3879.584. [DOI] [PubMed] [Google Scholar]
- KAMINSKI M., DURIEUX J. Etude comparative des sérums de poule, de coq, de poussin, d'embryon et du blanc d'oeuf. Exp Cell Res. 1956 Jun;10(3):590–618. doi: 10.1016/0014-4827(56)90038-6. [DOI] [PubMed] [Google Scholar]
- KAMINSKI M., DURIEUX J. Etude immunochimique et électrophorétique des constituants protéiques des divers liquides biologiques de l'oeuf de poule au cours de l'incubation. Bull Soc Chim Biol (Paris) 1954;36(8):1037–1051. [PubMed] [Google Scholar]
- KARRER H. E. Electron-microscopic observations on developing chick embryo liver. The Golgi complex and its possible role in the formation of glycogen. J Ultrastruct Res. 1960 Nov;4:149–165. doi: 10.1016/s0022-5320(60)90050-2. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B. On the RNA in cultured myeloma cells producing immunoglobulin. Biochim Biophys Acta. 1969 Jun 17;182(2):361–374. doi: 10.1016/0005-2787(69)90187-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- La Via M. F., Prater T. K. Polyribosome formation in relation to accumulation of plasma cells. J Reticuloendothel Soc. 1971 Jan;9(1):1–12. [PubMed] [Google Scholar]
- Loeb J. N., Howell R., Tomkins G. M. Free and membrane-bound ribosomes in rat liver. J Biol Chem. 1967 May 10;242(9):2069–2074. [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- Nihei T. In vitro amino acid incorporation into myosin by free polysomes of rat skeletal muscle. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1139–1144. doi: 10.1016/0006-291x(71)90581-x. [DOI] [PubMed] [Google Scholar]
- PALADE G. E. A small particulate component of the cytoplasm. J Biophys Biochem Cytol. 1955 Jan;1(1):59–68. doi: 10.1083/jcb.1.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALADE G. E. THE ORGANIZATION OF LIVING MATTER. Proc Natl Acad Sci U S A. 1964 Aug;52:613–634. doi: 10.1073/pnas.52.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER K. R. Electron microscopy of basophilic components of cytoplasm. J Histochem Cytochem. 1954 Sep;2(5):346–375. doi: 10.1177/2.5.346. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis: studies of Islet polyribosomes (nascent peptides-sucrose gradient analysis-gel filtration). Proc Natl Acad Sci U S A. 1972 Feb;69(2):505–509. doi: 10.1073/pnas.69.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
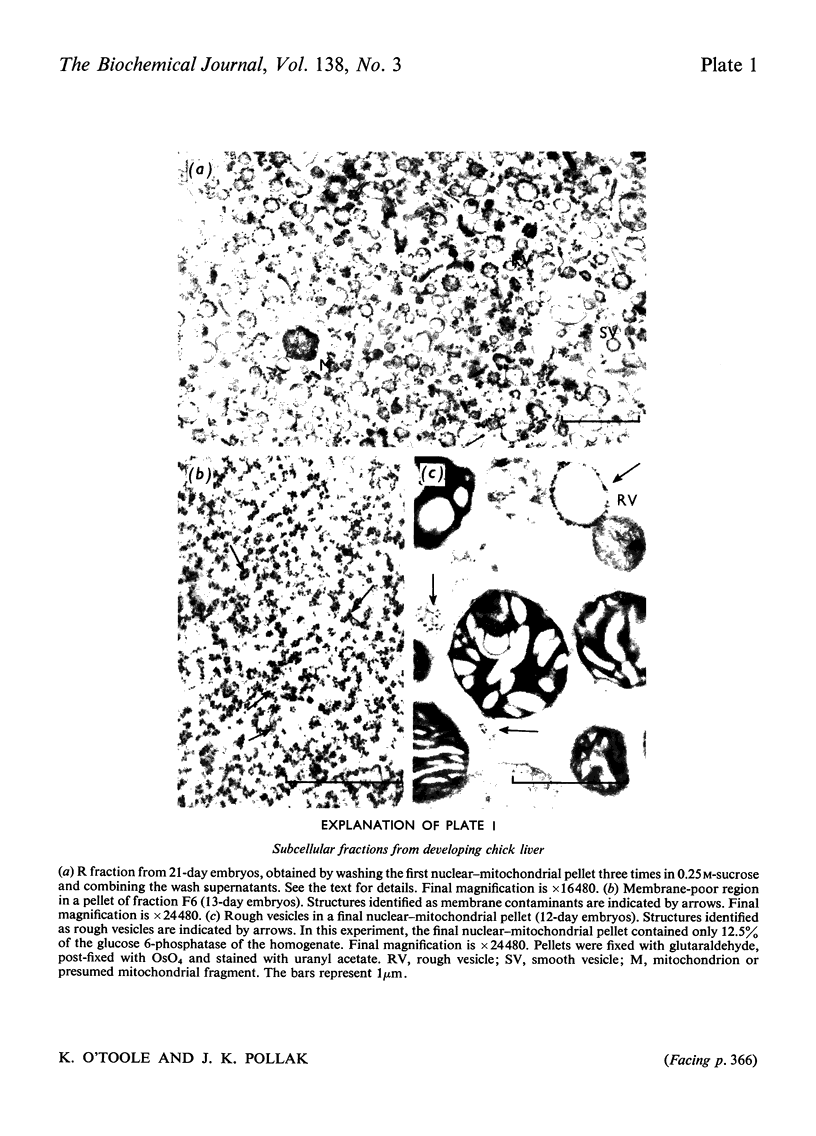
- Pollak J. K., Shorey C. D. Changes in ultrastructure of embryonic chick liver during morphogenesis. Aust J Exp Biol Med Sci. 1967 Aug;45(4):393–406. doi: 10.1038/icb.1967.38. [DOI] [PubMed] [Google Scholar]
- Pollak J. K., Ward D. B. Changes in the chemical composition and the enzymic activities of hepatic microsomes of the chick embryo during development. Biochem J. 1967 Jun;103(3):730–738. doi: 10.1042/bj1030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley G. C., Malt R. A. Development of the metanephric kidney. Protein and nucleic acid synthesis. J Cell Biol. 1968 Jun;37(3):703–715. doi: 10.1083/jcb.37.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryme I. F., Garatun-Tjeldstö O., Birckbichler P. J., Weltman J. K., Dowben R. M. Synthesis of immunoglobulins by membrane-bound polysomes and free polysomes from plasmacytoma cells. Eur J Biochem. 1973 Mar 1;33(2):374–378. doi: 10.1111/j.1432-1033.1973.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Ragnotti G., Lawford G. R., Campbell P. N. Biosynthesis of microsomal nicotinamide-adenine dinucleotide phosphate-cytochrome c reductase by membrane-bound and free polysomes from rat liver. Biochem J. 1969 Apr;112(2):139–147. doi: 10.1042/bj1120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M. Biosynthesis of serum proteins and ferritin by free and attached ribosomes of rat liver. J Biol Chem. 1969 Aug 25;244(16):4308–4315. [PubMed] [Google Scholar]
- Rosbash M., Penman S. Membrane-associated protein synthesis of mammalian cells. I. The two classes of membrane-associated ribosomes. J Mol Biol. 1971 Jul 28;59(2):227–241. doi: 10.1016/0022-2836(71)90048-9. [DOI] [PubMed] [Google Scholar]
- SCHJEIDE O. A., BINZ S., RAGAN N. Estrogen-induced serum protein synthesis in the liver of the chicken embryo. Growth. 1960 Dec;24:401–410. [PubMed] [Google Scholar]
- Sandström B., Westman J. Ultrastructure of the developing chicken liver before hatching. Z Zellforsch Mikrosk Anat. 1971;117(4):516–525. doi: 10.1007/BF00330712. [DOI] [PubMed] [Google Scholar]
- Sarma D. S., Reid I. M., Verney E., Sidransky H. Studies on the nature of attachment of ribosomes to membranes in liver. I. Influence of ethionine, sparsomycin, CCl 4 , and puromycin on membrane-bound polyribosomal disaggregation and on detachment of membrane-bound ribosomes from membranes. Lab Invest. 1972 Jul;27(1):39–47. [PubMed] [Google Scholar]
- Stephens R. J., Bils R. F. Ultrastructural changes in the developing chick liver. I. General cytology. J Ultrastruct Res. 1967 May;18(3):456–474. doi: 10.1016/s0022-5320(67)80130-8. [DOI] [PubMed] [Google Scholar]
- Takagi M., Ogata K. Isolation of serum albumin-synthesizing polysomes from rat liver. Biochem Biophys Res Commun. 1971 Jan 8;42(1):125–131. doi: 10.1016/0006-291x(71)90371-8. [DOI] [PubMed] [Google Scholar]
- Tata J. R. The formation, distribution and function of ribosomes and microsomal membranes during induced amphibian metamorphosis. Biochem J. 1967 Nov;105(2):783–801. doi: 10.1042/bj1050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLKIN E., COHN W. E. Estimation of nucleic acids. Methods Biochem Anal. 1954;1:287–305. doi: 10.1002/9780470110171.ch11. [DOI] [PubMed] [Google Scholar]
- Venkatesan N., Steele W. J. Free and membrane-bound polysomes of rat liver: separation in nearly quantitative yield and analysis of structure and function. Biochim Biophys Acta. 1972 Dec 22;287(3):526–537. doi: 10.1016/0005-2787(72)90298-5. [DOI] [PubMed] [Google Scholar]
- Vernie L. N., Bont W. S., Emmelot P. Ribosome monomers in rat liver following administration of dimethylnitrosamine. Cancer Res. 1971 Dec;31(12):2189–2195. [PubMed] [Google Scholar]
- Webb T. E., Blobel G., Potter V. R., Morris H. P. Polyribosomes in rat tissues. II. The polyribosome distribution in the minimal deviation hepatomas. Cancer Res. 1965 Sep;25(8):1219–1224. [PubMed] [Google Scholar]
- Wibo M., Amar-Costesec A., Berthet J., Beaufay H. Electron microscope examination of subcellular fractions. 3. Quantitative analysis of the microsomal fraction isolated from rat liver. J Cell Biol. 1971 Oct;51(1):52–71. doi: 10.1083/jcb.51.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]